Abstract
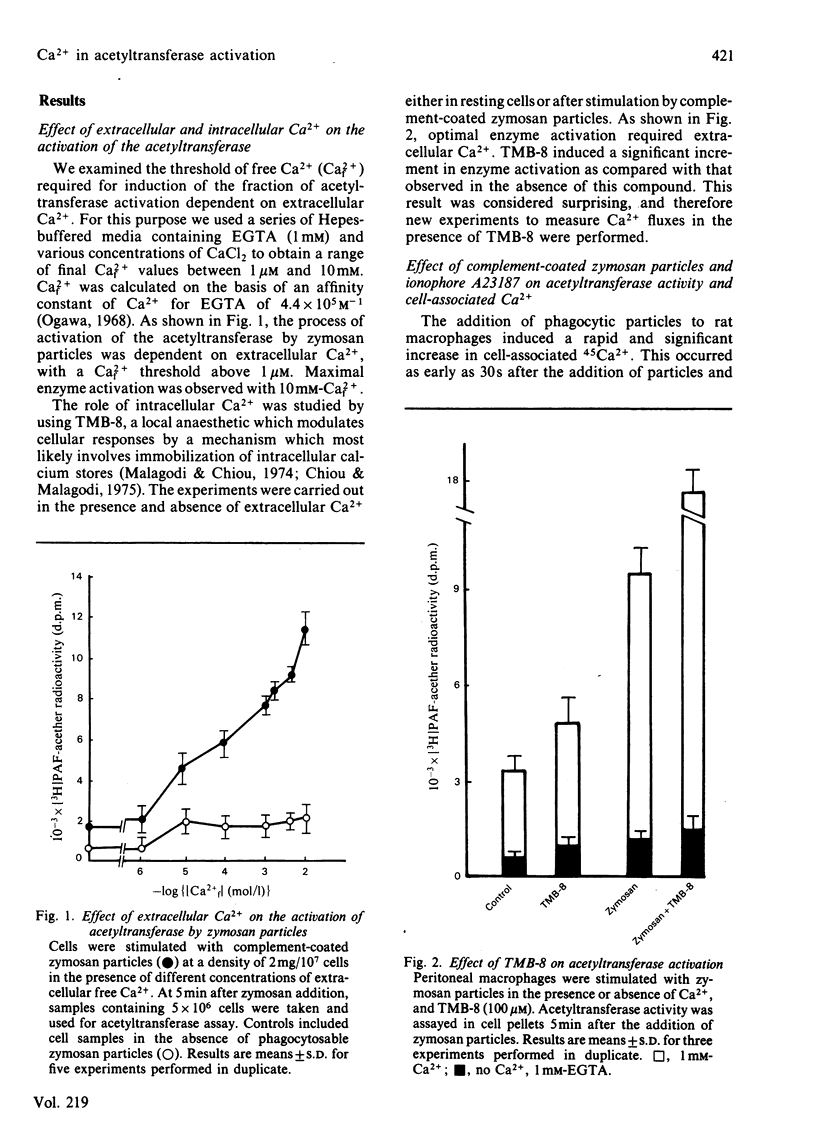
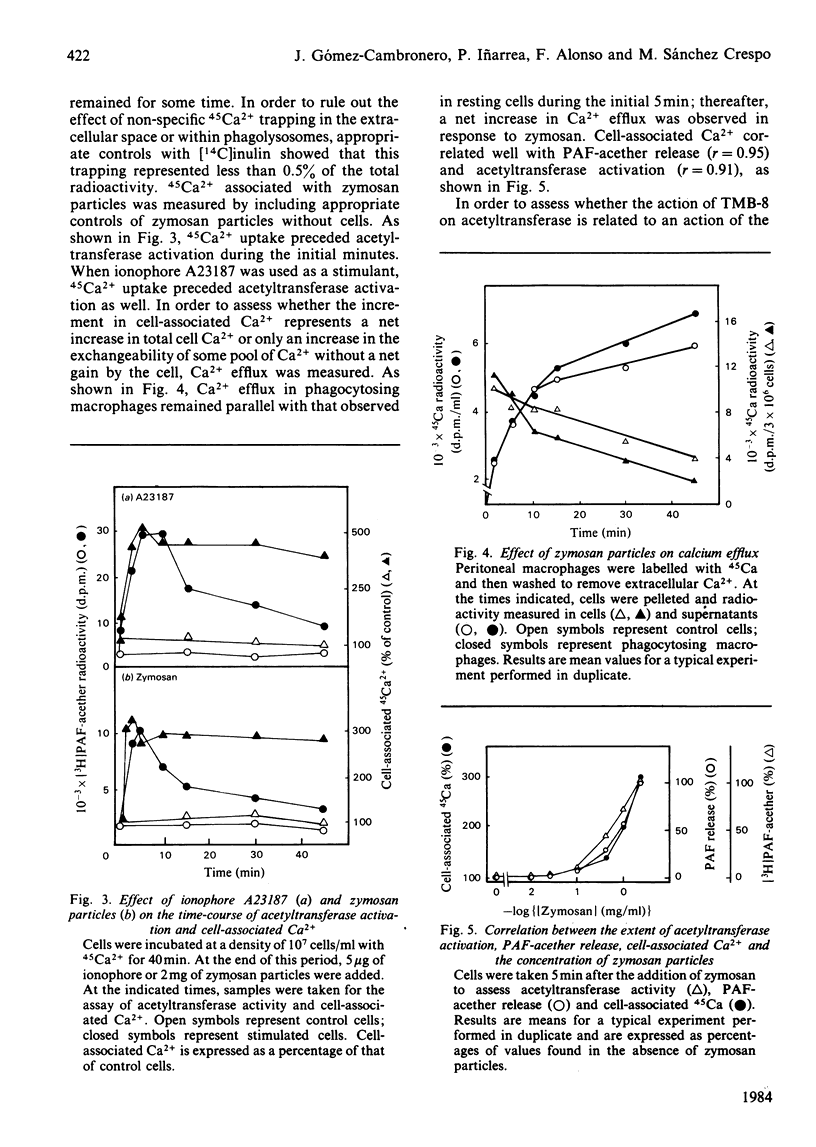
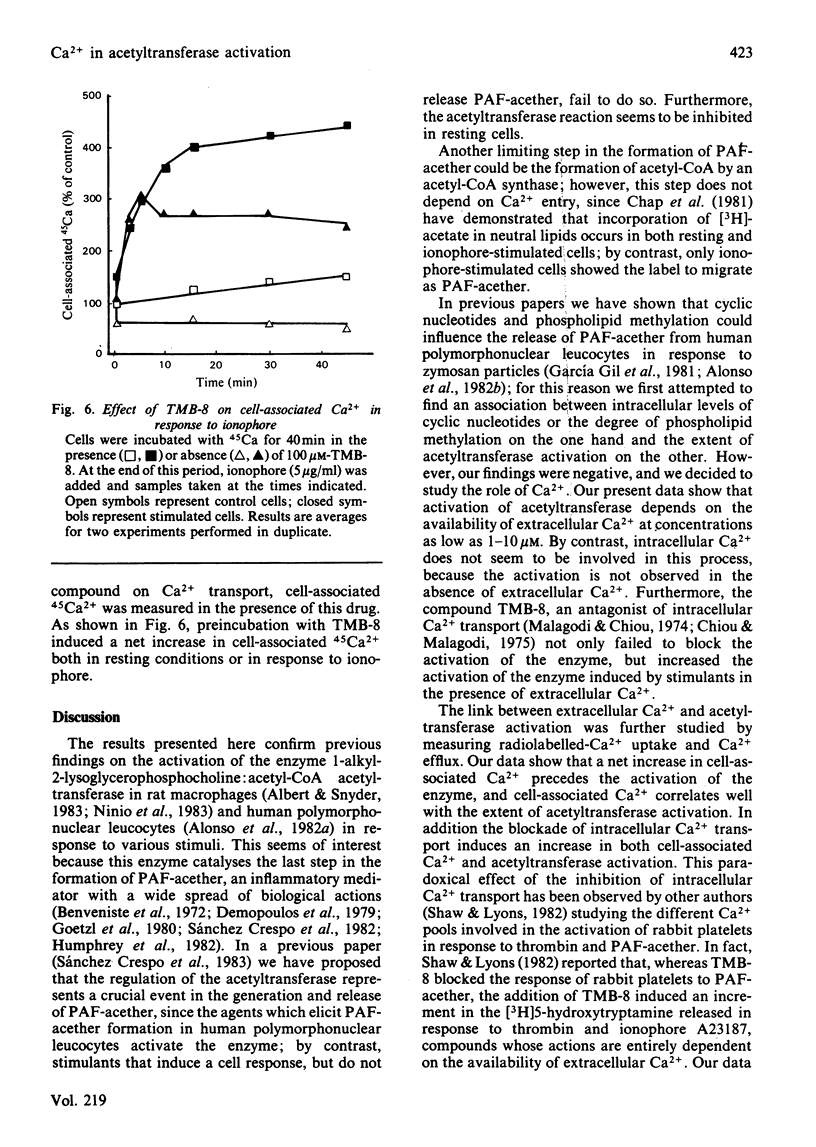
The role of Ca2+ in the activation of the enzyme lyso-(platelet-activating factor): acetyl-CoA acetyltransferase was studied in rat peritoneal macrophages in response to complement-coated zymosan particles and ionophore A23187. By using Ca2+-containing buffers, a threshold concentration of extracellular Ca2+ above 1 microM was found to be necessary to observe the activation of the enzyme in response to zymosan. By contrast, a significant role of intracellular Ca2+ in this process could be ruled out, since the putative intracellular calcium-transport antagonist TMB-8 [8-(NN-diethylamino)octyl-3,4,5-trimethoxybenzoate] did not inhibit the activation of the acetyltransferase induced by zymosan in the presence of extracellular Ca+. The link between acetyltransferase activation and extracellular Ca2+ transport was studied by measuring Ca2+ uptake in response to the stimuli. Zymosan particles induced a rapid increment in cell-associated Ca2+ which correlated well with the extent of acetyltransferase activation (r = 0.91) and with the release of platelet-activating factor (r = 0.95) in response to different doses of zymosan. Cellular Ca2+ efflux in response to zymosan particles was also measured and found to be increased, as compared with controls, when the activation of the acetyltransferase declined. In short, the data suggest that the entry of extracellular Ca2+ into the cell is a crucial event in the activation of acetyltransferase and, thereby, in the formation of platelet-activating factor in rat peritoneal macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Snyder F. Biosynthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine by rat alveolar macrophages. Phospholipase A2 and acetyltransferase activities during phagocytosis and ionophore stimulation. J Biol Chem. 1983 Jan 10;258(1):97–102. [PubMed] [Google Scholar]
- Alonso F., Gil M. G., Sánchez-Crespo M., Mato J. M. Activation of 1-alkyl-2-lysoglycero-3-phosphocholine. Acetyl-CoA transferase during phagocytosis in human polymorphonuclear leukocytes. J Biol Chem. 1982 Apr 10;257(7):3376–3378. [PubMed] [Google Scholar]
- Alonso F., Sánchez-Crespo M., Mato J. M. Modulatory role of cyclic AMP in the release of platelet-activating factor from human polymorphonuclear leucocytes. Immunology. 1982 Mar;45(3):493–500. [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. D., Fredholm B., Haegermark O. Studies on the time course of histamine release and morphological changes induced by histamine liberators in rat peritoneal mast cells. Acta Physiol Scand. 1967 Dec;71(4):270–282. doi: 10.1111/j.1748-1716.1967.tb03734.x. [DOI] [PubMed] [Google Scholar]
- Chap H., Mauco G., Simon M. F., Benveniste J., Douste-Blazy L. Biosynthetic labelling of platelet activating factor from radioactive acetate by stimulated platelets. Nature. 1981 Jan 22;289(5795):312–314. doi: 10.1038/289312a0. [DOI] [PubMed] [Google Scholar]
- Chiou C. Y., Malagodi M. H. Studies on the mechanism of action of a new Ca-2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol. 1975 Feb;53(2):279–285. doi: 10.1111/j.1476-5381.1975.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer E. B., Gallin J. I. Localization of submembranous cations to the leading end of human neutrophils during chemotaxis. J Cell Biol. 1979 Aug;82(2):369–379. doi: 10.1083/jcb.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Freedman M. H., Raff M. C. Induction of increased calcium uptake in mouse T lymphocytes by concanavalin A and its modulation by cyclic nucleotides. Nature. 1975 May 29;255(5507):378–382. doi: 10.1038/255378a0. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., Wiederhold M. L., Lipsky P. E., Rosenthal A. S. Spontaneous and induced membrane hyperpolarizations in macrophages. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):653–661. doi: 10.1002/jcp.1040860510. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Derian C. K., Tauber A. I., Valone F. H. Novel effects of 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphorycholine mediators on human leukocyte function: delineation of the specific roles of the acyl substituents. Biochem Biophys Res Commun. 1980 Jun 16;94(3):881–888. doi: 10.1016/0006-291x(80)91317-0. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Humphrey D. M., Hanahan D. J., Pinckard R. N. Induction of leukocytic infiltrates in rabbit skin by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1982 Sep;47(3):227–234. [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Malone B., Snyder F. Stimulation of calcium uptake by 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) in rabbit platelets: possible involvement of the lipoxygenase pathway. Arch Biochem Biophys. 1983 May;223(1):33–39. doi: 10.1016/0003-9861(83)90568-4. [DOI] [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca++ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in skeletal muscles. Pharmacology. 1974;12(1):20–31. doi: 10.1159/000136517. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Naccache P. H., Sha'afi R. I., Borgeat P., Goetzl E. J. Mono- and dihydroxyeicosatetraenoic acids alter calcium homeostasis in rabbit neutrophils. J Clin Invest. 1981 May;67(5):1584–1587. doi: 10.1172/JCI110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio E., Mencia-Huerta J. M., Benveniste J. Biosynthesis of platelet-activating factor (PAF-acether). V. Enhancement of acetyltransferase activity in murine peritoneal cells by calcium ionophore A23187. Biochim Biophys Acta. 1983 May 16;751(3):298–304. doi: 10.1016/0005-2760(83)90287-4. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Lyons R. M. Requirements for different Ca2+ pools in the activation of rabbit platelets. I. release reaction and protein phosphorylation. Biochim Biophys Acta. 1982 Feb 25;714(3):492–499. doi: 10.1016/0304-4165(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Sánchez-Crespo M., Alonso F., Iñarrea P., Alvarez V., Egido J. Vascular actions of synthetic PAF-acether (a synthetic platelet-activating factor) in the rat: evidence for a platelet independent mechanism. Immunopharmacology. 1982 Apr;4(2):173–185. doi: 10.1016/0162-3109(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]



