Abstract
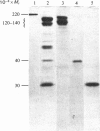
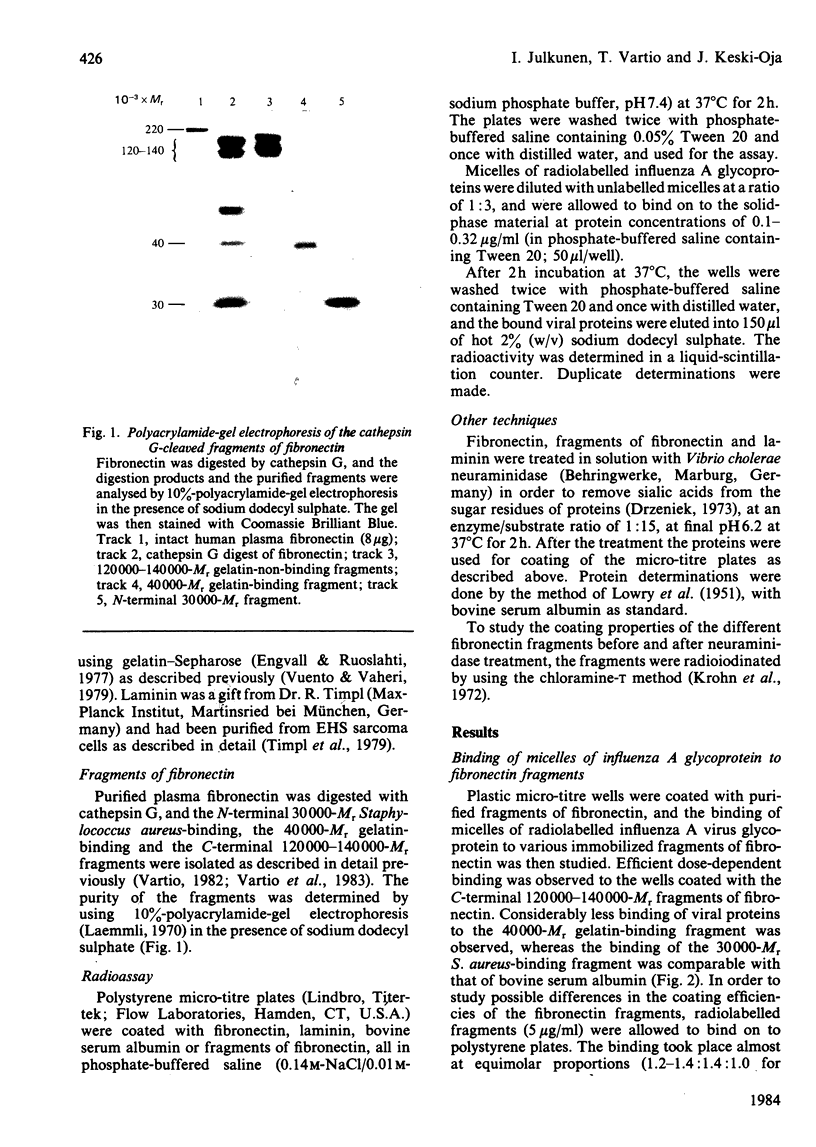
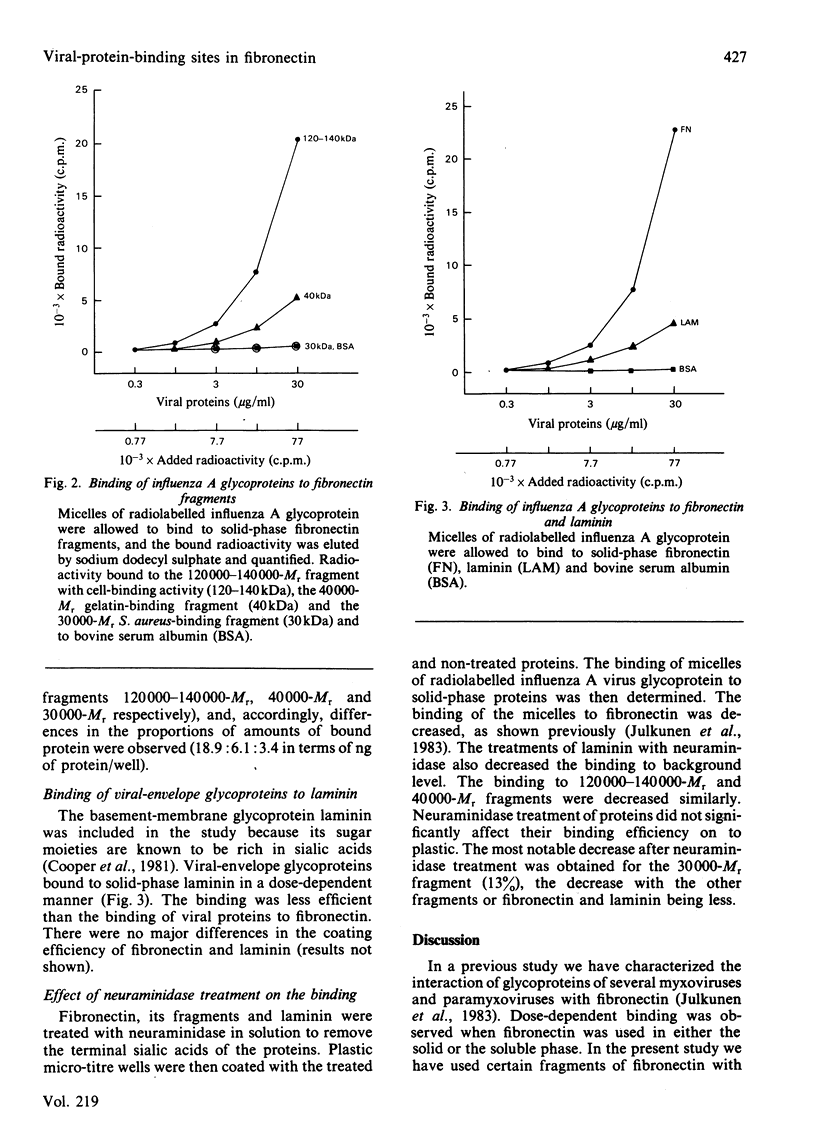
Purified viral-envelope glycoproteins from influenza A virus were found to bind to two fragments of the fibronectin molecule. Human plasma fibronectin was digested by leucocyte cathepsin G, and three different fragments, of Mr 30000, 40000 and 12000-140000, with specific binding functions were isolated. Micelles of radiolabelled influenza A glycoprotein were allowed to bind to these fragments immobilized on polystyrene micro-titre wells. The C-terminal 120000-14000-Mr fragments that carry the cell-binding activity bound viral proteins most efficiently, whereas the 40000-Mr gelatin-binding fragment bound considerably less. The N-terminal 30000-Mr Staphylococcus aureus-binding fragment was negative in the assays. Laminin, a basement-membrane protein, also bound viral proteins, though less effectively than fibronectin. The binding was abolished if laminin or fibronectin fragments were pretreated with neuraminidase. This suggests that the sialic acids in the sugar moieties of these glycoproteins are involved in the binding. The affinity of viral-envelope glycoproteins for certain domains of fibronectin and for laminin may play a role in virus-cell interactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Substrate specificity of neuraminidases. Histochem J. 1973 May;5(3):271–290. doi: 10.1007/BF01004994. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Isolation and biological characterization of active fragments of the adhesive glycoprotein fibronectin. Cell. 1979 Dec;18(4):1043–1051. doi: 10.1016/0092-8674(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Julkunen I., Hautanen A., Keski-Oja J. Interaction of viral envelope glycoproteins with fibronectin. Infect Immun. 1983 Jun;40(3):876–881. doi: 10.1128/iai.40.3.876-881.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn K., Sherman L., Welch M. Studies of radioiodinated fibrinogen. I. Physicochemical properties of the ICl, chloramine-T, and electrolytic reaction products. Biochim Biophys Acta. 1972 Dec 28;285(2):404–413. doi: 10.1016/0005-2795(72)90327-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luukkonen A., Gahmberg C. G., Renkonen O. Surface labeling of Semliki forest virus glycoproteins using galactose oxidase. Exposure of E3-glycoprotein. Virology. 1977 Jan;76(1):55–59. doi: 10.1016/0042-6822(77)90281-1. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Rohde H., Wick G., Timpl R. Immunochemical characterization of the basement membrane glycoprotein laminin. Eur J Biochem. 1979 Dec;102(1):195–201. doi: 10.1111/j.1432-1033.1979.tb06280.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Functional domain structure of fibronectin. Proc Natl Acad Sci U S A. 1980 May;77(5):2661–2665. doi: 10.1073/pnas.77.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Helenius A., Leonard K., Sarvas M., Gething M. J. Formation of protein micelles from amphiphilic membrane proteins. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5306–5310. doi: 10.1073/pnas.75.11.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vartio T. Characterization of the binding domains in the fragments cleaved by cathepsin G from human plasma fibronectin. Eur J Biochem. 1982 Apr 1;123(2):223–233. doi: 10.1111/j.1432-1033.1982.tb19757.x. [DOI] [PubMed] [Google Scholar]
- Vartio T., Salonen E. M., De Petro G., Barlati S., Miggiano V., Stähli C., Virgallita G., Takács B., Vaheri A. Monoclonal antibody against the N-terminal end of human plasma fibronectin. Biochem J. 1983 Oct 1;215(1):147–151. doi: 10.1042/bj2150147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Huard T. K. Macrophages express cell surface laminin. Exp Cell Res. 1983 Feb;143(2):475–479. doi: 10.1016/0014-4827(83)90077-0. [DOI] [PubMed] [Google Scholar]
- Wrann M. Methylation analysis of the carbohydrate portion of fibronectin isolated from human plasma. Biochem Biophys Res Commun. 1978 Sep 14;84(1):269–274. doi: 10.1016/0006-291x(78)90292-9. [DOI] [PubMed] [Google Scholar]