Abstract
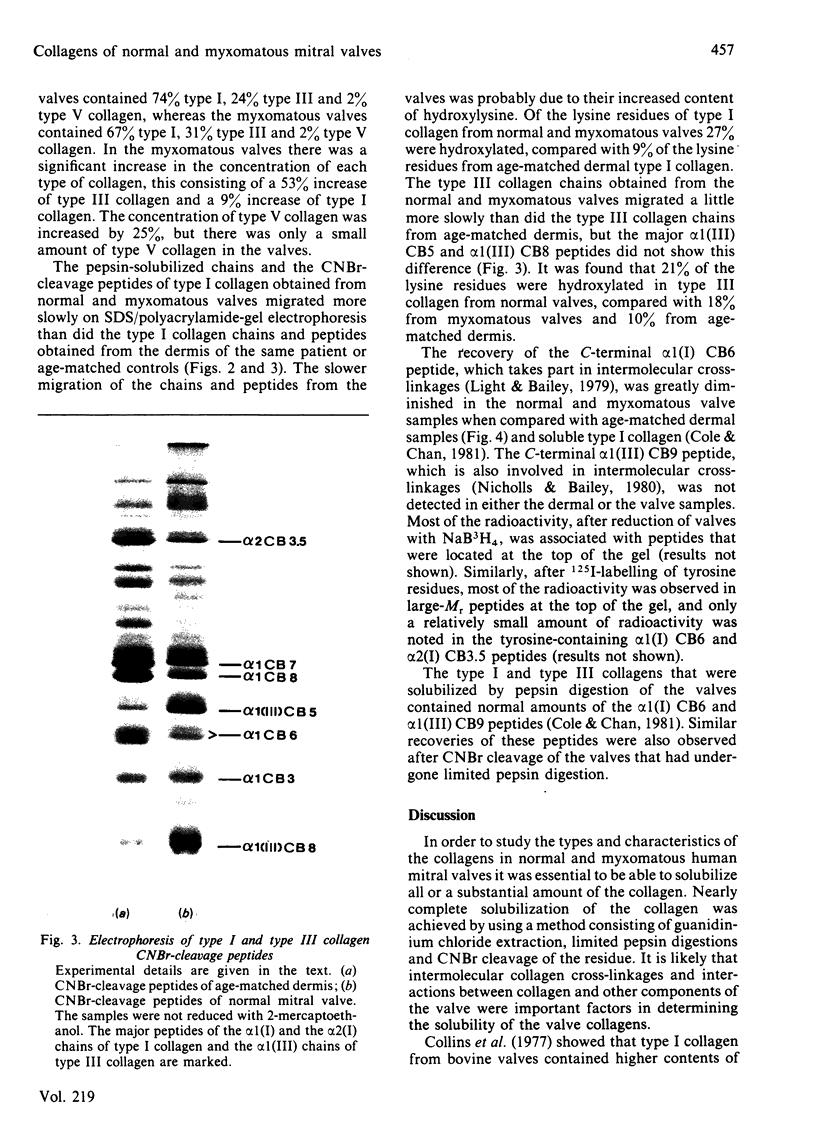
The collagens were studied in 13 normal and 19 myxomatous human mitral valves. The collagens of the valve were completely solubilized by using a method consisting of guanidinium chloride extraction, limited pepsin digestions and CNBr cleavage of the residue. The normal valves contained 74% type I, 24% type III and 2% type V collagen. The type I and type III collagens had similar solubility patterns, although only type I collagen was detected in the guanidinium chloride extract. Type V collagen was only detected in the first pepsin extract. The type I and III collagens had higher contents of hydroxylysine than did the same collagens from age-matched dermis. The two-dimensional electrophoretic 'maps' of CNBr-cleavage peptides showed low recoveries of the C-terminal alpha 1(I) CB6 and alpha 1(III) CB9 peptides, which are involved in forming intermolecular cross-linkages. Most of the reducible cross-linkages were present in large-Mr peptide complexes, and these complexes were shown by labelling with 125I to include the tyrosine-containing alpha 1(I) CB6 peptide. The myxomatous valves contained 67% type I, 31% type III and 2% type V collagens. There was a significant increase in the concentration of each type of collagen, which consisted of a 9% increase of type I collagen, a 53% increase of type III collagen and a 25% increase of type V collagen. The contents of hydroxylysine in type I and III collagens and the electrophoretic 'maps' of the CNBr-cleavage peptides involved in cross-linkages did not differ significantly from the results obtained from the normal valves. The biochemical findings suggest that there is an increased production of collagen, in particular type III collagen, and glycosaminoglycan as well as a proliferation of cells as part of a repair process in the myxomatous valves.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz H., Bächinger H. P., Glanville R., Kühn K. Physical evidence for the assembly of A and B chains of human placental collagen in a single triple helix. Eur J Biochem. 1978 Dec;92(2):563–567. doi: 10.1111/j.1432-1033.1978.tb12778.x. [DOI] [PubMed] [Google Scholar]
- Bonella D., Parker D. J., Davies M. J. Accumulation of procollagen in human floppy mitral valves. Lancet. 1980 Apr 19;1(8173):880–881. doi: 10.1016/s0140-6736(80)91382-3. [DOI] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- ChandraRajan J. Separation of type III collagen from type I collagen and pepsin by differential denaturation and renaturation. Biochem Biophys Res Commun. 1978 Jul 14;83(1):180–186. doi: 10.1016/0006-291x(78)90414-x. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Bean D. A. Analysis of collagen cyanogen bromide peptides using electrophoresis in continuous concave gradient polyacrylamide gels. Anal Biochem. 1979 Jan 1;92(1):183–188. doi: 10.1016/0003-2697(79)90642-0. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chan D. Analysis of the heterogeneity of human collagens by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1981 Aug 1;197(2):377–383. doi: 10.1042/bj1970377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D., Lindberg K., McLees B., Pinnell S. The collagen of heart valve. Biochim Biophys Acta. 1977 Nov 25;495(1):129–139. doi: 10.1016/0005-2795(77)90247-1. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Moore B. P., Braimbridge M. V. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978 May;40(5):468–481. doi: 10.1136/hrt.40.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Reducible crosslinks in hydroxylysine-deficient collagens of a heritable disorder of connective tissue. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2594–2598. doi: 10.1073/pnas.69.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay S., Rhodes R. K., Gay R. E., Miller E. J. Collagen molecules comprised of alpha 1(V)-chains (B-chains): an apparent localization in the exocytoskeleton. Coll Relat Res. 1981;1(1):53–58. doi: 10.1016/s0174-173x(80)80007-0. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Sanger F. The iodination of chymotrypsinogen. Biochem J. 1964 Jan;90(1):92–98. doi: 10.1042/bj0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D., Leier C. V., Baba N., Vasko J. S., Wooley C. F., Pinnell S. R. Altered collagen composition in a prolapsing mitral valve with ruptured chordae tendineae. Am J Med. 1979 Nov;67(5):863–866. doi: 10.1016/0002-9343(79)90746-0. [DOI] [PubMed] [Google Scholar]
- Henkel W., Glanville R. W. Covalent crosslinking between molecules of type I and type III collagen. The involvement of the N-terminal, nonhelical regions of the alpha 1 (I) and alpha 1 (III) chains in the formation of intermolecular crosslinks. Eur J Biochem. 1982 Feb;122(1):205–213. doi: 10.1111/j.1432-1033.1982.tb05868.x. [DOI] [PubMed] [Google Scholar]
- Hickey A. J., Wolfers J., Wilcken D. E. Mitral-valve prolapse: prevalence in an Australian population. Med J Aust. 1981 Jan 10;1(1):31–33. [PubMed] [Google Scholar]
- Jaffe A. S., Geltman E. M., Rodey G. E., Uitto J. Mitral valve prolapse: a consistent manifestation of type IV Ehlers-Danlos syndrome. The pathogenetic role of the abnormal production of type III collagen. Circulation. 1981 Jul;64(1):121–125. doi: 10.1161/01.cir.64.1.121. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Bashey R. I. Solubilization of bovine heart-value collagen. Biochem J. 1978 Jul 1;173(1):337–340. doi: 10.1042/bj1730337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. D., Clark M. A., Baba N., Kilman J. W., Wooley C. F. "Myxomatous" mitral valves: collagen dissolution as the primary defect. Circulation. 1982 Aug;66(2):288–296. doi: 10.1161/01.cir.66.2.288. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Cockerill P., McAnulty R. J., Hastings J. R. A simplified method for quantitation of the relative amounts of type I and type III collagen in small tissue samples. Anal Biochem. 1981 May 15;113(2):301–312. doi: 10.1016/0003-2697(81)90081-6. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Martin G. R., Kohn L. D., Byers P. H., McKusick V. A. Defect in conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science. 1973 Oct 19;182(4109):298–300. doi: 10.1126/science.182.4109.298. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Changes in crosslinking during aging in bovine tendon collagen. FEBS Lett. 1979 Jan 1;97(1):183–188. doi: 10.1016/0014-5793(79)80080-0. [DOI] [PubMed] [Google Scholar]
- Light N. D. Estimation of types I and III collagens in whole tissue by quantitation of CNBr peptides on SDS-polyacrylamide gels. Biochim Biophys Acta. 1982 Mar 18;702(1):30–36. doi: 10.1016/0167-4838(82)90024-3. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Bailey A. J. Identification of cyanogen bromide peptides involved in intermolecular cross-linking of bovine type III collagen. Biochem J. 1980 Jan 1;185(1):195–201. doi: 10.1042/bj1850195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Procacci P. M., Savran S. V., Schreiter S. L., Bryson A. L. Prevalence of clinical mitral-valve prolapse in 1169 young women. N Engl J Med. 1976 May 13;294(20):1086–1088. doi: 10.1056/NEJM197605132942004. [DOI] [PubMed] [Google Scholar]
- Roberts W. C., Perloff J. K. Mitral valvular disease. A clinicopathologic survey of the conditions causing the mitral valve to function abnormally. Ann Intern Med. 1972 Dec;77(6):939–975. doi: 10.7326/0003-4819-77-6-939. [DOI] [PubMed] [Google Scholar]
- Scheck M., Siegel R. C., Parker J., Chang Y. H., Fu J. C. Aortic aneurysm in Marfan's syndrome: changes in the ultrastructure and composition of collagen. J Anat. 1979 Oct;129(Pt 3):645–657. [PMC free article] [PubMed] [Google Scholar]
- Scott P. G., Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4(2):107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]