Abstract
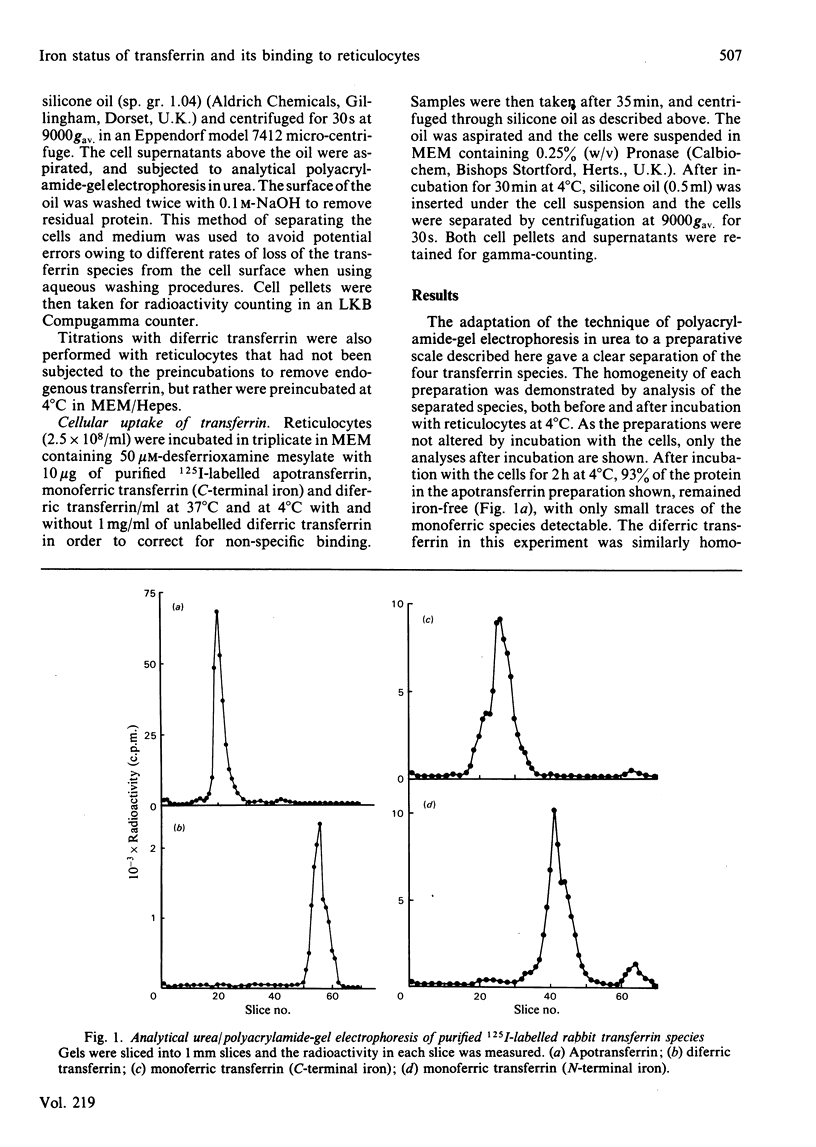
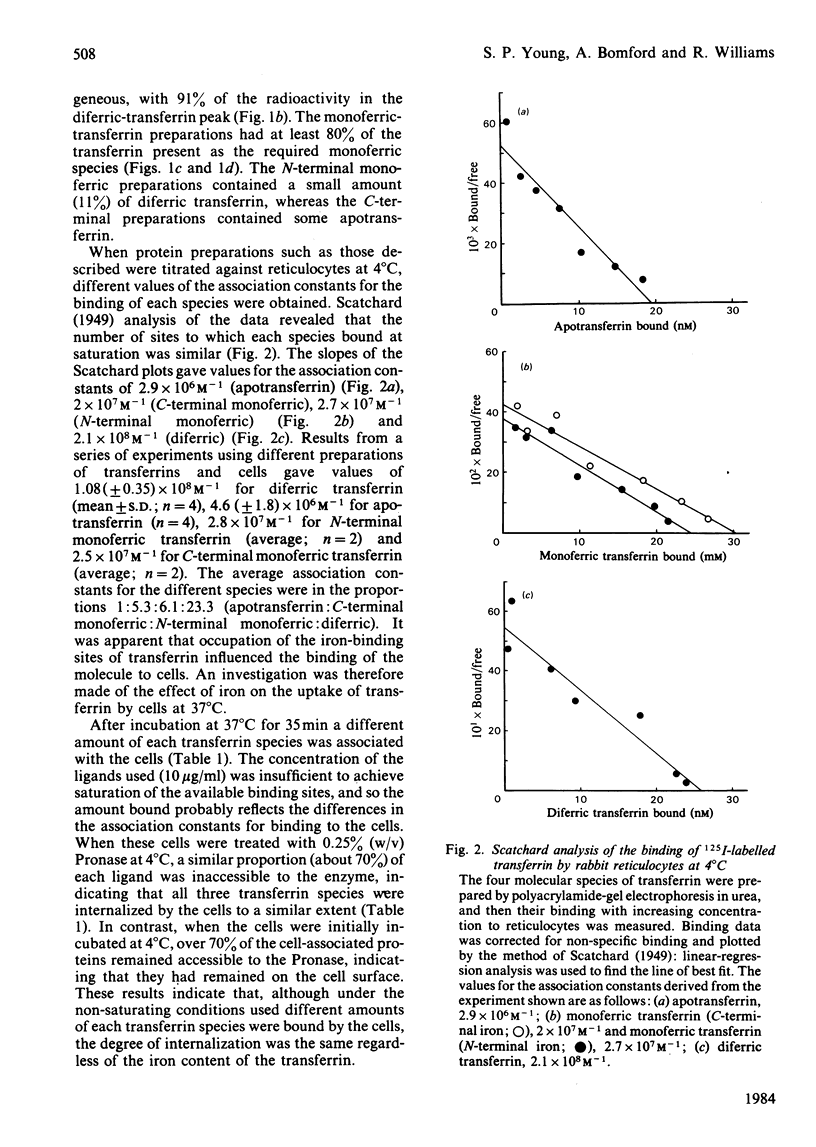
Polyacrylamide-gel electrophoresis in urea was used to prepare the four molecular species of transferrin:diferric transferrin, apotransferrin and the two monoferric transferrins with either the C-terminal or the N-terminal metal-binding site occupied. The interaction of these 125I-labelled proteins with rabbit reticulocytes was investigated. At 4 degrees C the average value for the association constant for the binding of transferrin to reticulocytes was found to increase with increasing iron content of the protein. The association constant for apotransferrin binding was 4.6 X 10(6)M-1, for monoferric (C-terminal iron) 2.5 X 10(7)M-1, for monoferric (N-terminal iron) 2.8 X 10(7)M-1 and for diferric transferrin, 1.1 X 10(8)M-1. These differences in the association constants did not affect the processing of the transferrin species by the cells at 37 degrees C. Accessibility of the proteins to extracellular proteinase indicated that the transferrin was internalized by the cells regardless of the iron content of the protein, since in each case 70% was inaccessible. Cycling of the cellular receptors may also occur in the absence of bound transferrin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E., Shaw D. C., Morgan E. H. Isolation and characterization of rabbit serum and milk transferrins. Evidence for difference in sialic acid content only. Biochemistry. 1968 Apr;7(4):1371–1378. doi: 10.1021/bi00844a019. [DOI] [PubMed] [Google Scholar]
- Bomford A., Young S. P., Nouri-Aria K., Williams R. Uptake and release of transferrin and iron by mitogen-stimulated human lymphocytes. Br J Haematol. 1983 Sep;55(1):93–101. doi: 10.1111/j.1365-2141.1983.tb01227.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Function of transferrin. Nature. 1968 Jun 29;218(5148):1211–1214. doi: 10.1038/2181211a0. [DOI] [PubMed] [Google Scholar]
- Fletcher J., Huehns E. R. Significance of the binding of iron by transferrin. Nature. 1967 Aug 5;215(5101):584–586. doi: 10.1038/215584a0. [DOI] [PubMed] [Google Scholar]
- HOSAIN F., FINCH C. A. FERROKINETICS: A STUDY OF TRANSPORT IRON IN PLASMA. J Lab Clin Med. 1964 Dec;64:905–912. [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. The mechanism of iron exchange between synthetic iron chelators and rabbit reticulocytes. Biochim Biophys Acta. 1974 Nov 27;373(1):84–99. doi: 10.1016/0005-2736(74)90108-4. [DOI] [PubMed] [Google Scholar]
- Huebers H. A., Csiba E., Huebers E., Finch C. A. Competitive advantage of diferric transferrin in delivering iron to reticulocytes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):300–304. doi: 10.1073/pnas.80.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebers H., Csiba E., Josephson B., Huebers E., Finch C. Interaction of human diferric transferrin with reticulocytes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):621–625. doi: 10.1073/pnas.78.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983 Aug 10;258(15):9108–9115. [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. The effect of metal attachment to human apotransferrin on its binding to reticulocytes. Biochim Biophys Acta. 1969 Nov 11;194(1):25–33. doi: 10.1016/0005-2795(69)90175-5. [DOI] [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- Lane R. S. Differences between human Fe1-transferrin molecules. Br J Haematol. 1975 Mar;29(3):511–520. doi: 10.1111/j.1365-2141.1975.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Leibman A., Aisen P. Distribution of iron between the binding sites of transferrin in serum: methods and results in normal human subjects. Blood. 1979 Jun;53(6):1058–1065. [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Van der Heul C., Kroos M. J., Van Noort W. L., Van Eijk H. G. No functional difference of the two iron-binding sites of human transferrin in vitro. Clin Sci (Lond) 1981 Feb;60(2):185–190. doi: 10.1042/cs0600185. [DOI] [PubMed] [Google Scholar]
- Young S. P., Aisen P. The interaction of transferrin with isolated hepatocytes. Biochim Biophys Acta. 1980 Dec 1;633(2):145–153. doi: 10.1016/0304-4165(80)90400-6. [DOI] [PubMed] [Google Scholar]
- Young S. P., Aisen P. Transferrin receptors and the uptake and release of iron by isolated hepatocytes. Hepatology. 1981 Mar-Apr;1(2):114–119. doi: 10.1002/hep.1840010205. [DOI] [PubMed] [Google Scholar]
- Young S. P. Evidence for the functional equivalence of the iron-binding sites of rat transferrin. Biochim Biophys Acta. 1982 Sep 17;718(1):35–41. doi: 10.1016/0304-4165(82)90006-x. [DOI] [PubMed] [Google Scholar]


