Abstract
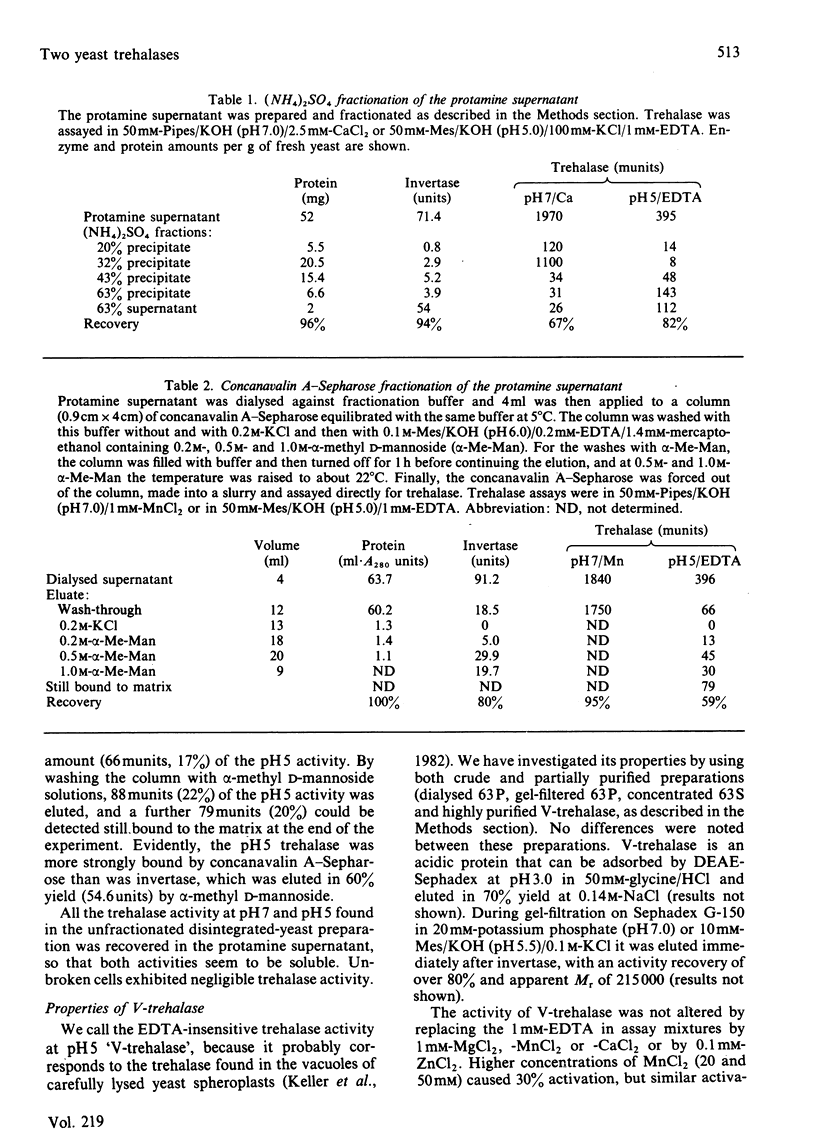
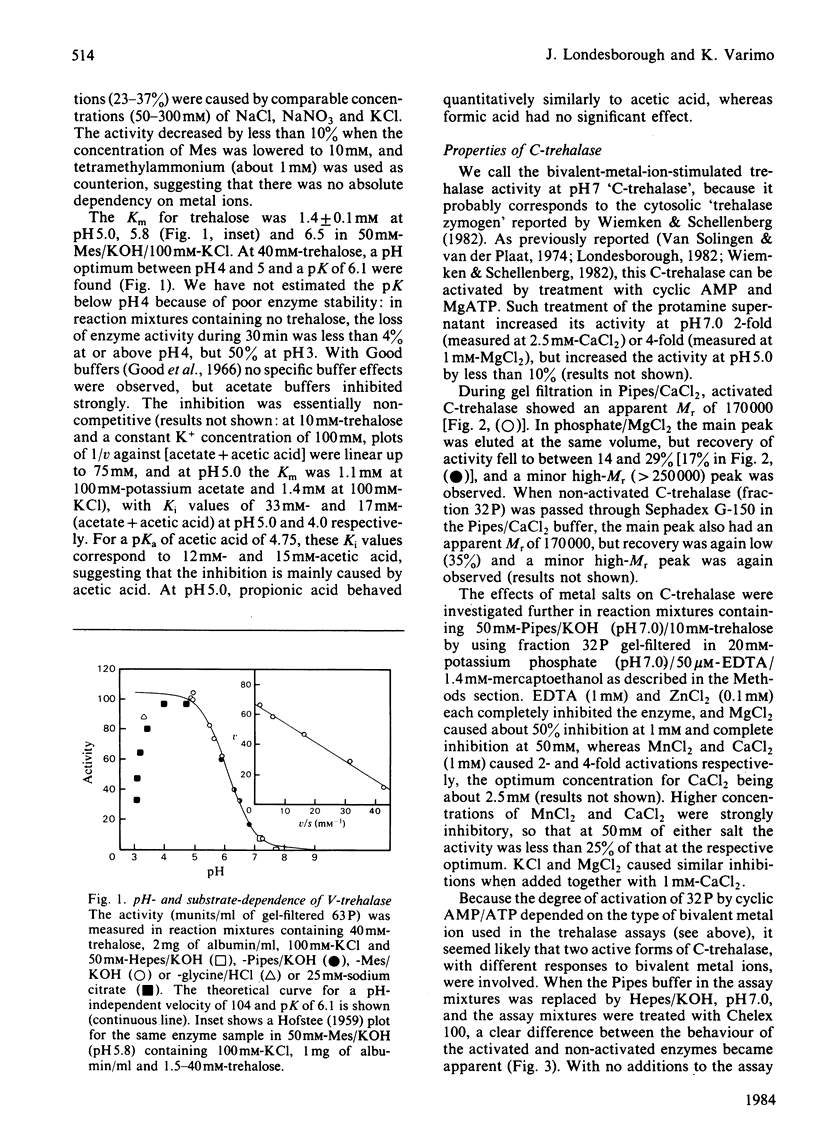
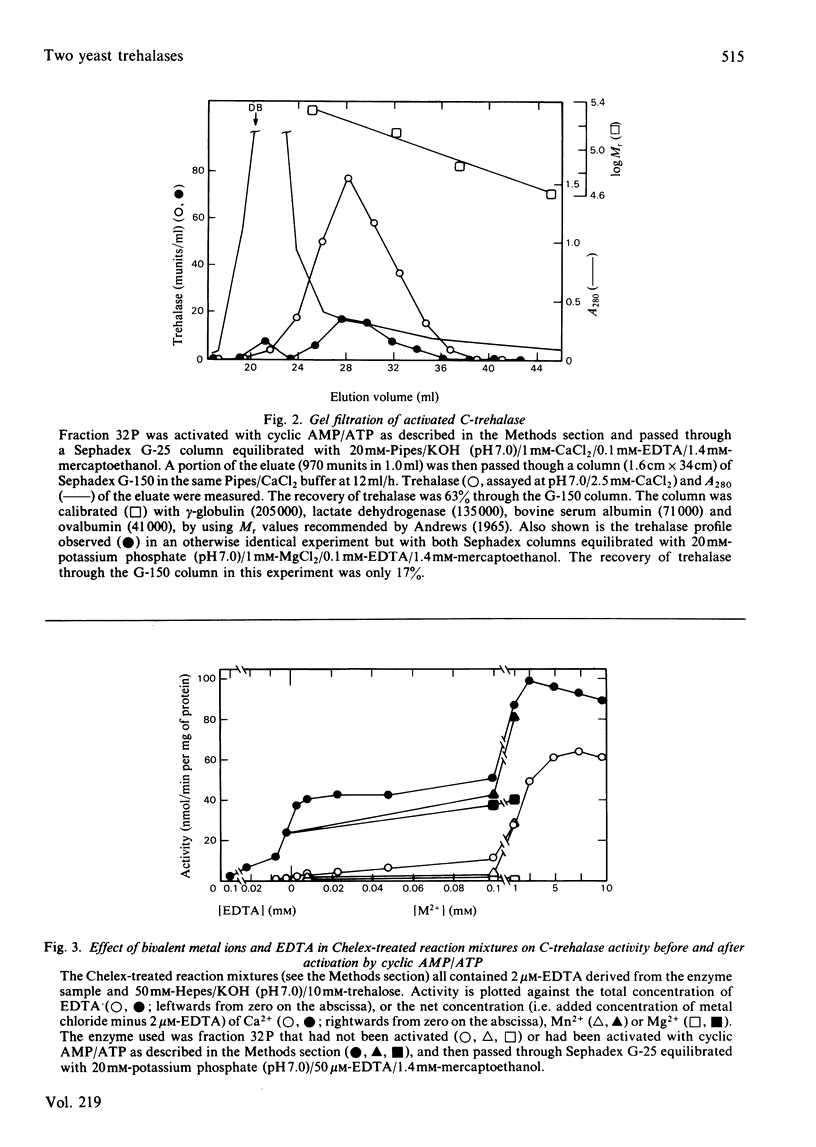
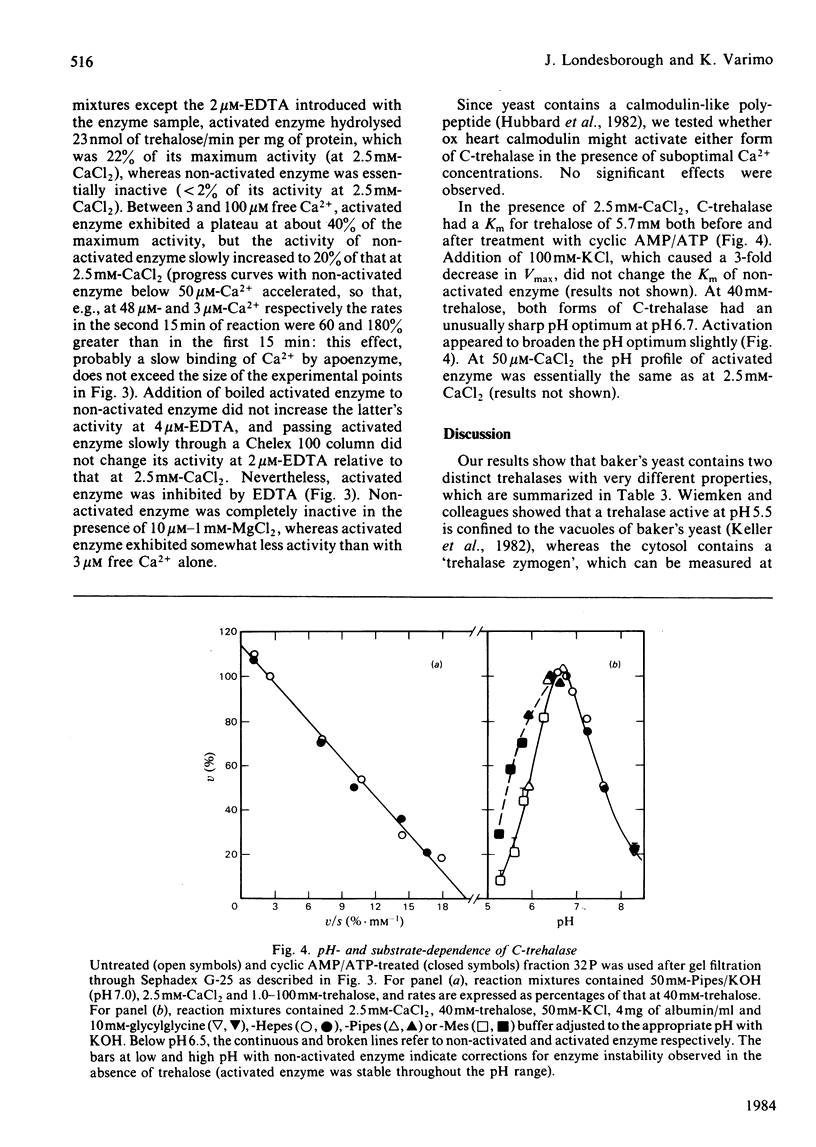
Trehalase activities at pH 5 (not inhibited by EDTA) and pH 7 (inhibited by EDTA) were present in the soluble fraction of disintegrated commercial baker's yeast. The pH 5 activity binds strongly to concanavalin A, is only partially salted out by saturated (NH4)2SO4, has an apparent Mr of 215000 (by gel filtration) and is an acidic protein. It has a Km of 1.4 mM, a broad pH optimum (at 40 mM-trehalose) between pH 4 and 5, and is activated by about 30% by 20-300 mM neutral salts such as KCl, NaNO3 and MnCl2. The enzyme is strongly inhibited by acetic acid/acetate buffers, with a Ki of about 15 mM-acetic acid. The pH 7 activity does not bind to concanavalin A, is salted out at 20-32% (w/v) (NH4)2SO4 and has an Mr of 170000 (by gel filtration). It is absolutely dependent on Ca2+ or Mn2+ ions (Mg2+ is ineffective) and strongly inhibited by neutral salts in the 20-100 mM range. It can be activated by treatment with MgATP in the presence of cyclic AMP. Activation decreases, but does not abolish, the Ca2+ requirement, and does not change the Km for trehalose (5.7 mM) or shift the sharp pH optimum at pH 6.7 (at 40 mM-trehalose).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigad G., Ziv O., Neufeld E. Intracellular trehalase of a hybrid yeast. Biochem J. 1965 Dec;97(3):715–722. doi: 10.1042/bj0970715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Taussig R., Kustu S., Botstein D. The secreted form of invertase in Saccharomyces cerevisiae is synthesized from mRNA encoding a signal sequence. Mol Cell Biol. 1983 Mar;3(3):439–447. doi: 10.1128/mcb.3.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- Hubbard M., Bradley M., Sullivan P., Shepherd M., Forrester I. Evidence for the occurrence of calmodulin in the yeasts Candida albicans and Saccharomyces cerevisiae. FEBS Lett. 1982 Jan 11;137(1):85–88. doi: 10.1016/0014-5793(82)80320-7. [DOI] [PubMed] [Google Scholar]
- KOTYK A. Intracellular pH of Baker's yeast. Folia Microbiol (Praha) 1963 Jan;8:27–31. doi: 10.1007/BF02868762. [DOI] [PubMed] [Google Scholar]
- Keller F., Schellenberg M., Wiemken A. Localization of trehalase in vacuoles and of trehalose in the cytosol of yeast (Saccharomyces cerevisiae). Arch Microbiol. 1982 Jun;131(4):298–301. doi: 10.1007/BF00411175. [DOI] [PubMed] [Google Scholar]
- Kelly P. J., Catley B. J. A purification of trehalase from Saccharomyces cerevisiae. Anal Biochem. 1976 May 7;72:353–358. doi: 10.1016/0003-2697(76)90541-8. [DOI] [PubMed] [Google Scholar]
- Londesborough J. Cyclic nucleotide-dependent inactivation of yeast fructose 1,6-bisphosphatase by ATP. FEBS Lett. 1982 Aug 2;144(2):269–272. doi: 10.1016/0014-5793(82)80652-2. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Calcium pumps: introduction. Fed Proc. 1980 May 15;39(7):2401–2402. [PubMed] [Google Scholar]
- Nieuwenhuis B. J., Weijers C. A., Borst-Pauwels G. W. Uptake and accumulation of Mn2+ and Sr2+ in Saccharomyces cerevisiae. Biochim Biophys Acta. 1981 Nov 20;649(1):83–88. doi: 10.1016/0005-2736(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Lichko L. P., Kadomtseva V. M., Kholodenko V. P., Titovsky V. T., Kulaev I. S. Energy-dependent transport of manganese into yeast cells and distribution of accumulated ions. Eur J Biochem. 1977 May 16;75(2):373–377. doi: 10.1111/j.1432-1033.1977.tb11538.x. [DOI] [PubMed] [Google Scholar]
- Ortiz C. H., Maia J. C., Tenan M. N., Braz-Padrão G. R., Mattoon J. R., Panek A. D. Regulation of yeast trehalase by a monocyclic, cyclic AMP-dependent phosphorylation-dephosphorylation cascade system. J Bacteriol. 1983 Feb;153(2):644–651. doi: 10.1128/jb.153.2.644-651.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANEK A., SOUZA N. O. PURIFICATION AND PROPERTIES OF BAKERS' YEAST TREHALASE. J Biol Chem. 1964 Jun;239:1671–1673. [PubMed] [Google Scholar]
- Panek A. D. Adenosine triphosphate inhibition of yeast trehalase. J Bacteriol. 1969 Sep;99(3):904–905. doi: 10.1128/jb.99.3.904-905.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavík J. Intracellular pH of yeast cells measured with fluorescent probes. FEBS Lett. 1982 Apr 5;140(1):22–26. doi: 10.1016/0014-5793(82)80512-7. [DOI] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Genetic and biochemical evidence that trehalase is a substrate of cAMP-dependent protein kinase in yeast. J Biol Chem. 1983 Sep 25;258(18):10867–10872. [PubMed] [Google Scholar]
- Wiemken A., Schellenberg M. Does a cyclic AMP-dependent phosphorylation initiate the transfer of trehalase from the cytosol into the vacuoles in Saccharomyces cerevisiae? FEBS Lett. 1982 Dec 27;150(2):329–331. doi: 10.1016/0014-5793(82)80762-x. [DOI] [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem Biophys Res Commun. 1975 Feb 3;62(3):553–560. doi: 10.1016/0006-291x(75)90434-9. [DOI] [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3',5'-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem Biophys Res Commun. 1974 Feb 4;56(3):580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]



