Abstract
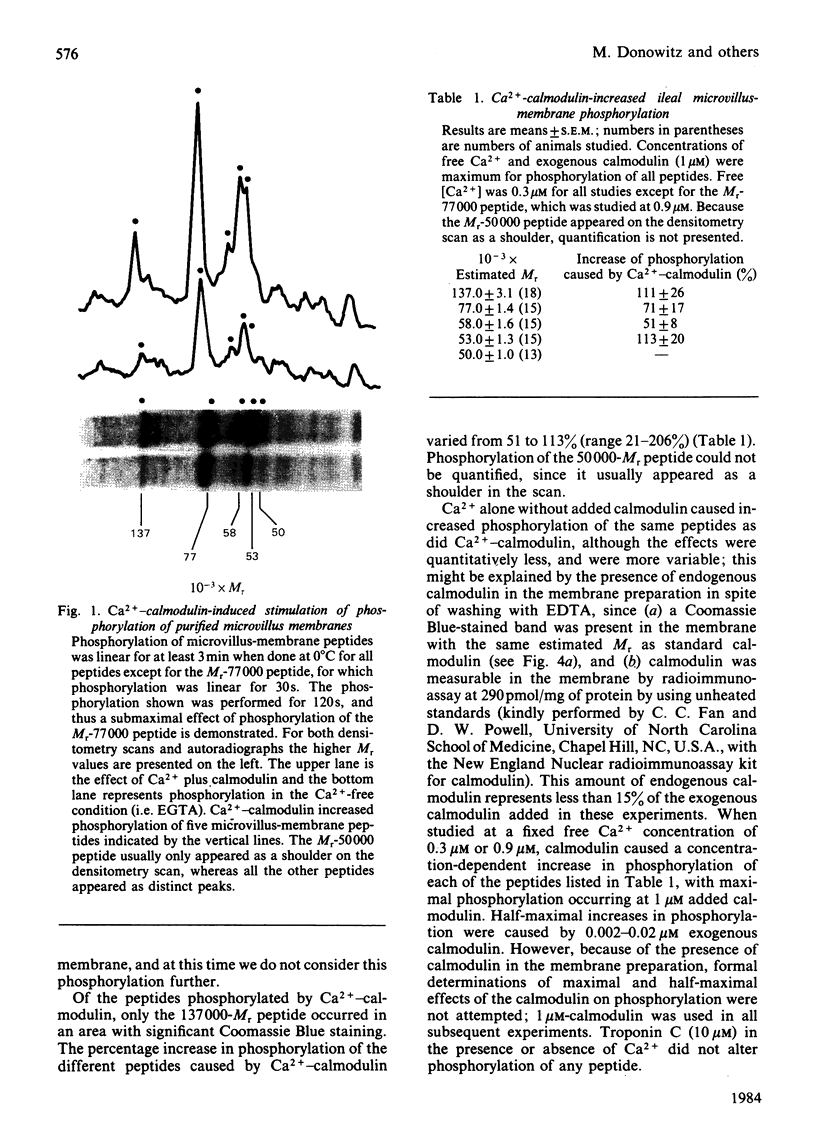
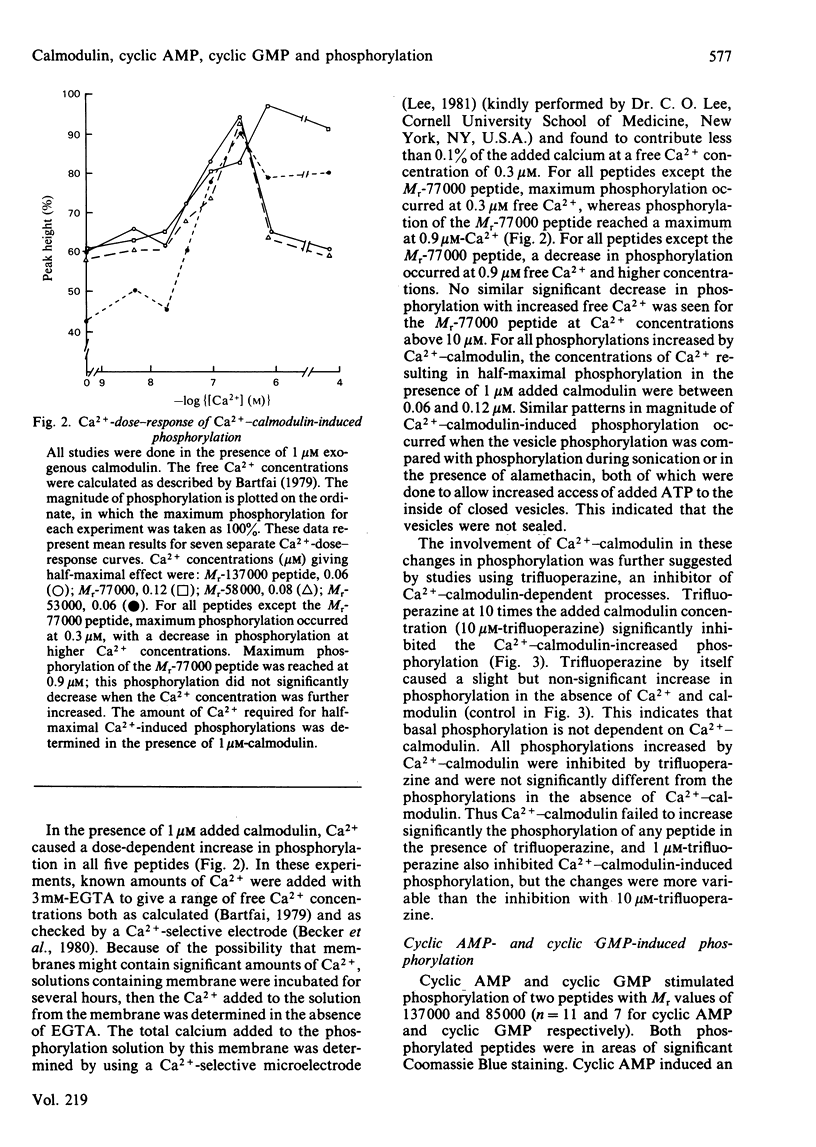
Evidence is available to suggest that Ca2+-calmodulin and cyclic nucleotides are involved in the regulation of ion transport in rabbit ileum. Since both Ca2+-calmodulin and cyclic nucleotides exert many of their effects by phosphorylation, the effects of Ca2+-calmodulin and cyclic nucleotides on phosphorylation of purified microvillus membrane from rabbit ileal mucosa were evaluated. Ca2+-calmodulin increased phosphorylation of five microvillus-membrane peptides, with Mr values of 137000, 77000, 58000, 53000 and 50000. The increases in phosphorylation caused by Ca2+-calmodulin were: Mr-137000 peptide, 111 +/- 26%; Mr-77000 peptide, 71 +/- 17%; Mr-58000 peptide, 51 +/- 8%; Mr-53000 peptide, 113 +/- 20%. These increases were maximal at 1 microM-calmodulin and 0.3-0.9 microM free Ca2+; concentrations of Ca2+ causing half-maximal effects on phosphorylation for the different peptides were 0.06-0.12 microM. Cyclic AMP and cyclic GMP increased phosphorylation of two peptides, of Mr 137000 and 85000. The concentrations of cyclic nucleotides giving half-maximal phosphorylation of the Mr-137000 peptide were 0.3 microM-cyclic AMP and 4.6 microM-cyclic GMP, and for the Mr-85000 peptide, 3.9 microM-cyclic AMP and 0.05 microM-cyclic GMP. The maximal increase in phosphorylation of the Mr-137000 peptide was 200% for cyclic AMP and 95% for cyclic GMP, and that of the Mr-85000 peptide was 220% for cyclic AMP and 120% for cyclic GMP. These studies demonstrate the existence of Ca2+-calmodulin-, cyclic AMP- and cyclic GMP-dependent protein kinases and substrate proteins in purified rabbit ileal microvillus membranes and that Ca2+ can regulate phosphorylation of these proteins over the presumed physiological concentration range of cytosol free Ca2+.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERS R. W., RODRIGUEZDE LORES, DEROBERTIS E. SODIUM-POTASSIUM-ACTIVATED ATPASE AND POTASSIUM-ACTIVATED P-NITROPHENYLPHOSPHATASE: A COMPARISON OF THEIR SUBCELLULAR LOCALIZATIONS IN RAT BRAIN. Proc Natl Acad Sci U S A. 1965 Mar;53:557–564. doi: 10.1073/pnas.53.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Leone G. R., Martin D. B. Identification and subcellular distribution of adipocyte peptides and phosphopeptides. J Biol Chem. 1976 Mar 10;251(5):1505–1510. [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Activation of protein kinase by physiological concentrations of cyclic AMP. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3580–3583. doi: 10.1073/pnas.71.9.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Chrisman T. D., Vandenheede J. R., Khandelwal R. L., Gella F. J., Upton J. D., Krebs E. G. Purification and regulatory properties of liver phosphorylase kinase. Adv Enzyme Regul. 1980;18:145–159. doi: 10.1016/0065-2571(80)90013-8. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Casnellie J. E., Catterall W. A. Selective phosphorylation of the alpha subunit of the sodium channel by cAMP-dependent protein kinase. J Biol Chem. 1982 Jul 25;257(14):7918–7921. [PubMed] [Google Scholar]
- Donowitz M., Asarkof N. Calcium dependence of basal electrolyte transport in rabbit ileum. Am J Physiol. 1982 Jul;243(1):G28–G35. doi: 10.1152/ajpgi.1982.243.1.G28. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N., Pike G. Calcium dependence of serotonin-induced changes in rabbit ileal electrolyte transport. J Clin Invest. 1980 Aug;66(2):341–352. doi: 10.1172/JCI109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M. Ca2+ in the control of active intestinal Na and Cl transport: involvement in neurohumoral action. Am J Physiol. 1983 Aug;245(2):G165–G177. doi: 10.1152/ajpgi.1983.245.2.G165. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cusolito S., Battisti L., Fogel R., Sharp G. W. Dopamine stimulation of active Na and Cl absorption in rabbit ileum: interaction with alpha 2-adrenergic and specific dopamine receptors. J Clin Invest. 1982 Apr;69(4):1008–1016. doi: 10.1172/JCI110504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Fogel R., Battisti L., Asarkof N. The neurohumoral secretagogues carbachol, substance P and neurotensin increase Ca++ influx and calcium content in rabbit ileum. Life Sci. 1982 Nov 1;31(18):1929–1937. doi: 10.1016/0024-3205(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Gensheimer H. P. Cyclic AMP-dependent protein kinase does not phosphorylate cyclic GMP-dependent protein kinase in vitro. FEBS Lett. 1983 Jan 10;151(1):71–75. doi: 10.1016/0014-5793(83)80345-7. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Ilundain A., Naftalin R. J. Role of Ca(2+)-dependent regulator protein in intestinal secretion. Nature. 1979 May 31;279(5712):446–448. doi: 10.1038/279446a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Lehrer S. S. Intrinsic fluorescence studies on troponin C. Arch Biochem Biophys. 1978 Apr 15;187(1):243–251. doi: 10.1016/0003-9861(78)90030-9. [DOI] [PubMed] [Google Scholar]
- Lee C. O. Ionic activities in cardiac muscle cells and application of ion-selective microelectrodes. Am J Physiol. 1981 Oct;241(4):H459–H478. doi: 10.1152/ajpheart.1981.241.4.H459. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Taylor A., Windhager E. E. Cytosolic calcium ion activity in epithelial cells of Necturus kidney. Nature. 1980 Oct 30;287(5785):859–861. doi: 10.1038/287859a0. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Uhm D. Y., Dresdner K. Sodium-calcium exchange in rabbit heart muscle cells: direct measurement of sarcoplasmic Ca2+ activity. Science. 1980 Aug 8;209(4457):699–701. doi: 10.1126/science.7394527. [DOI] [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Mircheff A. K., Wright E. M. Analytical isolation of plasma membranes of intestinal epithelial cells: identification of Na, K-ATPase rich membranes and the distribution of enzyme activities. J Membr Biol. 1976 Sep 17;28(4):309–333. doi: 10.1007/BF01869703. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S., Howe C. L. The brush border of intestinal epithelium: a model system for analysis of cell-surface architecture and motility. Methods Cell Biol. 1982;25(Pt B):143–174. doi: 10.1016/s0091-679x(08)61424-7. [DOI] [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Sharp G., Stiel D., Peters T. J. Cytochemical studies on the localization of alkaline phosphatase and HCO-3-activated adenosine triphosphatase in the brush border membrane of rat duodenal enterocytes. Histochem J. 1983 Nov;15(11):1131–1139. doi: 10.1007/BF01003976. [DOI] [PubMed] [Google Scholar]
- Shlatz L. J., Kimberg D. V., Cattieu K. A. Phosphorylation of specific rat intestinal microvillus and basal-lateral membrane proteins by cyclic nucleotides. Gastroenterology. 1979 Feb;76(2):293–299. [PubMed] [Google Scholar]
- Smith P. L., Blumberg J. B., Stoff J. S., Field M. Antisecretory effects of indomethacin on rabbit ileal mucosa in vitro. Gastroenterology. 1981 Feb;80(2):356–365. [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Katz A. M. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Apr 10;250(7):2640–2647. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Taylor L., Guerina V. J., Donowitz M., Cohen M., Sharp G. W. Calcium and calmodulin-dependent protein phosphorylation in rabbit ileum. FEBS Lett. 1981 Aug 31;131(2):322–324. doi: 10.1016/0014-5793(81)80395-x. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiser M. M., Neumeier M. M., Quaroni A., Kirsch K. Synthesis of plasmalemmal glycoproteins in intestinal epithelial cells. Separation of Golgi membranes from villus and crypt cell surface membranes; glycosyltransferase activity of surface membrane. J Cell Biol. 1978 Jun;77(3):722–734. doi: 10.1083/jcb.77.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge H. R. Cyclic GMP-dependent protein kinase in intestinal brushborders. Adv Cyclic Nucleotide Res. 1981;14:315–333. [PubMed] [Google Scholar]