Abstract
Biofilms are major virulence factors formed by pathogenic bacteria to invade their host and maintain their colony. While biofilms usually develop on diverse solid surfaces, floating biofilms, also called pellicles, are formed at the air–liquid interface. To address the problem of biofilm formation by bacterial pathogens, honey has been extensively studied. However, information on the effect of honey on biofilm formation by plant pathogens is scarce. This study aimed to determine the effects of manuka honey on biofilm and pellicle formation by Pectobacterium brasiliense and analyze the expression of genes encoding proteins needed to form biofilm by using semiquantitative PCR and RT-qPCR. Treatment with 5% (w/v) of manuka honey significantly decreased biofilm and pellicle formation by P. brasiliense. RT-qPCR results showed that the expression of bcsA, fis, hrpL, and expI decreased 7.07-fold, 5.71-fold, 13.11-fold, and 6.26-fold, respectively, after exposure to 5% (w/v) manuka honey. Our findings reveal that manuka honey may effectively inhibit biofilm and pellicle formation.
1. Introduction
A biofilm is a three-dimensional accumulation of bacterial communities that adheres to solid surfaces and is surrounded by a self-produced matrix of extracellular polymeric substances (EPSs) [1]. EPS is mainly composed of polysaccharides, proteins, nucleic acids, and lipids, all of which maintain the stability of the film and facilitate its cohesion and surface adhesion [2]. The combination of these biomolecules is called a matrixome and influences bacterial virulence [3]. Biofilms are an important component of bacterial functions. Bacterial transition from motility to biofilm formation happens when their motility is inhibited due to an increase in the levels of c-di-GMP, which in turn, slows down the bacteria and inhibits the expression of the flagellar genes [4]. This transition between the two states is an example of bacterial adaptation to environmental signals and stress [5]. Some Gram-negative bacteria, including pathogenic bacteria, can form a pellicle, which is a biofilm that floats on the air–liquid interface under static conditions [6]. Biofilms, then, contribute significantly to pathogenicity. Some pathogenic microorganisms can only regulate and express their virulence if environmental conditions and cell density are optimal. The production of biofilms is one of the most intriguing tactics to increase fitness against the harsh environments on or in the plant. Microbial biofilms can grow on the surfaces of leaves, roots, and plant tissues' intercellular spaces.
Plant-associated bacteria interact with the surface of plant tissues prior to pathogenesis process through symbiotic relationships and commensalism [7]. The surface components of plant tissues, the availability of nutrients and water, and the nature of bacterial colonies affect the formation of biofilm structures [8, 9]. Certain secondary plant metabolites, including alkaloids, flavonoids, phenols, glycosides, steroids, saponins, and terpenoids, have been shown to hinder biofilm formation by preventing and interrupting the mature biofilm and the bacterial cells' ability to disperse, mainly damaging the cell membranes [10, 11]. However, it should be noted that these plant-derived compounds are not directly released from the host plant into bacterial biofilm [12]. In addition, Guttman et al. [13] reported that the leaf texture with larger roughness of the abaxial leaf of Zantedeschia aethiopica is more resistant to Pectobacterium spp. than members of the other Araceae. Pectobacterium species can recognize hosts and express various virulence factors, and then plant hosts activate defense mechanisms in response to the identified bacterial strain [14]. Endophytic and pathogenic bacteria living in vascular tissues are known to be capable of invading plant tissues and forming biofilms. Inside the biofilm, phytopathogenic bacteria have higher virulence and pathogenicity that stand up to antimicrobials synthesized by the host plant and thus can act coordinated to colonize host plants and eventually infect them. All these processes are far more challenging for bacteria attempting them individually. One advantage of biofilm formation is that it shields connected bacteria from environmental stressors like UV radiation and desiccation [15, 16]. The formation of biofilms can cause blockage and tissue damage in xylem cells [17]. The ability to form robust biofilms inside xylem vessels in Xylella fastidiosa is the main cause of diseases, such as Pierce's disease and citrus variegated chlorosis; however, in certain instances, biofilms aid in limiting rapid growth and reducing self-virulence. This allows the pathogen to survive as a commensal endophyte in many plant species rather than instantly destroying the plant host [18]. Pectobacterium brasiliense, an phytopathogenic bacterium that is highly concentrated in the xylem, produces large amounts of plant cell-wall degrading enzymes that can cause necrosis of the vascular tissues [19, 20]. This pathogen has been widely reported to cause soft rot disease in potatoes, ornamental crops, and other economically valuable crops [21–24]. Biofilm formation by P. brasiliense plays an important role in bacterial colonization and disease progression [25]. P. brasiliense in susceptible potato cultivars colonizes xylem tissue to form biofilm-like aggregates that could lead to occlusion problems in some tissues [26, 27].
Honey has been extensively studied as a natural compound that could address the propensity of bacteria to form biofilms [28–30]. Manuka is a type of honey that has been widely investigated for its ability to inhibit the biofilm formation of various pathogenic bacteria. However, the effect of manuka honey on the biofilm formation of plant pathogens has not been extensively studied. Thus, the purpose of the present study is to determine the effects of manuka honey on the biofilm and pellicle formation of P. brasiliense and the expression of the related genes.
2. Materials and Methods
2.1. Bacterial Isolate and Growth Condition
P. brasiliense Pal3.4 used in this study was grown on yeast peptone agar medium (YPA; 0.5% yeast extract, 1% polypeptone, 1.5% agar). A single bacterial colony was incubated for 1-2 days at room temperature (±27°C) on a new YPA medium to maintain the isolate's viability and purity. Colonies of P. brasiliense were scraped from forty-eight-hour-old isolates and suspended in sterile water to an OD600 nm of 0.2 (108 CFU/mL) measured with a spectrophotometer (Genesys 10S UV-VIS, Thermo Fisher Scientific, USA). A 50 μL of bacterial suspension was then grown in 5 mL of yeast peptone broth (YPB) medium. Manuka honey (Streamland UMF 20+) was added to the medium at a concentration of 5% (w/v) as previously described [31]. A control treatment without adding manuka honey (0% w/v) to the medium was also prepared.
2.2. Assessment of Biofilm Formation by Crystal Violet Staining Assay
The biofilm formation protocol was adapted from the work of O'Toole and Kolter [32] with slight modifications. Biofilm formation was assayed in terms of the ability of cells to adhere to a bottle made of polyvinyl chloride (PVC). An overnight culture grown in SOBG medium (20 g/L tryptone, 5 g/L yeast, 0.5 g/L NaCl, 2.4 g/L MgSO4, 0.18 g/L KCl, 40% glycerol) was diluted (1:10 v/v) with the same medium containing 5% (w/v), 10% (w/v), 25% (w/v), 50% (w/v), and 75% (w/v) concentration of manuka honey as a treatment. The honey was replaced with sterile distilled water in the control treatment. The cultures (5 mL, approximately 105 CFU) were poured into PVC bottles and incubated for 48 h at room temperature without shaking. The cultures were removed after incubation, and the absorbance was measured with a spectrophotometer (Genesys 10S UV-VIS, Thermo Fisher Scientific, USA) in OD600nm. The bottle was dried for 15 min, rinsed thrice with sterile distilled water, added with 7.5 mL of 1% crystal violet solution, and allowed to stand for 20 min. The dye stained the cells but not the plastic surface. The bottles were washed three times with sterile distilled water until no color was observed in the rinse water. The final wash was done with 7.5 mL of 96% ethanol solution and left for 2 min. 3 mL of this ethanol solution was measured with a spectrophotometer at the absorbance of 600 nm using a Genesys 10S UV-VIS (Thermo Fisher Scientific, USA).
2.3. Assessment of Pellicle Formation
The pellicle formation assay was adapted from the previously described protocol [33]. In brief, overnight bacterial cultures were diluted at a ratio of 1:100 (v/v) in 5 mL of SOBG medium containing 5% manuka honey (w/v) as treatment and without manuka honey as control and grown in the dark in glass tubes for 72 h at room temperature without shaking. Finally, the appearance of pellicles on water surface of treatment and control was recorded.
2.4. Selection and Primer Design of Genes Involved in Biofilm and Pellicle Formation
PCR primers were designed to amplify genes involved in biofilm and pellicle formation by P. brasiliense, including bcsA (cellulose synthase), fis (DNA-binding protein), hrpL (alternative sigma factor), and expI (quorum-sensing (QS) signal generator, acyl-homoserine lactone (AHL)) [34–37]. The recA (Recombinase A) gene, a known housekeeping gene in P. brasiliense, was used as an internal control. The primer was designed on the basis of the complete genome sequence of P. brasiliense type strain LMG 21371T (accession number GCA_000754695.1), which is available at the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/). The selected primer candidates were searched using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm the identity of the gene to be used. The primers were designed using Primer3Plus software (https://primer3plus.com/cgi-bin/dev/primer3plus.cgi), and the annealing temperature of each primer was optimized using a gradient thermal cycler [38]. The primer sequences are shown in Table 1.
Table 1.
Primer sequences used for the RT-qPCR analysis of genes related to biofilm and pellicle formation in Pectobacterium brasiliense.
| Gene | Accession | Function | Sequence (5′⟶ 3′) |
|---|---|---|---|
| bcsA | 57242389 | Cellulose synthase | F: AAGAAATGGTGCGGGGTTTG |
| R: TCAACAACGCCCTGAAACAG | |||
|
| |||
| fis | 57242014 | DNA-binding protein | F: ACAACGCGTGAATTCTGACG |
| R: TCCTGACCGTTCAATTGAGC | |||
|
| |||
| hrpL | AJ496800 | Alternative sigma factor | F: AAGTCAGCGGTCCTTGAAAC |
| R: TCCAACTGCAATGCGAGATC | |||
|
| |||
| expI | LC387225.1 | Quorum-sensing signal generator, acyl-homoserine lactone | F: TGTCCCGGTAATCATGTTAGGG |
| R: AATTGGGCCGTGCAATGTAC | |||
|
| |||
| recA ∗ | 57243346 | Recombinase A | F: TGCGTTTATCGATGCTGAGC |
| R: AGCGCGTTAATGCATCACAG | |||
∗ recA was used as a reference gene.
2.5. Bacterial RNA Isolation and cDNA Synthesis
P. brasiliense was grown in YPB medium for 12 h with or without the addition of 5% manuka honey. The bacterium was harvested by centrifugation of 100,00 × g for 1 min, and RNA was isolated using GENEzol Reagent (Geneaid, Taiwan) according to the manufacturer's instructions. The quality and quantity of RNA were calculated using a MaestroNano spectrophotometer (MaestroGen, Taiwan) [39].
2.6. Analysis of Biofilm and Pellicle Gene Expression
Bacterial RNA was synthesized into cDNA via the PCR reverse-transcription method using ReverTra Ace-α-® (TOYOBO, Japan) with the primer sequences presented in Table 1. In this study, gene expression analysis was carried out using two methods, namely, semiquantitative gene expression levels and quantitative gene relative expression. Semiquantitative analysis of the expression of genes encoding for biofilm and pellicle formation was performed using conventional PCR (Bio-Rad T100, Germany) with GoTaq Green Master Mix (Promega, USA). PCR was performed as follows: initial denaturation at 95°C for 3 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing (Table 1) for 40 s, and extension at 72°C for 40 s. Visualization was performed by electrophoresis and UV transillumination (Bio-Rad, USA) [40]. ImageJ software [41] was used to quantify gel images of biofilm and pellicle gene expression in comparison with that of recA. Gene expression was clustered into biofilm and pellicle formation–associated genes with those of recA. Expression levels were quantified from gel images from pellicle and biofilm-related genes compared to its reference gene. Quantitative analysis was performed using a CFX96 Touch real-time qPCR (Bio-Rad, USA) with THUNDERBIRD SYBR qPCR Mix (TOYOBO, Japan) and the recA gene as an internal standard. The program used included initial denaturation at 95°C for 1 min, followed by 39 cycles of denaturation at 95°C for 15 s, annealing at 59°C for 30 s, and extension at 72°C for 5 s and final extension at 72°C for 5 min. Results were analyzed as described by Livak and Schmittgen [42] comparing the CT of the target gene with the CT of the reference gene. ΔCT = Ct target gene − Ct internal control, ΔΔCT = ΔCT treatment − ΔCT control, and relative expression = 2−ΔΔCT. The fold change differences between 0% and 5% treatments were calculated by dividing the expression fold change of 0% treatment by the expression fold change of 5% treatment for each biofilm and pellicle-related genes.
3. Results
3.1. Reduction in Biofilm Formation After Honey Treatment
The biofilm formation of P. brasiliense in the SOBG medium was observed as a purple ring on the top of the PVC surface. After staining with CV, treatment of 5% manuka honey reduced biofilm formation, which was adhered to the PVC surface, followed by 10%, 25%, 50%, and 75%, while the control was not (Figure 1(a)). Biofilm formation measurement showed that all treatments decreased in absorbance, and the concentration of bacteria was also reduced (Figure 1(b)). Absorbance measurement showed that 7.98 × 108 CFU/mL of control can form biofilm formation adhered to PVC surface at 0.513 of OD600nm, and treatment with 5% manuka honey can reduce the bacteria to 4.27 × 108 CFU/mL and biofilm formation decreased to 0.384. In addition, treatment with 10%, 25%, 50%, and 75% showed similar reduction results (Figure 1(b)). This result showed that the control treatment did not show any inhibition of biofilm formation compared to the manuka honey treatment.
Figure 1.
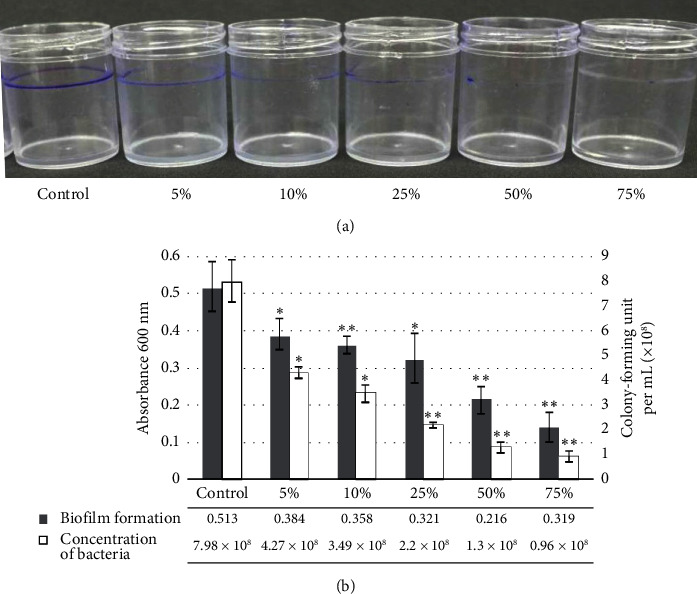
Assay of biofilm formation by Pectobacterium brasiliense by crystal violet staining. Treatments: 5%, 10%, 25%, 50%, and 75% honey; control, no honey. Biofilm formation was characterized as a purple ring appearing around the sides of the polyvinyl chloride bottles (a) as well as by solubilizing the dye in ethanol and determining the absorbance at 600 nm; bacterial concentration was also measured (b). Three independent experiments gave similar results. ∗(p < 0.05) and ∗∗(p < 0.01) are significantly different from the control as evaluated using t-test.
3.2. Pellicle Formation
A pellicle is a robust layer of connected cells covering the surface of a liquid. After 72 h of incubation in test tubes, P. brasiliense demonstrated pellicle formation, as evidenced by the formation of a thick aggregate of cells on the surface of the liquid SOBG culture medium without manuka honey treatment. Compared with the control treatment, reductions in pellicle formation were observed on the surface of the liquid medium treated with 5% manuka honey (Figure 2). These results indicated that manuka honey inhibits the pellicle formation of P. brasiliense.
Figure 2.
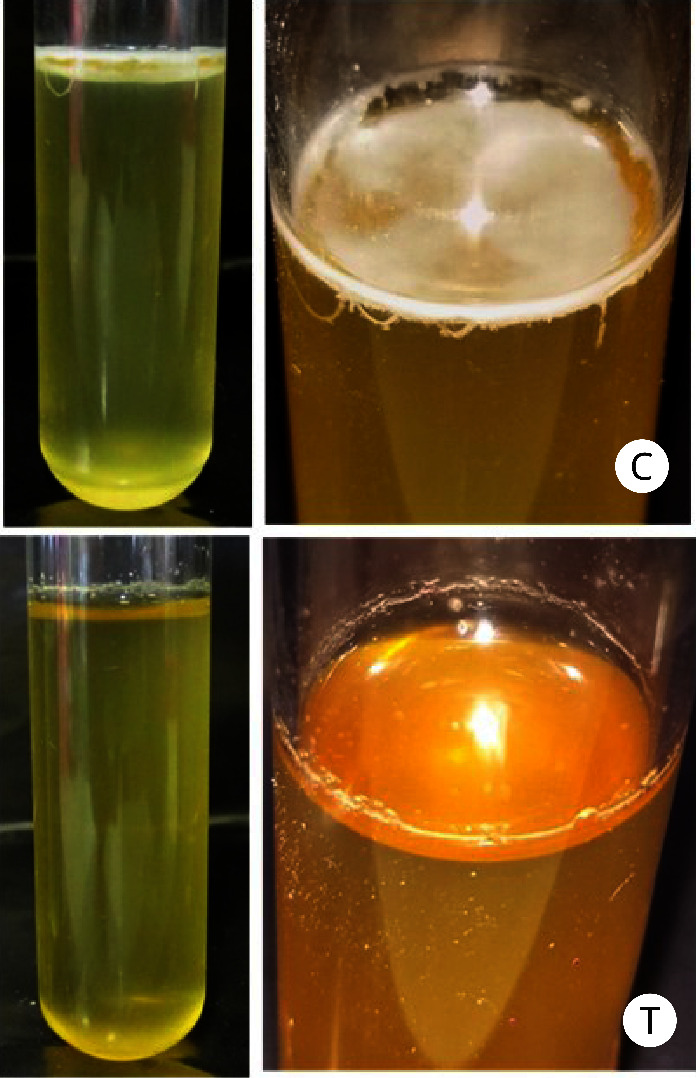
Pellicle formation by Pectobacterium brasiliense grown in SOBG medium and analyzed after 72 h of incubation at room temperature in the absence of light. A pellicle developed at the liquid–air interface in control (without manuka honey) treatment (C). No pellicle formation was observed in the culture treated with 5% manuka honey (T). Three independent experiments gave similar results.
3.3. Semiquantitative Analysis of Gene Expression
The semiquantitative analysis of bcsA, fis, hrpL, and expI gene expression was done to identify the effect of manuka honey on the expression of genes involved in biofilm and pellicle formation. The results suggested differences in expression among four of the genes studied (i.e., bcsA (p = 0.0453), fis (p = 0.0093), hrpL (p = 0.135), and expI (p = 0.0496)) without and with 5% manuka honey treatment (Figure 3(a)). Specifically, higher expression was observed in genes without manuka honey treatment than in genes with honey treatment (Figure 3(b)). The semiquantitative test results suggested that manuka honey reduces the expression of bcsA, fis, hrpL, and expI.
Figure 3.
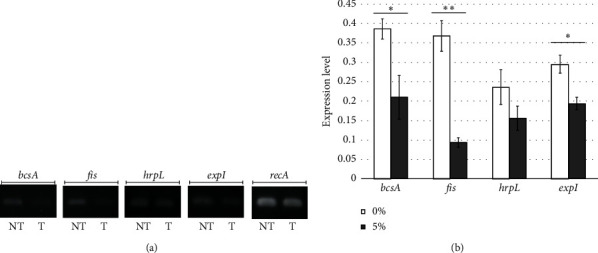
Semiquantitative analysis of the expression of genes involved in biofilm and pellicle formation in Pectobacterium brasiliense. (a) Visualization of electrophoresed gels without (NT) and with (T) 5% manuka honey treatment. (b) Estimation of gene expression level without (0%) and with manuka honey treatment (5%) using ImageJ software. Expression levels were quantified from gel images from pellicle and biofilm-related genes compared to its reference gene (recA). ∗(p < 0.05) and ∗∗(p < 0.01) are significantly different from the control as evaluated using t-test.
3.4. Quantitative Analysis of Gene Expression
We quantitatively analyzed the expression of the genes by using RT-qPCR to confirm the results of the semiquantitative test. The results showed a significant decrease in the expression of four genes of P. brasiliense, including bcsA (7.07-fold; p = 0.0198), fis (5.71-fold; p = 0.0446), hrpL (13.11-fold, p = 0.0163), and expI (6.26-fold; p = 0.0084), following treatment with 5% manuka honey (Figure 4). These results confirmed that manuka honey reduced the expression of genes involved in biofilm and pellicle formation.
Figure 4.
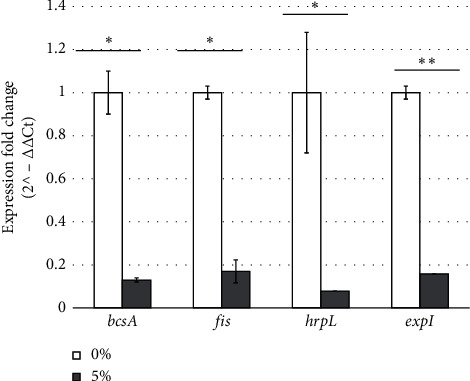
Alterations in the expression profiles of genes involved in the biofilm and pellicle formation of Pectobacterium brasiliense treated with 5% manuka honey as determined by RT-qPCR. All genes were downregulated, and different degrees of downregulation were observed. The expression of bcsA, fis, hrpL, and expI decreased by 7.07-fold, 5.71-fold, 13.11-fold, and 6.26-fold, respectively, after exposure to 5% (w/v) manuka honey. The fold change differences were calculated by dividing the expression fold change of 0% treatment by the expression fold change of 5% treatment for each biofilm and pellicle-related gene. ∗(p < 0.05) and ∗∗(p < 0.01) are significantly different from the control as evaluated using t-test.
4. Discussion
Biofilms and pellicles are formed by bacteria as a means of protection and self-defense against environmental factors. In this study, the observed biofilm in the SOBG medium is similar to that of the air–liquid interface (pellicle), which forms purple rings on the top of the PVC surface when staining with CV; however, the difference between treatment and control is well observed. Under the conditions tested, treatment with manuka honey at the lowest concentration tested (i.e., 5%) reduced biofilm formation, while treatment at the highest concentration tested (i.e., 75%) completely inhibited biofilm formation in P. brasiliense. The higher the concentration of manuka honey, the greater the extent of reduction of biofilm formation. This result may be attributed to various compounds acting together in manuka honey that can inhibit the formation of biofilms by pathogenic bacteria. Treatment with 5% manuka honey could also inhibit pellicle formation under static conditions. These findings might help to understand and identify the potential management of biofilm. In Streptococcus pyogenes, manuka honey can inhibit biofilm by inhibiting adherence, intracellular aggregation, and bacterial attachment to human tissue protein fibronectin [28]. Decreases in biofilm formation are influenced by methylglyoxal (MGO) and the sugar components of manuka honey; studies indicate that anti-biofilm activity is achieved not by a single inhibitor but by a combination of complex synergistic components [43, 44].
As described by Ava et al. [31], manuka honey could inhibit the growth of P. brasiliense. In this study, we described that biofilm formation was not only affected by cell number but also by the decreased expression level of biofilm-related genes. The decrease in biofilm and pellicle formation observed in this study is supported by decreases in the expression of bcsA, fis, hrpL, and expI at the semiquantitative and quantitative levels under the conditions tested. The bcsA gene encodes for bacterial cellulose synthase (bcs), which plays a role in pellicle and biofilm formation. The bcsA gene is encoded by the bcs operon, and bcsA transcription is controlled by cytR homolog; also, reducing bcsA expression is followed by reducing biofilm formation at the air–liquid interface [45]. Lv et al. [46] suggested that bacterial cellulose is involved in the formation of bacterial or biofilm communities. Besides BcsA, Fis is also closely related to bcs operons during biofilm/pellicle formation. The expression of the bcs operon is induced in the biofilm, directly regulated by Fis, and directly suppressed by interacting with the missing operator from the bcs operon in plant pathogenic Pectobacterium [47]. In Dickeya zeae, the fis deletion mutant produces fewer surface-attached biofilms compared with the wild-type variant [48].
The formation of biofilms in P. brasiliense in this study was influenced by QS, as indicated by the observed decrease in the expression of QS-related genes. The expression level of expI is positively correlated with biofilm. The expI functions to synthesize AHL, which is a signal released by bacteria for QS [45, 46]. Due to the high concentration of cells in biofilm, QS cell density-dependent regulation of gene expression plays a crucial role in physiological biofilm formation [49]. Since the cells in the biofilm aggregates are remarkably similar and are connected through a self-generated extracellular matrix, the biofilm represents an ecological environment related to QS [50, 51]. In addition, this study showed that pellicle formation also has a positive correlation with the expression of hrpL. Some studies reported that one of the factors that delay or reduce biofilm production at the air–liquid interface (pellicle) is the low expression of hrpL [52]. In plant pathogenic Pectobacteriaceae, such as Pectobacterium, hrpL encodes alternate sigma factor that functions as the main regulator genes in hrp (hypersensitive response and pathogenicity) cluster and also regulates the expression of type III secretion system (T3SS) [53, 54]. T3SS is required for the formation of bacterial aggregates at the air–liquid interface [55].
Air–liquid interface formation by plant pathogenic bacteria has been shown to be positively related to their virulence in plants [56, 57]. Biofilm-like structures of P. brasiliense in the xylem vessels of host plants may become important virulence factors for pathogenesis. Islamov et al. [58] reported that bacterial emboli or biofilm-like structures will be created when soft rot phytopathogenic bacteria colonize the xylem vessels of the host plant.
5. Conclusion
The treatment with 5% manuka honey could reduce the formation of biofilms and pellicles by P. brasiliense. This decrease is accompanied by reduction in the semiquantitative and quantitative gene expression of bcsA, fis, hrpL, and expI. In this study, there was no evidence that honey accelerated direct control of soft rot disease in planta; however, this study can be a basis for further research to understand the role of honey in suppressing biofilm and bacterial pathogenesis. Future studies on the direct application of honey to plant tissue using the bioactive compound of manuka honey in the prevention of bacterial soft rot might be required.
Acknowledgments
This study was funded by Universitas Gadjah Mada under RTA Program with the Grant Number 2488/UN1.P.III/DIT-LIT/PT/2020.
Data Availability Statement
All data are provided in the article.
Disclosure
This manuscript represents part of the thesis of SA and INSP.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by Universitas Gadjah Mada under RTA Program with the Grant Number 2488/UN1.P.III/DITLIT/PT/2020.
References
- 1.Castiblanco L. F., Sundin G. W. New Insights on Molecular Regulation of Biofilm Formation in Plant-Associated Bacteria. Journal of Integrative Plant Biology . 2016;58(4):362–372. doi: 10.1111/jipb.12428. [DOI] [PubMed] [Google Scholar]
- 2.Flemming H. C., Wingender J. The Biofilm Matrix. Nature Reviews Microbiology . 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 3.Karygianni L., Ren Z., Koo H., Thurnheer T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends in Microbiology . 2020;28(8):668–681. doi: 10.1016/j.tim.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Guttenplan S. B., Kearns D. B. Regulation of Flagellar Motility during Biofilm Formation. FEMS Microbiology Reviews . 2013;37(6):849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi E., Paroni M., Landini P. Biofilm and Motility in Response to Environmental and Host-Related Signals in Gram Negative Opportunistic Pathogens. Journal of Applied Microbiology . 2018;125(6):1587–1602. doi: 10.1111/jam.14089. [DOI] [PubMed] [Google Scholar]
- 6.Armitano J., Méjean V., Jourlin-Castelli C. Minireview: Gram-Negative Bacteria Can Also Form Pellicles. Environmental Microbiology Reports . 2014;6(6):534–544. doi: 10.1111/1758-2229.12171. [DOI] [PubMed] [Google Scholar]
- 7.Navitasari L., Joko T., Murti R. H., Arwiyanto T. Rhizobacterial Community Structure in Grafted Tomato Plants Infected by Ralstonia solanacearum. Biodiversitas Journal of Biological Diversity . 2020;21(10):4888–4895. doi: 10.13057/biodiv/d211055. [DOI] [Google Scholar]
- 8.Ramey B. E., Koutsoudis M., Bodman S. B. V., Fuqua C. Biofilm Formation in Plant-Microbe Associations. Current Opinion in Microbiology . 2004;7(6):602–609. doi: 10.1016/j.mib.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Rahma A. A., Suryanti, Somowiyarj S., Joko T. Induced Disease Resistance and Promotion of Shallot Growth by Bacillus velezensis B-27. Pakistan Journal of Biological Sciences . 2020;23(9):1113–1121. doi: 10.3923/pjbs.2020.1113.1121. [DOI] [PubMed] [Google Scholar]
- 10.de Melo A. L. F., Rossato L., Barbosa M. D. S., et al. From the Environment to the Hospital: How Plants Can Help to Fight Bacteria Biofilm. Microbiological Research . 2022;261:p. 127074. doi: 10.1016/j.micres.2022.127074. [DOI] [PubMed] [Google Scholar]
- 11.Kaur S., Samota M. K., Choudhary M., et al. How Do Plants Defend Themselves against Pathogens-Biochemical Mechanisms and Genetic Interventions. Physiology and Molecular Biology of Plants . 2022;28(2):485–504. doi: 10.1007/s12298-022-01146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilmiah H. H., Sulistyaningsih E., Joko T. Fruit Morphology, Antioxidant Activity, Total Phenolic and Flavonoid Contents of Salacca zalacca (Gaertner) Voss by Applications of Goat Manures and Bacillus velezensis B-27. Caraka Tani: Journal of Sustainable Agriculture . 2021;36(2):270–282. doi: 10.20961/carakatani.v36i2.43798. [DOI] [Google Scholar]
- 13.Guttman Y., Joshi J. R., Chriker N., et al. Ecological Adaptations Influence the Susceptibility of Plants in the Genus Zantedeschia to Soft Rot Pectobacterium spp. Horticulture Research . 2021;8(1):13–12. doi: 10.1038/s41438-020-00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khadka N., Joshi J. R., Reznik N., et al. Host Specificity and Differential Pathogenicity of Pectobacterium Strains from Dicot and Monocot Hosts. Microorganisms . 2020;8(10):1479–1522. doi: 10.3390/microorganisms8101479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaron S., Römling U. Biofilm Formation by Enteric Pathogens and its Role in Plant Colonization and Persistence. Microbial Biotechnology . 2014;7(6):496–516. doi: 10.1111/1751-7915.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carezzano M. E., Paletti Rovey M. F., Cappellari L. D. R., et al. Biofilm-forming Ability of Phytopathogenic Bacteria: A Review of its Involvement in Plant Stress. Plants . 2023;12(11):p. 2207. doi: 10.3390/plants12112207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danhorn T., Fuqua C. Biofilm Formation by Plant-Associated Bacteria. Annual Review of Microbiology . 2007;61(1):401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- 18.Mina I. R., Jara N. P., Criollo J. E., Castillo J. A. The Critical Role of Biofilms in Bacterial Vascular Plant Pathogenesis. Plant Pathology . 2019;68(8):1439–1447. doi: 10.1111/ppa.13073. [DOI] [Google Scholar]
- 19.Charkowski A. O. The Changing Face of Bacterial Soft-Rot Diseases. Annual Review of Phytopathology . 2018;56(1):269–288. doi: 10.1146/annurev-phyto-080417-045906. [DOI] [PubMed] [Google Scholar]
- 20.Joko T., Umehara M., Murata T., Etoh H., Izumori K., Tsuyumu S. Hyperinduction of Pectate Lyase in Dickeya chrysanthemi EC16 by Plant-Derived Sugars. Journal of Plant Interactions . 2018;13(1):141–150. doi: 10.1080/17429145.2018.1444206. [DOI] [Google Scholar]
- 21.Naas H., Sebaihia M., Orfei B., Rezzonico F., Buonaurio R., Moretti C. Pectobacterium carotovorum subsp. brasiliense and Pectobacterium carotovorum subsp. carotovorum as Causal Agents of Potato Soft Rot in Algeria. European Journal of Plant Pathology . 2018;151(4):1027–1034. doi: 10.1007/s10658-018-1438-3. [DOI] [Google Scholar]
- 22.Kwenda S., Motlolometsi T. V., Birch P. R. J., Moleleki L. N. RNA-Seq Profiling Reveals Defense Responses in a Tolerant Potato Cultivar to Stem Infection by Pectobacterium carotovorum ssp. Frontiers in Plant Science . 2016;7:p. 1905. doi: 10.3389/fpls.2016.01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joko T., Soffan A., Rohman M. S. A Novel Subspecies-specific Primer Targeting the Gyrase B Gene for the Detection of Pectobacterium carotovorum subsp. brasiliense. Biodiversitas Journal of Biological Diversity . 2019;20(10):3042–3048. doi: 10.13057/biodiv/d201037. [DOI] [Google Scholar]
- 24.Onkendi E. M., Ramesh A. M., Kwenda S., Naidoo S., Moleleki L. Draft Genome Sequence of a Virulent Pectobacterium carotovorum subsp. brasiliense Isolate Causing Soft Rot of Cucumber. Genome Announcements . 2016;4(1):015300-15–e1615. doi: 10.1128/genomeA.01530-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanui C. K., Shyntum D. Y., Priem S. L., Theron J., Moleleki L. N. Influence of the Ferric Uptake Regulator (Fur) Protein on Pathogenicity in Pectobacterium carotovorum subsp. brasiliense. PLoS One . 2017;12(5):p. e0177647. doi: 10.1371/journal.pone.0177647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubheka G. C., Coutinho T. A., Moleleki N., Moleleki L. N. Colonization Patterns of an mCherry-Tagged Pectobacterium carotovorum subsp. brasiliense Strain in Potato Plants. Phytopathology . 2013;103(12):1268–1279. doi: 10.1094/PHYTO-02-13-0049-R. [DOI] [PubMed] [Google Scholar]
- 27.Moleleki L. N., Pretorius R. G., Tanui C. K., Mosina G., Theron J. A Quorum Sensing-Defective Mutant of Pectobacterium carotovorum ssp. brasiliense 1692 Is Attenuated in Virulence and Unable to Occlude Xylem Tissue of Susceptible Potato Plant Stems. Molecular Plant Pathology . 2017;18(1):32–44. doi: 10.1111/mpp.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassar H. M., Li M., Gregory R. L. Effect of Honey on Streptococcus Mutans Growth and Biofilm Formation. Applied and Environmental Microbiology . 2012;78(2):536–540. doi: 10.1128/AEM.05538-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper R., Henriques N. F. B., Peters A., Cooper R. A. Honey Modulates Biofilms of Pseudomonas aeruginosa in a Time and Dose Dependent Manner. Journal of ApiProduct and ApiMedical Science . 2009;1(1):6–10. doi: 10.3896/IBRA.4.01.1.03. [DOI] [Google Scholar]
- 30.Truchado P., Gil-Izquierdo A., Tomás-Barberán F., Allende A. Inhibition by Chestnut Honey of N-Acyl-L-Homoserine Lactones and Biofilm Formation in Erwinia carotovora, Yersinia enterocolitica, and Aeromonas hydrophila. Journal of Agricultural and Food Chemistry . 2009;57(23):11186–11193. doi: 10.1021/jf9029139. [DOI] [PubMed] [Google Scholar]
- 31.Ava S., Subandiyah S., Rohman M. S., Ogawa N., Joko T. Manuka Honey Reduces the Virulence of Pectobacterium brasiliense by Suppressing Genes that Encodes Plant Cell-Wall Degrading Enzymes. ASEAN Journal on Science and Technology for Development . 2022;39(3):119–124. doi: 10.29037/ajstd.880. [DOI] [Google Scholar]
- 32.O’Toole G. A., Kolter R. Initiation of Biofilm Formation in Pseudomonas fluorescens WCS365 Proceeds via Multiple, Convergent Signalling Pathways: A Genetic Analysis. Molecular Microbiology . 1998;28(3):449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 33.Joko T., Hirata H., Tsuyumu S. A Sugar Transporter (MfsX) Is Also Required by Dickeya dadantii 3937 for in Planta Fitness. Journal of General Plant Pathology . 2007;73(4):274–280. doi: 10.1007/s10327-007-0019-7. [DOI] [Google Scholar]
- 34.Omadjela O., Narahari A., Strumillo J., et al. BcsA and BcsB Form the Catalytically Active Core of Bacterial Cellulose Synthase Sufficient for In Vitro Cellulose Synthesis. Proceedings of the National Academy of Sciences . 2013;110(44):17856–17861. doi: 10.1073/pnas.1314063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prigent-Combaret C., Zghidi-Abouzid O., Effantin G., Lejeune P., Reverchon S., Nasser W. The Nucleoid-Associated Protein Fis Directly Modulates the Synthesis of Cellulose, an Essential Component of Pellicle-Biofilms in the Phytopathogenic Bacterium Dickeya dadantii. Molecular Microbiology . 2012;86(1):172–186. doi: 10.1111/j.1365-2958.2012.08182.x. [DOI] [PubMed] [Google Scholar]
- 36.McNally R. R., Toth I. K., Cock P. J. A., Pritchard L., et al. Genetic Characterization of the HrpL Regulon of the Fire Blight Pathogen Erwinia amylovora Reveals Novel Virulence Factors. Molecular Plant Pathology . 2012;13(2):160–173. doi: 10.1111/j.1364-3703.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirhonen M., Flego D., Heikinheimo R., Palva E. T. A Small Diffusible Signal Molecule Is Responsible for the Global Control of Virulence and Exoenzyme Production in the Plant Pathogen Erwinia Carotovora. The EMBO Journal . 1993;12(6):2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widyaningsih S., Utami S. N. H., Joko T., Subandiyah S. Development of Disease and Growth on Six Scion/Rootstock Combinations of Citrus Seedlings under Huanglongbing Pressure. Journal of Agricultural Science . 2017;9(6):229–238. doi: 10.5539/jas.v9n6p229. [DOI] [Google Scholar]
- 39.Prakoso A. B., Anjani A. A., Joko T., Drenth A., Soffan A., Subandiyah S. Transcriptomic Data of the Musa balbisiana Cultivar Kepok Inoculated with Ralstonia syzigii subsp. celebesensis and Ralstonia solanacearum. Data in Brief . 2020;29:p. 105366. doi: 10.1016/j.dib.2020.105366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trianom B., Arwiyanto T., Joko T. Morphological and Molecular Characterization of Sumatra Disease of Clove in Central Java, Indonesia. Tropical Life Sciences Research . 2019;30(2):107–118. doi: 10.21315/tlsr2019.30.2.8. [DOI] [Google Scholar]
- 41.Schneider C. A., Rasband W. S., Eliceiri K. W. NIH Image to ImageJ: 25 Years of Image Analysis. Nature Methods . 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak K. J., Schmittgen T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods . 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Cooper R. A., Jenkins L., Henriques A. F., Duggan R. S., Burton N. F. Absence of Bacterial Resistance to Medical-Grade Manuka Honey. European Journal of Clinical Microbiology & Infectious Diseases . 2010;29(10):1237–1241. doi: 10.1007/s10096-010-0992-1. [DOI] [PubMed] [Google Scholar]
- 44.Johnston M., McBride M., Dahiya D., Owusu-Apenten R., Singh Nigam P. Antibacterial Activity of Manuka Honey and its Components: An Overview. AIMS Microbiology . 2018;4(4):655–664. doi: 10.3934/microbiol.2018.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haque M. M., Hirata H., Tsuyumu S. SlyA Regulates motA and motB, Virulence and Stress-Related Genes under Conditions Induced by the PhoP-PhoQ System in Dickeya dadantii 3937. Research in Microbiology . 2015;166(6):467–475. doi: 10.1016/j.resmic.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Lv M., Chen Y., Liao L., et al. Fis Is a Global Regulator Critical for Modulation of Virulence Factor Production and Pathogenicity of Dickeya zeae. Scientific Reports . 2018;8(1):p. 341. doi: 10.1038/s41598-017-18578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi J. R., Burdman S., Lipsky A., Yariv S., Yedidia I. Plant Phenolic Acids Affect the Virulence of Pectobacterium aroidearum and P. carotovorum ssp. brasiliense via Quorum Sensing Regulation. Molecular Plant Pathology . 2016;17(4):487–500. doi: 10.1111/mpp.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi J. R., Khazanov N., Charkowski A., Faigenboim A., Senderowitz H., Yedidia I. Interkingdom Signaling Interference: The Effect of Plant-Derived Small Molecules on Quorum Sensing in Plant-Pathogenic Bacteria. Annual Review of Phytopathology . 2021;59(1):153–190. doi: 10.1146/annurev-phyto-020620-095740. [DOI] [PubMed] [Google Scholar]
- 49.Borges A., Abreu A., Dias C., Saavedra M., Borges F., Simoes M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules . 2016;21(7):p. 877. doi: 10.3390/molecules21070877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Zhao X. Effects of Quorum Sensing on the Biofilm Formation and Viable but Non-culturable State. Food Research International . 2020;137:p. 109742. doi: 10.1016/j.foodres.2020.109742. [DOI] [PubMed] [Google Scholar]
- 51.Passos da Silva D., Schofield M., Parsek M., Tseng B. An Update on the Sociomicrobiology of Quorum Sensing in Gram-Negative Biofilm Development. Pathogens . 2017;6(4):p. 51. doi: 10.3390/pathogens6040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S., Peng Q., Zhang Q., et al. Dynamic Regulation of GacA in Type III Secretion, Pectinase Gene Expression, Pellicle Formation, and Pathogenicity of Dickeya dadantii (Erwinia chrysanthemi 3937) Molecular Plant-Microbe Interactions . 2008;21(1):133–142. doi: 10.1094/MPMI-21-1-0133. [DOI] [PubMed] [Google Scholar]
- 53.Chatterjee A., Cui Y., Chaudhuri S., Chatterjee A. K. Identification of Regulators of Hrp/hop Genes of Erwinia carotovora ssp. Carotovora and Characterization of HrpL(Ecc) (SigmaL(EccM)), an Alternative Sigma Factor. Molecular Plant Pathology . 2002;3(5):359–370. doi: 10.1046/j.1364-3703.2002.00128.x. [DOI] [PubMed] [Google Scholar]
- 54.Hogan C. S., Mole B. M., Grant S. R., Willis D. K., Charkowski A. O. The Type III Secreted Effector DspE Is Required Early in Solanum tuberosum Leaf Infection by Pectobacterium carotovorum to Cause Cell Death, and Requires Wx(3–6)D/E Motifs. PLoS One . 2013;8(6):p. e65534. doi: 10.1371/journal.pone.0065534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mee-Ngan Y., Rojas C. M., Yang C.-H., Charkowski A. O. Harpin Mediates Cell Aggregation in Erwinia chrysanthemi 3937. Journal of Bacteriology . 2006;188(6):2280–2284. doi: 10.1128/JB.188.6.2280-2284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manjurul Haque M., Hirata H., Tsuyumu S. Role of PhoP-PhoQ Two-Component System in Pellicle Formation, Virulence and Survival in Harsh Environments of Dickeya dadantii 3937. Journal of General Plant Pathology . 2012;78(3):176–189. doi: 10.1007/s10327-012-0372-z. [DOI] [Google Scholar]
- 57.Jahn C. E., Willis D. K., Charkowski A. O. The Flagellar Sigma Factor FliA Is Required for Dickeya dadantii Virulence. Molecular Plant-Microbe Interactions . 2008;21(11):1431–1442. doi: 10.1094/MPMI-21-11-1431. [DOI] [PubMed] [Google Scholar]
- 58.Islamov B., Petrova O., Mikshina P., et al. The Role of Pectobacterium atrosepticum Exopolysaccharides in Plant–Pathogen Interactions. International Journal of Molecular Sciences . 2021;22(23):p. 12781. doi: 10.3390/ijms222312781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in the article.


