Abstract
Globally, gastric cancer (GC) ranks among the most prevalent forms of malignancy, posing a significant health burden. Epigenetic modifications, predominantly characterized by alterations in DNA methylation patterns, have been linked to a diverse array of neoplastic processes. Here, we undertake a comprehensive analysis of the DNA methylation signature in GC, with the aim to discover the potential diagnostic epigenetic biomarkers. Utilizing the Illumina 935 K BeadChip, we conducted a genome-wide exploration of DNA methylation patterns in four paired samples of GC tissues and adjacent non-cancerous counterparts. The bisulfite-pyrosequencing (n = 7) was employed to the quantification for methylated gene. The pubic databases including GWAS Catalog, TCGA and GEO were used. The immunohistochemistry and qRT-PCR analysis were performed. In contrast to adjacent tissues, GC tissues manifested pronounced hypermethylation patterns specifically within the promoter cytosine-phosphate-guanine (CpG) islands, indicating localized epigenetic alterations. DNA methylome analysis further revealed 4432 differentially-methylated probes (DMPs), with the gene PRKCB exhibited the most prominent average DNA methylation disparity (mean Δβ = 0.353). Pyrosequencing validation confirmed three DMPs within the PRKCB promoter (cg08406370, cg00735962, and cg18526361). Notably, the mean methylation levels of PRKCB were inversely correlated with mRNA expression levels in the GWAS Catalog. Furthermore, both mRNA and protein expression levels of PRKCB were significantly reduced in GCs when compared to their adjacent non-cancerous counterparts, verified by TCGA and GEO database. Our study reveals significant DNA methylation alterations in GC and emphasizes the pivotal role of PRKCB gene hypermethylation in conferring GC risk, which offers fresh perspectives for advancing diagnostic approaches and therapeutic strategies for GC.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78135-6.
Keywords: Genome-wide DNA methylation, Gastric cancer, PRKCB
Subject terms: Gastrointestinal cancer, Tumour biomarkers, Gastroenterology
Introduction
Gastric cancer (GC) stands as a formidable malignancy, occupying the fifth position among the primary causes of cancer-associated mortality worldwide. Its rapid progression into advanced stages, coupled with a high propensity for metastasis, contributes significantly to its devastating impact1,2. Despite great achievements in diagnostic and therapeutic methods, the prognosis of GC patients remains disappointing3. Delayed diagnosis and the absence of efficient therapies contribute to the high mortality rate of GC patients4. The exploration of epigenomic regulation has reinforced the potential for utilizing epigenetic drugs to treat solid tumors5. Furthermore, understanding the regulation of gene expression may aid doctors in determining which patients are most likely to benefit from epigenetic drug therapies.
DNA methylation, particularly targeting promoter-associated cytosine-phosphate-guanine (CpG) islands, is recognized as a pivotal epigenetic modification that exerts significant influence on the regulation of gene expression6. The methylation profile of cancer is unique, characterized by overall hypomethylated DNA and hypermethylated CpG islands7. This hypermethylation in promoter CpG islands and the simultaneous demethylation in gene bodies are linked to gene silencing8. However, the relationship between DNA methylation and cancer gene expression is notably intricate, defying simple model categorizations9 and leading to distinct tumor-specific DNA methylation signatures that form the basis for utilizing DNA methylation in cancer classification10. Despite these advancements, there remains a dearth of comprehensive data on the genome-wide methylome of GC. Therefore, a detailed exploration of DNA methylation patterns across the entire genome in GC could significantly enhance our comprehension of GC pathogenesis and facilitate the identification of key epigenetic drivers.
In the present study, we applied the Human Methylation 935 K BeadChip to matched GC and adjacent normal tissues, with an aim to identify the differentially methylated CpGs in GC. The bisulfite-pyrosequencing was performed to quantitatively evaluate the methylation levels of the CpG islands. Additionally, we employed immunohistochemistry and qRT-PCR anlysis to assessed the protein and mRNA levels of candidate differentially methylated gene (DMG) in expanded human GC tissues cohort. The findings of this study could provide novel insight for diagnostic biomarkers and potential therapeutic targets for GC.
Study population
A total of fifty-four pairs of fresh gastric cancer and adjacent non-cancerous counterparts were collected from the First Affiliated Hospital of Nanchang University. The eligibility criterion for participation entailed a confirmed histopathological diagnosis of gastric cancer, with no prior history of radiotherapy, chemotherapy, or targeted therapeutic interventions. A comprehensive genome-wide methylation profiling was conducted on four pairs of tissues, aiming to discern methylation patterns across the entire genome. Immunohistochemistry and qRT-PCR were performed on fifty and twenty-seven tumors and adjacent non-cancerous tissues respectively. All the sample slides were reviewed by the same experienced pathologist. The clinicopathological profiles of all patients for MethylationEPIC v2.0 (935 K) BeadChip (n = 4) and patients for IHC analysis (n = 50) were shown in Tables S1 and S2, respectively. All the participants signed informed consents and the research guideline was granted approval by the Ethics Committee of the First Affiliated Hospital of Nanchang University under the reference number (2023) CDYFYYLK (01–009). Research had been performed in accordance with the Declaration of Helsinki.
MethylationEPIC v2.0(935 K) BeadChip and bioinformatic analysis
To evaluate DNA concentration and maintain its integrity, we utilized a NanoDrop 2000 spectrophotometer (Waltham, MA, USA) and agarose gel electrophoresis, respectively. Subsequently, the DNA underwent bisulfite treatment. The converted DNA was then subjected to analysis on an Illumina Infinium MethylationEPIC v2.0 (935 K) BeadChip platform (Illumina). Following this, the chip was scanned using the Illumina iSCAN system to obtain Idat files. These files were imported into R and preprocessed with the ChAMP package (version 2.29.1) to extract raw data. The raw data underwent normalization utilizing the BMIQ method. Statistical comparisons between the two groups for continuous variables were performed using t-tests. To identify significantly differential methylation sites (DMSs), we applied a threshold of |Δβ| > 0.1 and a p-value < 0.05. For more stringent criteria, a |Δβ| > 0.2 or higher has been chosen to screen differentially methylated probes (DMPs). The methylation status of differentially methylated genes was listed in Table S3.
Bisulfite-pyrosequencing
A quantity of 500 nanograms of total genomic DNA extracted from each sample underwent chemical modification through bisulfite conversion. Confirmation of the amplified PCR product’s authenticity and integrity was successfully achieved through the employment of 1% agarose gel electrophoresis, resulting in the clear visualization of a single, distinct band on the gel. In accordance with the manufacturer’s specified protocols, the biotinylated PCR product underwent purification to obtain single-stranded DNA, which served as the template for subsequent pyrosequencing reactions. Subsequently, pyrosequencing was performed to analyze the methylation status of the target DNA sequences. The PyroMark Q96 system from QIAGEN automatically processed and calculated the methylation level for each individual CpG site. The outcomes of this analysis were presented in a graphical format known as a pyrogram, which visually displayed the methylation percentage for each targeted site. Table S4 provides a comprehensive list of the primers employed in the pyrosequencing procedure.
Public data analysis
Gene expression and DNA methylation data of GC patients were obtained from the GWAS Catalog (https://www.ebi.ac.uk/gwas/). Gene expression data for GC patients was downloaded from the TCGA database and the GEO dataset (GSE122401, GSE127857). The data processing details were mentioned in our previous study11.
Immunohistochemistry
Matched samples of gastric cancer tissues and their adjacent non-cancerous counterparts were initially fixed in formalin. Subsequently, these tissues underwent dehydration, paraffin embedding, and were then sliced into 4-micrometer thick sections. After deparaffinization and rehydration steps, antigen retrieval was carried out by placing the sections in a pressure cooker containing 0.01 mol/L citrate buffer at a pH of 9.0 for a duration of 3 min. To eliminate any potential interference from endogenous peroxidase activity, a solution containing 3% hydrogen peroxide was utilized to block such activity. Then, slides were treated with the primary PRKCB antibody (A21241, 1:400, ABclonal, Wuhan, China) at 4 °C overnight. The sections underwent incubation with a secondary antibody at 37 °C for 30 min, followed by visualization using 3,3-diaminobenzidine and subsequent counterstaining with hematoxylin. The assessment of PRKCB expression encompassed both the intensity and the proportion of positive cells. The immunohistochemistry score for PRKCB was then derived by multiplying the percentage and intensity scores. Each slide was independently evaluated and scored by two seasoned pathologists. A score of 0–2 is considered as negative (−). A score of 3–5 is considered as positive (+). A score of 6–8 indicates moderately positive (++). A score of 9–12 is considered as strongly positive (+++).
qRT-PCR analysis
To conduct the qRT-PCR analysis, we followed the methodology outlined in our previously published work12. Briefly, total RNA was isolated using the TRIZOL reagent sourced from TIANGEN Biotech, Beijing, China. Subsequently, qPCR assays were carried out adhering strictly to the manufacturer’s protocol. The GAPDH served as a control. The primer sequences for qPCR are shown in Table S5.
Statistical analysis and data visualization
The statistical analysis and data visualization tasks were executed utilizing GraphPad Prism software (www.graphpad.com, version 7, San Diego, USA) and RStudio (www.rstudio.com, version 4.4.1) within the R statistical environment. GSE127857 methylation dataset was selected for external validation. Impute package was used to interpolate methylation data. Differential analysis of methylation data was performed using ChAMP (version 2.34.0). Tidyverse package was used to organize and visualize data. Receiver operating characteristic (ROC) curve was plotted using plotROC package and used to estimate the diagnostic values of methylation levels of PRKCB. Unless specified otherwise, all quantitative data were reported in the format of mean ± SEM. In cases involving continuous data, a two-sided paired Student’s t-test was used for statistical analysis. A statistical significance threshold of p < 0.05 was established, with any p-value falling below this level deemed to indicate a statistically significant result.
Results
DNA methylation landscape of GC patients
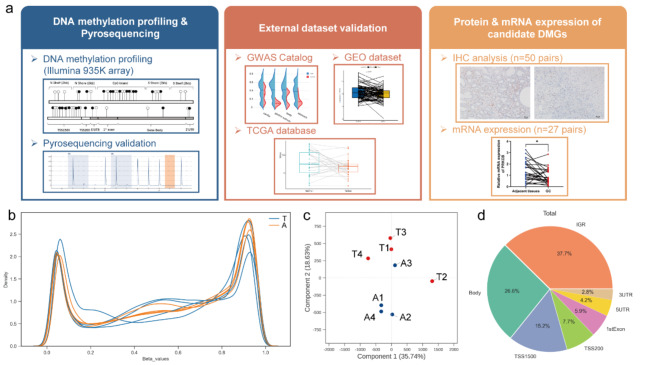
To gain a comprehensive understanding of the genome-wide DNA methylation patterns associated with GC, we conducted a DNA methylome analysis utilizing four paired tumor and its adjacent non- cancerous tissue from patients diagnosed with GC (Fig. 1a). The raw DNA methylation data were deposited in the GEO database (GSE272207). The methylation levels observed across all targeted sites within the array exhibited a characteristic bimodal distribution pattern, as has been previously reported in the context of GC tissues and their adjacent normal counterparts (Fig. 1b). We found significant genome-wide DNA methylation variations between paired samples (Fig. 1c). The DMSs in different regions of the gene were in order as follows: Intergenic region (37.7%), Body region (26.6%), transcription start sites 1500 (TSS1500) (15.2%), transcription start sites 200 (TSS200) (7.7%), 1stExon (5.9%), 5′ untranslated regions (5’UTR) (4.2%), 3′ untranslated regions (3′UTR) (2.8%) (Fig. 1d).
Fig. 1.
The DNA methylation landscape of gastric cancer. (a) Study design and overview of the experimental workflow. Based on the result of 935k DNA methylation profiling (n = 8), we use internal (immunohistochemistry and qRT-PCR) and external (GWAS Catalog, GEO dataset and TCGA database) data to verify our findings. (b) A density plot shows the beta values of both gastric cancer tissues and adjacent tissues. The bimodal distribution shows great detection efficiency. (c) A PCA plot grounded on the CpG sites present in the 935 K BeadChip, with a clear demarcation discernible between gastric cancer tissues and adjacent tissues. (d) Different methylation sites in different regions of the gene. T, tumor tissue; A, adjacent tissue.
Hypermethylated DMPs are enriched in gene promoters
With the specific goal of identifying DNA methylation changes that are distinctive to GC, we conducted a comparative analysis between GC and adjacent non-cancerous tissues. This analysis yielded a total of 4432 differentially methylated probes (DMPs), each satisfying the criteria of an adjusted p-value less than 0.05 and an absolute change in beta value (|Δβ|) greater than 0.2 (see Additional file 1 for details). These represented 0.51% of the total CpG probes (n = 869,932). Consistent with previous research13, 3224 (72.7%) DMPs were hypomethylated in GCs and occupied a majority of the differences. In contrast, 1208 (27.3%) DMPs were hypermethylated (Fig. 2a).
Fig. 2.
Differentially methylated probes (DMPs) in gastric cancer. (a) Volcano plot of DMPs containing 3224 hypomethylated and 1208 hypermethylated DMPs. Percentages of hypermethylated and hypomethylated DMPs are shown at the top. (b) The distribution of hypermethylated and hypomethylated DMPs in gene regions and bar charts show the DMPs in different genomic regions. (c) Heatmap cluster analysis of hypermethylated (left panel) and hypomethylated (right panel) DMPs, respectively. Each row indicates an individual DMP, and each column represents a color-coded sample. The unscaled DNA methylation levels are shown on a heat map from the unmethylated state (blue color) to the methylated state (red color).
Recognizing the crucial influence of genomic location on the DNA methylation landscape and its subsequent functional implications, we embarked on a study aimed at deciphering the distribution patterns of DMPs throughout the genome.
As depicted in Fig. 2b, our analysis revealed a notable enrichment of hypermethylated DMPs within gene promoters, specifically in regions such as TSS1500, TSS200, 5’UTR, and 1stExon. Conversely, we observed an overrepresentation of hypomethylated DMPs in intergenic regions. These findings suggest that DNA methylation alterations in promoters, which are critical for the regulation of gene expression, may play a particularly important role in GC. The hypermethylation of promoters can lead to the silencing of tumor suppressor genes, while hypomethylation in intergenic regions may contribute to the deregulation of gene expression through mechanisms such as altered chromatin structure or the activation of oncogenes. The application of heatmap visualization and hierarchical clustering to the analysis of promoter CpG islands, focusing on both hypermethylated (depicted in the left panel) and hypomethylated (illustrated in the right panel) DMPs, revealed distinct methylation patterns differentiating GC tissues from their adjacent non-cancerous counterparts (Fig. 2c). These findings highlight the crucial role of hypermethylation of promoter CpG islands in GC progression.
Promoter of PRKCB is hypermethylated in GC
To identify the DMGs with high confidence between GC and adjacent normal tissues, we applied rigorous filtering criteria. Specifically, we selected DMPs located within promoter CpG islands (CGIs) that exhibited an absolute change in β-value (|Δβ|) greater than 0.3, and further narrowed down to genes that possessed at least three such DMPs (Fig. 3a). Identifying five candidate DMGs with significant hypermethylation in your study is an important step towards understanding the epigenetic changes, including PRKCB, IKAROS family zinc finger 1 (IKZF1), fms related receptor tyrosine kinase 3 (FLT3), sphingomyelin phosphodiesterase 5 (SMPD5) and potassium calcium-activated channel subfamily N member 2 (KCNN2). The methylation level of DMPs was shown in Table S3. Among the analyzed genes, PRKCB demonstrated the most pronounced average alteration in DNA methylation levels, highlighting its potential significance in the epigenetic context (mean |Δβ| = 0.353; Fig. 3b). Moreover, in the gene PRKCB, the aggregate DNA methylation level across the three DMPs was notably elevated in GC samples compared to their adjacent normal counterparts (Fig. 3c). To quantify the methylation status of candidate DMPs within the promoter region of PRKCB, we employed bisulfite-pyrosequencing in an expanded cohort of GC and paired adjacent normal tissues (Fig. 3d). As a result, the methylation status of all three DMPs within the PRKCB promoter (cg08406370, cg00735962, and cg18526361) showed significant hypermethylation in GC samples relative to their paired normal tissues. In line with these findings, the hypermethylation levels of PRKCB-cg08406370 and PRKCB-cg00735962 in GC were corroborated by the data from the gastric carcinoma methylome chip sequencing cohort from public GEO database (GSE127857) (Fig. 3e). Furthermore, ROC curve analysis showed that the AUC values of PRKCB-cg08406370 and PRKCB-cg00735962 were 0.76 and 0.81 respectively, suggesting the favorable capability of PRKCB methylation in GC diagnosis (Fig. 3f). Taken together, these findings indicated the hypermethylation of these CpG loci within the PRKCB promoter in GC, which emerges as a promising biomarker for GC diagnosis.
Fig. 3.
DNA methylation of the PRKCB promoter in gastric cancer. (a) Schematic diagram showing the filtering scheme for DMGs in this study. (b) DNA methylation level and the average change of PRKCB, IKZF1, FLT3, SMPD5 and KCNN2 in gastric cancer tissues and adjacent tissues. (c) The scatter plot shows the average PRKCB gene methylation (vertical axis) of different samples (dots) in three prominent DMPs (horizontal axis). (d) DNA methylation levels of PRKCB-cg08406370, PRKCB-cg00735962, and PRKCB-cg18526361 by pyrosequencing. P-values are shown between the indicated groups, determined by a two-sided paired Student’s t-test. *P < 0.05. (e) DNA methylation levels of PRKCB-cg08406370 (P = 0.00096) and PRKCB-cg00735962 (P = 0.00024) was higher in gastric cancer tissues compared with adjacent normal tissue. (f) ROC curve of the Δβ methylation values for PRKCB-cg08406370 and PRKCB-cg00735962 in gastric cancer patients. AUC of the ROC curves: 0.81 for PRKCB-cg00735962, 0.76 for PRKCB-cg08406370. AUC, area under the curve; ROC, receiver operating characteristic.
PRKCB is downregulated in GC tissues and is negatively associated with DNA methylation
To verify the methylation modification and expression of PRKCB in GC tissues, the GWAS Catalog was used. The GWAS Catalog, accessible at www.ebi.ac.uk/gwas, represents the most extensive and comprehensive repository of publicly available genome-wide association study (GWAS) data. Data is obtained using deep learning techniques for publication identification and then checked by expert scientists, or directly submitted by authors. Until 2022, the GWAS Catalog includes approximately 400,000 SNP-trait associations derived from over 45,000 individual GWAS studies across approximately 6000 publications14. We obtained mRNA expression and DNA methylation profiles of PRKCB in GC from the GWAS Catalog. Upon analysis, we observed a significant downregulation of PRKCB mRNA expression in GC samples in comparison to their adjacent normal tissue counterparts (Fig. 4a). Of note, GC tissues from different sites, including the gastric antrum and body, all exhibited higher methylation levels of PRKCB than those in adjacent tissues (Fig. 4b). We identified a statistically noteworthy inverse relationship between the mean DNA methylation and the corresponding gene expression, indicating that increased methylation may negatively regulate gene transcription (Fig. 4c). These results suggest that the downregulation of PRKCB is closely associated with its hypermethylation level.
Fig. 4.
Correlation of PRKCB mRNA expression and DNA methylation levels in gastric cancer from GWAS Catalog. (a) The mRNA expression level of PRKCB was decreased in gastric cancer tissues compared with adjacent normal tissues (P = 0.077). The p-value was calculated by the two-sided paired Student’s t-test. (b) PRKCB DNA methylation states at four distinct locations of the gastric cancer. (c) Scatter plots showing a correlation between the DNA methylation and expression of PRKCB.
To further investigate the inverse correlation between DNA methylation and expression of PRKCB, we assessed PRKCB protein levels in fifty paired GC and adjacent tissues using immunohistochemistry. Notably, PRKCB was distributed in the cytoplasm in the majority of gastric tissues (Fig. 5a). Compared with the adjacent normal tissues, a substantial decreased in PRKCB protein levels was observed in GC samples (P < 0.0001; Fig. 5b). Moreover, PRKCB expression was not associated with patients’ age, gender, location of stomach, the Lauren classification and invasion depth of GC (Table S2). In concordance with our previous findings, qRT-PCR analysis revealed a statistically significant downregulation of PRKCB mRNA expression in GC tissues compared to their paired adjacent normal tissues. This consistent result further strengthens the evidence for the involvement of PRKCB in GC pathogenesis (Fig. 5c, p < 0.05). In addition, we also obtained mRNA expression profiles of PRKCB in GC cohorts from the TCGA database. The expression of PRKCB was remarkably reduced in GC tissues compared to adjacent tissues (Fig. 5d). Moreover, PRKCB was lower in intestinal-type GC than in diffuse GC (Fig. 5e). Downregulation of PRKCB in GC tissues was also supported by comparing the tumor specimens with the adjacent stomach samples from GEO datasets (GSE122401) (Fig. 5f). Collectively, these findings suggested that PRKCB expression is significantly downregulated in GC.
Fig. 5.
Expression of PRKCB between gastric cancer and adjacent tissue. (a) Representative images depicting immunostaining for PRKCB in paired tumor-adjacent tissues (scale bars, 10 μm). (b) The immunohistochemistry analysis score of PRKCB (n = 50) is given. P-values are shown between the indicated groups, determined by a two-sided paired Student’s t-test. ****P < 0.0001. (c) The PRKCB mRNA expression level of paired tumor-adjacent tissues (n = 27). P-values are shown between the indicated groups, determined by a two-sided paired Student’s t-test. *P < 0.05. (d) The relative PRKCB expression level of adjacent tissue-gastric cancer group and (e) diffuse type-intestinal type group from TCGA database. *P < 0.05, ****P < 0.0001. (f) The relative PRKCB expression level of adjacent tissue-gastric cancer group from the GEO dataset (GSE122401). IHC immunohistochemistry, GC gastric cancer, AT adjacent tissue.
Discussion
The loss of gene expression caused by methylation modification plays a role in the occurrence and progression of tumors15. In recent years, methylation has offered a potential link between genetics and the phenotype of GC16,17. Early methylation detection has been used for the diagnosis of some cancers such as colorectal cancer18 and hepatocellular carcinoma19. The 935 K BeadChip has been optimized with versions such as 450 K and 850 K BeadChips and its stability is unmatched by existing technologies20. To the best of our knowledge, this is the first comprehensive study that uses the MethylationEPIC v2.0(935 K) BeadChip to provide distinct DNA methylation profiles of GC and adjacent tissues. In our study, we investigated DNA methylation abnormalities in GC and found that hypermethylation of the PRKCB is associated with the development of GC, which provides novel insights into the diagnosis and treatment of GC.
The pattern of DNA methylation in GC has a substantial potential to serve as a tool for early diagnosis and clinical monitoring of cancer21. There is a growing body of evidence indicating that methylation alterations in GC are not merely coincidental occurrences but rather play an active and pivotal role in the promotion of carcinogenesis. This suggests that methylation patterns may serve as critical regulators of GC development22. For instance, in GC and its precancerous lesions, promoter methylation silences well-known tumor suppressors or tumor-related genes such as CDH1, FBX032, CDH11 and MLH123–26. In the context of cancer, aberrant DNA methylation patterns can be broadly categorized into two distinct groups: global DNA hypomethylation and regional hypermethylation. Global DNA hypomethylation primarily targets CpG dinucleotides located within repetitive sequences, which are typically methylated in normal tissue, indicating a widespread decrease in methylation levels across the genome27. Conversely, regional hypermethylation has relatively more studies in carcinogenesis, predominantly occurs at promoter CpG islands and leads to gene inactivation without altering the genetic sequence15,28. Based on DNA methylation, researchers have already established a diagnostic model for the early detection of GC lymph node metastasis22. Our study demonstrates distinct DNA methylation profiles between GC and adjacent tissues. Particularly noteworthy is our observation of increased DNA methylation in promoter regions while noting decreased methylation levels in intergenic regions. This pattern resembles the general characterization of the methylation in cancer29. Our findings indicate that aberrant DNA methylation occurs in GC tissue. This observation holds potential implications for clinical applications and underscores the significance of exploring DNA methylation as a potential biomarker or therapeutic target in GC.
With the objective of discovering specific biomarkers for the diagnosis of GC, we initially filtered out DMPs with large methylation differences (Δβ). Subsequently, we retained candidate DMGs that exhibited at least 3 DMPs in promoter-associated CGIs to undergo further validation. This approach identified five genes (PRKCB, IKZF1, FLT3, SMPD5, and KCNN2) for potential diagnostic utility. Previous studies indicate that PRKCB comprises several serine/threonine kinases that can be triggered by calcium and diacylglycerol30. IKZF1 encodes the transcription factor IKAROS, a zinc finger DNA-binding protein playing a critical role in the development of lineage-restricted mature lymphocytes31. FLT3, a class III receptor tyrosine kinase, is mainly found within the hematopoietic system and serves a pivotal function in maintaining the vitality and integrity of stem and progenitor cells32. SMPD5 encodes neutral sphingomyelinase 5 and localizes to both mitochondria and the endoplasmic reticulum33. KCNN2 signifies the small conductance calcium-activated potassium channel 234.
Notably, among the five candidate DMGs, PRKCB displayed the highest average alteration in DNA methylation, highlighting its potential significance. Moreover, all three candidate DMPs of PRKCB were verified by pyrosequencing (cg08406370, cg00735962, cg18526361). Our observations align with previous studies that have recognized hypermethylation of PRKCB gene in a variety of diseases, including different cancer type35–37. The findings of this study on the methylation of PRKCB cg08406370 site in GC have been confirmed by earlier research evidence to also exist in lung carcinoma38. Notably, we analyzed methylation chip data from GC tissues carried out by Antonios and his colleagues (sourced from the GEO dataset GSE127857)37, confirming the hypermethylation of PRKCB gene cg08406370 and cg00735962 sites in GC. However, research evidence has shown methylation at the PRKCB gene cg03217795 site in prostate cancer39. This discrepancy with our findings may be attributed to the heterogeneity among different tumors.
Our study encompassed an investigation into the distinctive expression pattern of the PRKCB protein within GC tissues, with the aim of gaining further insights. In the validation cohort, the qRT-PCR and immunohistochemistry analysis indicated a common decrease in the mRNA and protein expression level of PRKCB in GC when compared to their adjacent counterparts. Of note, GC exhibited lower mRNA expression and higher DNA methylation level of PRKCB than adjacent tissue in the GWAS Catalog. The observed inverse correlation between PRKCB mRNA expression and the extent of DNA methylation underscores the pivotal role of this epigenetic modification in modulating PRKCB’s gene expression patterns. Intriguingly, ROC curve of PRKCB methylation sites also presented good diagnostic performance. In line with our findings, Wang et al. showed hypermethylation and lower expression of PRKCB in lung adenocarcinoma40. PRKCB plays important roles in cell life and survival, particularly in regulating cell survival and apoptosis41. Higher expression of PRKCB is also associated with better overall survival in breast cancer42. PRKCB is also involved in controlling B cell fate by remodeling mitochondria and reprogramming metabolites43. PRKCB-mediated non-muscle heavy chain myosin-IIA phosphorylation contributes to breast cancer metastasis44. Additionally, research suggests that PRKCB may modulate GC development via circRNA–miRNA–mRNA networks45. Our data support the potential role of PRKCB hypermethylation in GC initiation and progression, and the underlying molecular mechanisms will be elucidated in the future.
The limitation of this study is as follows: the criteria used were p value < 0.05 and |Δβ| > 0.2 for DMPs. However, it is noted that thresholds may vary across studies13. Therefore, future investigations should consider exploring additional genes by fine-tuning the threshold criteria.
In summary, our study offers a comprehensive overview of the genome-wide alterations in DNA methylation patterns observed in GC. Based on our findings, we postulate that aberrant hypermethylation and diminished expression of the PRKCB gene may play a contributory role in the pathogenesis of GC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Key Laboratory Project of Digestive Diseases in Jiangxi Province (2024SSY06101), and Jiangxi Clinical Research Center for Gastroenterology (20223BCG74011).
Author contributions
NSL and YZ conceived and designed the study. LYL and XF performed the methylation chip sequencing and bioinformatic analysis. LYL, HW, XBX and YAZ collected human specimens and analyzed immunohistochemical data. SHC, XF, HJK and YH assisted with data analyses. LYL and NSL interpreted the data and drafted the manuscript. NSL, NHL and YZ supervised and oversaw the study. CH, CX and JPL revised the manuscript. All authors provided intellectual content, and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82260119 and 82170580), Natural Science Foundation of Jiangxi Province (20224ACB216004), Academic and Technical Leader of major disciplines in Jiangxi Province (20225BCJ23021), Chunhui Program of the Ministry of Education (HZKY20220394) and Jiangxi Provincial Health Commission project (202410013).
Data availability
The datasets generated during and/or analyzed during the current study are included within the article and additional files. The raw DNA methylation data were deposited in the GEO database (GSE272207). To review GEO accession GSE272207:Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272207Entertokencpojiyumlpwbtqh into the box.
Declarations
Ethics approval and consent to participate
All experimental procedures were conducted according to the guidelines approved by the Ethics Committee (2023CDYFYYLK01-009) of the First Affiliated Hospital of Nanchang University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Leyan Li and Xiao Fei.
Contributor Information
Yin Zhu, Email: ndyfy01977@ncu.edu.cn.
Nianshuang Li, Email: ndyfy05390@ncu.edu.cn.
References
- 1.Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74 (3), 229–263. 10.3322/caac.21834 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Machlowska, J., Baj, J., Sitarz, M., Maciejewski, R. & Sitarz, R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci.21 (11), 4012. 10.3390/ijms21114012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang, W. J. et al. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol.29 (16), 2452–2468. 10.3748/wjg.v29.i16.2452 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Erp, N. F. et al. Time to diagnosis of symptomatic gastric and oesophageal cancer in the Netherlands: where is the room for improvement? United Eur. Gastroenterol. J.8 (5), 607–620. 10.1177/2050640620917804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Y. et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal. Transduct. Target. Ther.4, 62. 10.1038/s41392-019-0095-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiyama, A. & Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet.37 (11), 1012–1027. 10.1016/j.tig.2021.05.002 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Bure, I. V. & Nemtsova, M. V. Methylation and noncoding RNAs in gastric cancer: everything is connected. Int. J. Mol. Sci.22 (11), 5683. 10.3390/ijms22115683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg, M. V. C. & Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell. Biol.20 (10), 590–607. 10.1038/s41580-019-0159-6 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Spainhour, J. C., Lim, H. S., Yi, S. V. & Qiu, P. Correlation patterns between DNA methylation and gene expression in the Cancer Genome Atlas. Cancer Inf.18, 1176935119828776. 10.1177/1176935119828776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello, J. F. et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat. Genet.24 (2), 132–138. 10.1038/72785 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Wang, H. et al. Identification and validation of immune cells and hub genes in gastric cancer microenvironment. Dis. Mark.. 2022, 8639323. 10.1155/2022/8639323 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, N. et al. Integrative analysis of differential lncRNA/mRNA expression profiling in Helicobacter pylori infection-associated gastric carcinogenesis. Front. Microbiol.11, 880. 10.3389/fmicb.2020.00880 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi, Z. et al. DNA methylation profiling identifies epigenetic signatures of early gastric cancer. Virchows Arch.484 (4), 687–695. 10.1007/s00428-024-03765-0 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Sollis, E. et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res.51 (D1), D977–D985. 10.1093/nar/gkac1010 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura, J., Tanaka, T., Kitajima, Y., Noshiro, H. & Miyazaki, K. Methylation-mediated gene silencing as biomarkers of gastric cancer: a review. World J. Gastroenterol.20 (34), 11991–12006. 10.3748/wjg.v20.i34.11991 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, R. et al. Genome-wide DNA methylation profiling of gastric cardia cancer. J. Gastro Hepatol.38 (2), 290–300. 10.1111/jgh.16054 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Sogutlu, F., Pekerbas, M. & Biray Avci, C. Epigenetic signatures in gastric cancer: current knowledge and future perspectives. Expert Rev. Mol. Diagn.22 (12), 1063–1075. 10.1080/14737159.2022.2159381 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Luo, H. et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci. Transl. Med.12 (524), eaax7533. 10.1126/scitranslmed.aax7533 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Wang, P. et al. Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection. Sci. Transl Med.14 (672), eabp8704. 10.1126/scitranslmed.abp8704 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Murat, K. et al. Ewastools: Infinium Human methylation BeadChip pipeline for population epigenetics integrated into Galaxy. Gigascience. 9 (5), giaa049. 10.1093/gigascience/giaa049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, T., Chen, X., Gu, M., Deng, A. & Qian, C. Identification of the subtypes of gastric cancer based on DNA methylation and the prediction of prognosis. Clin. Epigenet.12 (1), 161. 10.1186/s13148-020-00940-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, S. et al. A novel DNA methylation signature associated with lymph node metastasis status in early gastric cancer. Clin. Epigenetics. 14 (1), 18. 10.1186/s13148-021-01219-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng, W. et al. The clinicopathological significance of CDH1 in gastric cancer: a meta-analysis and systematic review. DDDT Published Online April. 2149. 10.2147/DDDT.S75429 (2015). [DOI] [PMC free article] [PubMed]
- 24.Guo, W. et al. FBXO32, a new TGF-β/Smad signaling pathway target gene, is epigenetically inactivated in gastric cardia adenocarcinoma. neo. 62 (04), 646–657. 10.4149/neo_2015_078 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Yuan, S., Li, L., Xiang, S., Jia, H. & Luo, T. Cadherin-11 is inactivated due to promoter methylation and functions in colorectal cancer as a tumour suppressor. CMAR. 11, 2517–2529. 10.2147/CMAR.S193921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Y. et al. Predictive value of CHFR and MLH1 methylation in human gastric cancer. Gastr. Cancer. 18 (2), 280–287. 10.1007/s10120-014-0370-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besselink, N. et al. The genome-wide mutational consequences of DNA hypomethylation. Sci. Rep.13 (1), 6874. 10.1038/s41598-023-33932-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Y. Q. et al. Hypermethylation of TGF-β1 gene promoter in gastric cancer. World J. Gastroenterol.19 (33), 5557–5564. 10.3748/wjg.v19.i33.5557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, X. et al. DNA methylation drives a new path in gastric cancer early detection: current impact and prospects. Genes Dis.11 (2), 847–860. 10.1016/j.gendis.2023.02.038 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker, P. J. et al. Equivocal, explicit and emergent actions of PKC isoforms in cancer. Nat. Rev. Cancer. 21 (1), 51–63. 10.1038/s41568-020-00310-4 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Paolino, J., Tsai, H. K., Harris, M. H. & Pikman, Y. IKZF1 alterations and therapeutic targeting in B-cell acute lymphoblastic leukemia. Biomedicines. 12 (1), 89. 10.3390/biomedicines12010089 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung, P. J. et al. FLT3 tyrosine kinase inhibition modulates PRC2 and promotes differentiation in acute myeloid leukemia. Leukemia. 38 (2), 291–301. 10.1038/s41375-023-02131-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Airola, M. V. & Hannun, Y. A. Sphingolipid metabolism and neutral sphingomyelinases. Handb. Exp. Pharmacol. (215), 57–76. 10.1007/978-3-7091-1368-4_3 (2013). [DOI] [PMC free article] [PubMed]
- 34.Mochel, F. et al. Variants in the SK2 channel gene (KCNN2) lead to dominant neurodevelopmental movement disorders. Brain. 143 (12), 3564–3573. 10.1093/brain/awaa346 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Daniunaite, K. et al. Promoter methylation of PRKCB, ADAMTS12, and NAALAD2 is specific to prostate cancer and predicts biochemical disease recurrence. Int. J. Mol. Sci.22 (11), 6091. 10.3390/ijms22116091 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao, Q. et al. Hyper-methylation of AVPR1A and PKCΒ gene associated with insensitivity to arginine vasopressin in human pre-eclamptic placental vasculature. EBioMedicine. 44, 574–581. 10.1016/j.ebiom.2019.05.056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez, A. et al. Follow-Up study confirms the presence of gastric cancer DNA methylation Hallmarks in high-risk precursor lesions. Cancers (Basel). 13 (11), 2760. 10.3390/cancers13112760 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, S. et al. Diagnostic role of wnt pathway gene promoter methylation in non small cell lung cancer. Oncotarget. 8 (22), 36354–36367. 10.18632/oncotarget.16754 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.FitzGerald, L. M. et al. Detection of differentially methylated CpGs between tumour and adjacent benign cells in diagnostic prostate cancer samples. Sci. Rep.14 (1), 17877. 10.1038/s41598-024-66488-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, J. et al. PRKCB is relevant to prognosis of lung adenocarcinoma through methylation and immune infiltration. Thorac. Cancer. 13 (12), 1837–1849. 10.1111/1759-7714.14466 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tkachenko, A. Apoptosis and eryptosis: similarities and differences. Apoptosis Published Online November. 3010.1007/s10495-023-01915-4 (2023). [DOI] [PubMed]
- 42.Mao, X. H. et al. Identification of differentially methylated genes as diagnostic and prognostic biomarkers of breast cancer. World J. Surg. Oncol.19 (1), 29. 10.1186/s12957-021-02124-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsui, C. et al. Protein kinase C-β dictates B cell fate by regulating mitochondrial remodeling, metabolic reprogramming, and heme biosynthesis. Immunity. 48 (6), 1144–1159e5. 10.1016/j.immuni.2018.04.031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, L. et al. HBXIP blocks myosin-IIA assembly by phosphorylating and interacting with NMHC-IIA in breast cancer metastasis. Acta Pharm. Sin B. 13 (3), 1053–1070. 10.1016/j.apsb.2022.11.025 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong, Z. et al. Identification of circRNA–miRNA–mRNA networks contributes to explore underlying pathogenesis and therapy strategy of gastric cancer. J. Transl. Med.19 (1), 226. 10.1186/s12967-021-02903-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are included within the article and additional files. The raw DNA methylation data were deposited in the GEO database (GSE272207). To review GEO accession GSE272207:Go to https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272207Entertokencpojiyumlpwbtqh into the box.