Abstract
Hepatic dysregulation of lipid metabolism exacerbates inflammation and enhances the progression of metabolic dysfunction-associated steatotic liver disease (MASLD). STAT3 has been linked to lipid metabolism and inflammation. Jolkinolide B (JB), derived from Euphorbia fischeriana, is known for its pharmacological anti-inflammatory and anti-tumor properties. Therefore, this study investigated whether JB affects MASLD prevention by regulating STAT3 signaling. JB attenuated steatosis and inflammatory responses in palmitic acid (PA)-treated hepatocytes. Additionally, JB treatment reduced the mRNA expression of de-novo lipogenic genes, such as acetyl-CoA carboxylase and stearoyl-CoA desaturase 1. Interestingly, JB-mediated reduction in inflammation and lipogenesis was dependent on STAT3 signaling. JB consistently modulated mitochondrial dysfunction and the mRNA expression of inflammatory cytokines by inhibiting PA-induced JAK/STAT3 activation. This study suggests that JB is a potential therapeutic agent to prevent major stages of MASLD through inhibition of JAK/STAT3 signaling in hepatocytes.
Keywords: MASLD, Jolkinolide B, Lipid accumulation, Inflammation
INTRODUCTION
MASLD is a highly prevalent chronic liver disease worldwide and has emerged as a significant clinical and economic burden (Teng et al., 2023). The disease spectrum of MASLD ranges from simple steatosis to hepatocyte ballooning, metabolic dysfunction-associated steatohepatitis (MASH) with liver inflammation, and steatosis with fibrosis (Vuppalanchi and Chalasani, 2009). Fatty liver is characterized by an increased content of hepatocellular lipids, which can be associated with steatohepatitis and hepatocellular damage, eventually leading to fibrosis and cirrhosis. Therefore, blocking diseases at each MASLD stage—fatty liver, steatohepatitis, and cirrhosis—is the core of MASLD treatment (Abd El-Kader and El-Den Ashmawy, 2015) . Based on these research directions, lipid-lowering agents and statins, cytoprotective agents, antioxidants (vitamin E), PPARγ antagonists (rosiglitazone and pioglitazone), and insulin sensitizers (metformin and GLP-1 receptor agonists) have been identified (Makri et al., 2022). However, these drugs do not target the liver and may increase the risk of side effects. Therefore, the development of safe and effective treatments, such as natural herbal medicines, is required.
Palmitic acid (PA, C16:0) is the most abundant natural saturated fatty acid in the liver triglycerides of patients with MASLD (Liang et al., 2021). It overexpresses intracytoplasmic lipid accumulation and activates fat synthesis genes in liver tissue and is widely used in PA-induced models to stimulate lipotoxicity, steatosis, and inflammation (Zhao et al., 2022). PA induces lipid accumulation and important metabolic changes, including upregulation of Srebp-1c, acetyl-CoA carboxylase (Acc), and stearoyl-CoA desaturase 1 (Scd1) (Flowers and Ntambi, 2008). Lipid accumulation in hepatocytes exacerbates mitochondrial damage and stimulates the production of mitochondrial reactive oxygen species (mtROS), which impede β-oxidation (Wang et al., 2020). Therefore, PA-induced models have often been used to confirm lipotoxicity-induced hepatocyte apoptosis, mitochondrial dysfunction, and identify the pathological mechanisms of diseases (Yang et al., 2022).
In addition, PA activates the JAK2/STAT3 signaling pathway, which influences cellular functions in response to extracellular cytokines and growth factors, including cell growth, differentiation, and death (Hu et al., 2021). Although it mainly has direct effects on the inflammatory response, it also promotes the synthesis of new fatty acids by increasing the expression of fat genes (Kojta et al., 2020). Based on this evidence, the mechanism was investigated, and evaluation of single compounds capable of preventing or treating liver disease by inhibiting hepatic lipid accumulation and inflammation via the PA-induced STAT3 pathway as an upstream signal has been recommended (Liu et al., 2023).
Jolkinolide B (JB), isolated from the roots of Euphorbia fischeriana Steud, is a natural compound with a typical ent-abietane diterpenoid structure (Wang et al., 2010). It is mainly known for its pharmacological mechanisms such as anti-inflammatory responses and anti-tumor activity and is associated with inhibition of the JAK/STAT, NF-κB, and PI3K/Akt/mTOR pathways. JB has been studied in various cell lines, such as breast cancer MCF-cells and BT474, prostate cancer LNCap, lung cancer A549, melanoma B16F10, leukemia U937, and gastric cancer MKN45, because of its strong pharmacological activity (Wang et al., 2022). In this study, we investigated the potential protective effects of JB against lipid accumulation and anti-lipid toxicity in hepatocytes induced by PA. Additionally, we aimed to explore the underlying signaling pathway mechanisms involved in the effect of JB on lipid accumulation and inflammation.
MATERIALS AND METHODS
Isolation of Jolkinolide B from Euphorbia fischeriana
The dried and powdered roots of E. fischeriana (3.0 kg) were extracted with 18 L of MeOH (×3) at room temperature for 3 days, and then the organic solvent was evaporated under vacuum (450 g). The residue was suspended in water and partitioned successively with 2 L of n-hexane (×2, EFH), CH2Cl2 (×2, EFC), and EtOAc (×2, EFE). The CH2Cl2-soluble fraction (EFC, 64 g) was subjected to silica gel chromatography with elution using CH2Cl2-MeOH gradient system (90:1→0:1) to afford four fractions (EFC1~EFC4). EFC2 (12 g) was fractionated into 12 sub-fractions (EFC2-1~EFC2-12) by MPLC with a Lichroprep RP-18 column using a MeOH-H2O gradient system (20:80→100:0). Jolkinolide B (54 mg) was obtained from EFC2-11 (370 mg) by recrystallization in MeOH. The structure of Jolkinolide B (Fig. 1A) was determined by the comparison of its physicochemical and spectroscopic data (Supplementary Fig. 1-3) (Geng et al., 2011).
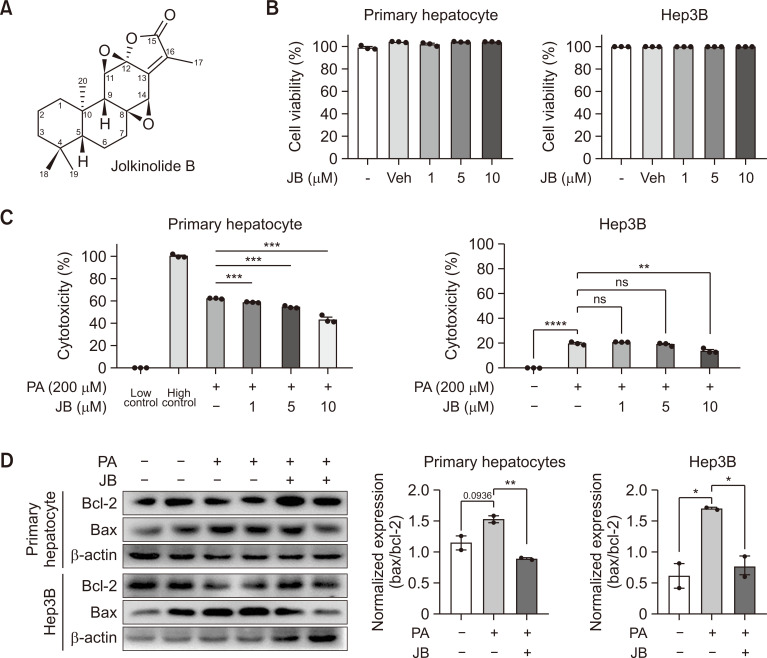
Fig. 1.
Jolkinolide B attenuates hepatocellular lipotoxicity. (A) JB structure. (B) Hepatocytes were treated with JB for each concentration (1, 5, and 10 μM), and cell viability was measured. (C) Cytotoxicity assay in hepatocytes were incubated with PA for 24 h with different concentrations (0, 1, 5, and 10 μM) of JB. Low control refers to cells under normal conditions, while the High control represents 100% cell death. (D) Whole cell lysates from primary hepatocytes and Hep3B cells were extracted and analyzed by immunoblotting of Bax/Bcl-2 after PA (200 μM) and JB (10 μM). The data are expressed as means ± sem. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Jolkinolide B: White needle; 1H NMR (500 MHz, CDCl3) δH 4.06 (1H, H-11), 3.70 (1H, H-14), 2.31 (1H, H-9), 2.10 (3H, H-17), 2.02 (1H, H-7a), 1.95 (1H, H-3a), 1.84 (1H, H-6a), 1.59 (2H, H-2), 1.57 (1H, H-7b), 1.50 (1H, H-6b), 1.47 (1H, H-1a), 1.32 (1H, H-1b), 1.26 (1H, H-3b), 1.13 (1H, H-5), 0.96 (3H, H-18), 0.87 (3H, H-19), 0.84 (3H, H-20); 13C NMR (125 MHz, CDCl3) δC 169.5 (C-16), 148.6 (C-13), 130.2 (C-15), 85.2 (C-12), 66.0 (C-8), 60.9 (C-11), 55.3 (C-14), 53.5 (C-5), 48.0 (C-9), 41.3 (C-3), 39.2 (C-10), 39.1 (C-1), 35.6 (C-7), 33.5 (C-4), 33.4 (C-18), 21.8 (C-19), 20.8 (C-6), 18.4 (C-2), 15.4 (C-20), 8.7 (C-17); ESIMS m/z 353 [M+Na]+.
Mouse hepatocyte isolation
Primary hepatocytes were isolated from C57BL/6 mice and tested as previously described. Isolated hepatocytes were resuspended in complete M199 medium (Corning, NY, USA). First, to confirm lipid accumulation, primary hepatocytes (1×105 cells/mL) were incubated with PA (200 µM) and JB (10 µM) at 37°C for 24 h to stain Oil Red O and BODIPY. Under the same conditions, mRNA gene levels related to lipogenesis and inflammation were measured. Second, to confirm mitochondrial dysfunction, primary hepatocytes (2×104 cells/mL) were incubated with PA (200 µM) and JB (10 µM) at 37°C for 24 h, followed by Mitosox staining and protein level checking.
Hep3B cell culture
Hep3B was purchased from a company (ATCC, HB-8064, Manassas, VA, USA) and incubated at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 5% CO2. To measure lipogenic genes and anti-inflammatory mRNA levels, PA and JB were cultured with Hep3B (1×105 cells/mL) for 24 h. To demonstrate the relevant gene protein levels, Hep3B (4×105 cells/mL) was incubated with PA and JB and cultured for 24 h at 37°C.
Cytotoxicity assay
Primary hepatocytes were analyzed as previously described. Subsequently, Hep3B was suspended in DMEM containing 10% FBS, plated on a 12-well plate (1×105 cells/mL), and incubated for 24 h. JB was then added to the FBS-free medium and reacted for 24 h. The supernatant was collected and subjected to a Quanti-LDH cytotoxicity assay (Cat. No. BCT-LDHP100) and measured using a microplate reader (VersaMax Microplate Reader, CA, USA).
Lipid staining (Oil red O and BODIPY)
After the reaction was complete, the culture medium of the primary hepatocytes was aspirated and washed twice with 1 x phosphate-buffered saline (PBS). After fixing with 4% paraformaldehyde for 20 min, the medium was washed again with PBS and incubated for 3 min with 60% isopropanol. The cells were then stained with Oil Red O solution at room temperature for 10 min and washed five times with H2O. Cell images were taken using a Leica microsystem (DE/DMi8, Leica Camera AG, Wetzlar, Germany), and after image capture, quantification was performed using the Image J software (National Institute of Health, Bethesda, MD, USA).
After the reaction, the culture medium of the primary hepatocytes was removed, and the cells were washed three times with 1 x PBS. After fixation with 4% paraformaldehyde for 30 min, the medium was washed with PBS three times, stained with DAPI (4′,6-diamidino-2-phenylindole, 1 µg) and BODIPY (2 µM) solution for 20 min in a light-shielded state, and again washed with PBS three times. Cell images were captured using a Leica microsystem (DE/DMi8). After capturing four or more images, the area in which the lipid droplets were stained was quantified using the Image J software. Values were quantified relative to the fluorescence signals of the controls.
Quantitative Real-Time PCR analysis
Total RNA was extracted from Primary Hepatocytes and Hep3B cells using RiboEX reagent (Cat. No. 301-001) and purified using a Hybrid-R kit (Cat. No. 305-101). The extracted RNA was reverse transcribed into complementary DNA (cDNA) using a high capacity cDNA Reverse Transcription Kit (Cat. No. 4368813) with the CFX Connect real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Target gene expression was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. The primer sequences are in the Table 1.
Table 1.
Primer sequences of the genes used for quantitative real-time polymerase chain reaction
| Gene | Forward (5’ to 3’) | Reverse (3’ to 5’) |
|---|---|---|
| Human | ||
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| SREBP-1c | ACAGTGACTTCCCTGGCCTAT | GCATGGACGGGTACATCTTCAA |
| ACC | TCGCTTTGGGGGAAATAAAGTG | ACCACCTACGGATAGACCGC |
| SCD1 | TGTGTCCCAGATGCTGTCATTA | CGTGGCAATGCGTTGTTTATG |
| CPT1α | TCCAGTTGGCTTATCGTGGTG | TCCAGAGTCCGATTGATTTTTGC |
| IL-6 | AAGCCAGAGCTGTGCAGATG | TGTCCTGCAGCCACTGGTTC |
| TNF-α | CTGCCTGCTGCACTTTGGAG | ACATGGGCTACAGGCTTGTCA |
| SOCS3 | CCTCAAGACCTTCAGCTCCA | TCACTGCGCTCCAGTAGAAG |
| MT-ND1 | ACCCCCGATTCCGCTACGACCAAC | GGTTTGAGGGGGAATGCTGGA |
| Mouse | ||
| GAPDH | GCCAAGCCTGCTTCTTACTC | TGAGGGCAATTCCAGCCTTA |
| SREBP-1c | ACACTTCTGGAGACATCGCA | CGGATGAGGTTCCAAAGCAG |
| ACC | CTCCCGATTCATAATTGGGTCTG | TCGACCTTGTTTTACTAGGTGC |
| SCD1 | TTCTTGCGATACACTCTGGTGC | CGGGATTGAATGTTCTTGTCGT |
| STAT5a | CAGATGCAAGTGTTGTATGGGC | GCTGGCTCTCGATCCACTG |
| CPT1α | CGGTTCAAGAATGGCATCATC | TCACACCCACCACCACGAT |
| IL-6 | ACAAAGCCAGAGTCCTTCAGAGAG | TTGGATGGTCTTGGTCCTTAGCC |
| TNF-α | CCAACGGCATGGATCTCAAA | CCCTTGAAGAGAACCTGGGA |
| SOCS3 | GCAGGAGAGCGGATTCTACT | TGGATGCGTAGGTTCTTGGT |
| MT-ND1 | CTAGCAGAAACAAACCGGGC | CCGGCTGCGTATTCTACGTT |
| 16s rRNA | CCGCAAGGGAAAGATGAAAGAC | TCGTTTGGTTTCGGGGTTTC |
| HK2 | GCCAGCCTCTCCTGATTTTAG | GGGAACACAAAAGACCTCTTCT |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SREBP-1c, Sterol regulatory element-binding transcription factor 1; ACCα, 1-Aminocyclopropane-1-Carboxylate; SCD1, Stearoyl-CoA desaturase-1; IL-6, Interleukin 6; TNF-α, Tumor necrosis factor-alpha; SOCS3, Suppressor of cytokine signaling 3; MT-ND1, Mitochondrially encoded NADH dehydrogenase 1; 16s rRNA, 16S ribosomal RNA; HK2, Hexokinase 2.
Mitochondrial superoxide indicator
mtROS can cause oxidative damage to mitochondrial DNA and affect the respiratory chain, leading to disease. To determine whether JB affected mitochondrial function, the fluoroprobe MitoSOX Red (M36008, Invitrogen, CA, USA) was used, which is a superoxide indicator and fluorescent dye that targets the mitochondria of living cells. We detected the generation of red fluorescence through oxidation of the MitoSOX reagent by mitochondrial superoxide. Quantitative values were obtained using the Image J software.
mtDNA/nDNA ratio quantification
DNA was isolated from hepatocytes and Hep3B cells using the Total DNA Isolation Kit (GeneAllExgene™ Cell SV mini, Cat # 106-101, Seoul, Korea). DNA was subjected to real-time PCR on a CFX-linked real-time PCR detection system (Bio-Rad) using a SYBR qPCR master mix (TB Green, TAKARA Bio, Kusatsu, Japan) to determine the mtDNA/nDNA ratio. A comparison of ND1 and 16S rRNA DNA expression relative to HK DNA expression will give a measure of mtDNA copy number to nDNA copy number ratio (Quiros et al., 2017). All primers used are in the supplementary material.
Western blot assay
Primary hepatocytes and Hep3B cells were washed with PBS and homogenized on ice using a radioimmunoprecipitation assay buffer. The cell lysates were centrifuged at 13,000 rpm for 15 min. The total protein concentration was quantified using a BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, CA, USA). Equal amounts of protein were separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and blocked with 5% skim milk for 1 h at room temperature. After blocking, membranes were incubated with specific primary antibodies overnight at 4°C. The Phospho-JAK2 (AP0531), JAK2 (A19629), Phospho-STAT3 (AP0705), STAT3 (A1192), and Actin (#3700) antibodies used were diluted 1:1000 in a tris-buffered saline/Tween solution with 2% BSA. The secondary antibody was then reacted for 1 h, enhanced chemiluminescence was applied, and a signal was detected using ChemiDoc (Bio-Rad).
Cellular triglyceride assay
Isolated primary hepatocytes were seeded at 6-well plate and incubates at 37 degrees for overnight. After then, 200 uM PA (P0500, Sigma, St. Louis, USA) and 10 uM JB (Seoul, Korea) were treated for 24 h. Cellular triglyceride Assay was performed according to the instruction manual (BM-TGR-100, BIOMAX, Guri, Korea).
Statistical analysis
All data are presented by means of SEM (Standard error of the mean) and analyzed with Student’s t-test using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was set at p<0.05.
RESULTS
JB attenuates hepatocellular lipotoxicity
JB is a bioactive diterpene that has not been found to be cytotoxic in hepatocytes up to a concentration of 10 µM (Fig. 1A, 1B). We found that JB reduced PA-induced cytotoxicity in hepatocytes in a dose-dependent (Fig. 1C). Additionally, it blocked the PA-mediated induction of Bax and the reduction of Bcl-2 (Fig. 1D), thereby reducing the Bax/Bcl-2 ratio in primary hepatocytes. Collectively, these results suggest that JB exerts protective effects against hepatocellular lipotoxicity.
JB improves lipid-induced mitochondrial dysfunction
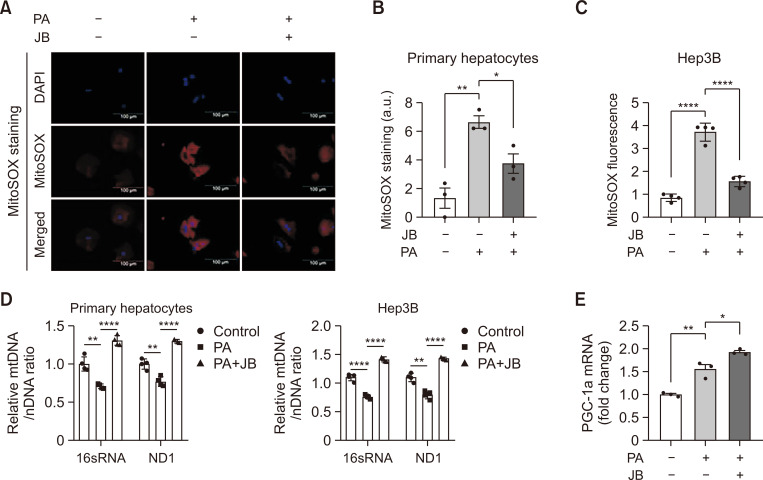
Lipotoxicity increases mitochondrial ROS (mtROS) levels, causing mitochondrial respiratory impairment and intracellular homeostasis by reducing mitochondrial DNA (mtDNA) production (Quan et al., 2020). The effect of JB on mitochondrial dysfunction based on the inhibition of lipid accumulation was investigated. JB reduced the PA-induced mtROS production and mitigated mitochondrial damage, as demonstrated by MitoSox staining (Fig. 2A). JB also reduced the mtROS levels in these cells (Fig. 2B, 2C). A decrease in mtDNA with increased mtROS impairs cell and tissue function, thus contributing to the accumulation of damaged mitochondria. We thus measured the mitochondrial content in cells as expressed as the mitochondrial DNA/nuclear DNA (mtDNA/nDNA) ratio. JB prevented the PA-inhibited mtDNA content, as measured by the ratio of mtDNA to nDNA (Fig. 2D). In addition, JB treatment enhanced PA-induced PGC-1a mRNA expression as shown in Fig. 2E. Taken together, these results suggest that JB protects against lipid-induced mitochondrial dysfunction.
Fig. 2.
Jolkinolide B improves lipid-induced mitochondrial dysfunction. Hep3B and primary hepatocytes treat with PA (200 μM) and JB (10 μM). (A) mtROS was detected by Mitosox staining. The images were shown at 20× magnifications for each experimental condition (scale bar=100 μm). (B) Mitosox quantification was performed using the Image J software. (C) Expression of Mitosox red fluorescence for mtROS in Hep3B cells was measured with a microplate reader. (D) Relative mtDNA/nDNA ratio from primary hepatocytes and Hep3B cells under treated with PA (200 μM) and JB (10 μM) for 24 h. A comparison of ND1 and 16S rRNA DNA expression relative to HK DNA expression will give a measure of mtDNA copy number to nDNA copy number ratio. (E) The mRNA expression of PGC-1a in primary hepatocytes under PA (200 μM) and JB (10 μM) treatment for 24 h. Relative mRNA expression levels were normalized to GAPDH levels. The data are expressed as means ± sem. *p<0.05, **p<0.01, and ****p<0.0001.
JB inhibits PA-induced lipid accumulation and lipogenesis
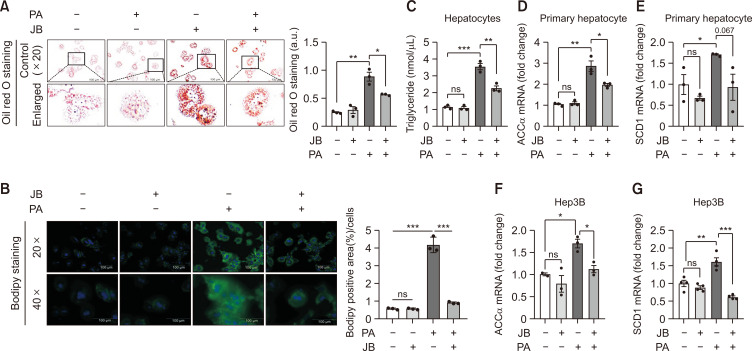
PA is a saturated fatty acid that causes lipotoxicity and metabolic disorders in hepatocytes (Chen et al., 2018). We thus treated mouse primary hepatocytes with PA to investigate the role of JB on lipid accumulation in vitro. Lipid accumulation was assessed by Oil Red O staining and BODIPY staining (Fig. 3A, 3B), which suggests that JB treatment alleviated PA-induced lipid accumulation. In addition, cellular triglyceride assay was performed as shown in Fig. 3C, which suggested that JB treatment improved PA-induced triglyceride level accumulation. Acc and Scd1 are important transcription factors that regulate lipogenic enzyme expression during steatosis. Therefore, we investigated the effects of JB on the expression of the key genes involved in lipogenesis. JB treatment downregulated the PA-induced hepatic expression of Acc and Scd1 (Fig. 3D-3G). These data indicate that JB inhibited PA-induced intracellular lipid deposition by regulating lipogenesis.
Fig. 3.
Jolkinolide B inhibits PA-induced lipid accumulation and lipogenesis. (A) Primary hepatocyte lipid accumulation was presented by Oil Red O staining. Red-stained intracellular fat content was quantified with a fluorescence microscope. The images were shown at 20× magnifications for each experimental condition (scale bar=100 μm). (B) Primary hepatocyte lipid accumulation was presented by BODIPY staining. Lipid droplet-stained BODIPY was imaged with a fluorescence microscope. BODIPY expression was quantified using Image J. The images were shown at 20× and 40× magnifications for each experimental condition (scale bar=100 μm). (C) Cellular TG content measurement was performed in primary hepatocytes under PA and JB treatment. (D, E) Lipogenesis genes were measured using qRT-PCR in primary hepatocytes. (F, G) Expression of the lipogenic genes Acc and Scd1 determined by qRT-PCR in Hep3B cells. Relative mRNA expression levels were normalized to GAPDH levels. The data are expressed as means ± sem. *p<0.05, **p<0.01, and ***p<0.001.
JB enhances anti-steatosis effects through JAK/STAT3 signaling
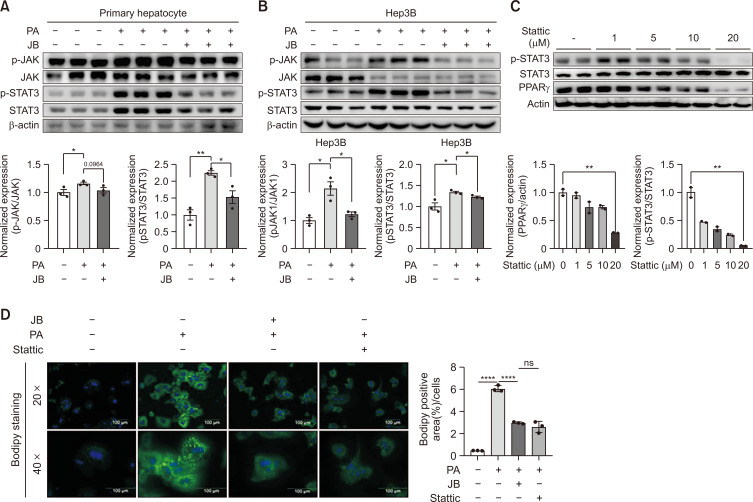
Previous research indicates that the JAK/STAT3 signaling pathway is involved in lipid metabolism. To explore whether JB treatment alleviates hepatic steatosis by targeting the JAK/STAT3 pathway, we detected the expression level of JAK–STAT signaling pathway-related target proteins using western blotting. The results showed that JB treatment greatly inhibited the expression of JAK–STAT signaling pathway-related target proteins in PA-treated primary hepatocytes and Hep3B cells (Fig. 4A, 4B). To further confirm this pathway, STAT3 inhibitor (Stattic), was used and the results compared with those for JB. As shown in Fig. 4C and 4D, Stattic treatment had the same effect on inhibiting PA-induced lipid accumulation as JB treatment. Taken together, these results demonstrate that JB modulates the JAK–STAT signaling pathway in PA-induced steatosis.
Fig. 4.
JB alleviates hepatic steatosis by preventing JAK/STAT3 activity. JB alleviates hepatic steatosis by preventing JAK/STAT3 activity. (A) Relative protein expression levels of JAK and STAT3 and β-Actin were analyzed as immunoblot data concentrations. Values are presented as the means ± SEM and results are expressed by at least three independent experiments. (B) The protein expression levels of pSTAT3 and PPARgamma for each concentration of Stattic (0, 1, 5, 10, and 20 μM) were confirmed by immunoblot. (C) After 24 h of treatment with either Stattic or JB in the PA-treated group in primary hepatocytes. (D) lipid accumulation contents were compared through BODIPY staining. The images were shown at 20× and 40× magnifications for each experimental condition (scale bar=100 μm). Relative mRNA expression levels were normalized to mouse GAPDH levels. The data are expressed as means ± sem. *p<0.05, **p<0.01, and ****p<0.0001.
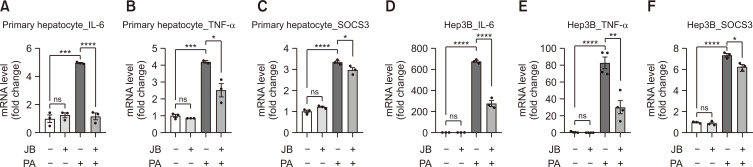
JB suppresses inflammatory responses
To investigate the anti-inflammatory effects of JB, we utilized PA-induced liver inflammation. Compared with the PA group, JB treatment downregulated mRNA expression of inflammatory cytokines, such as Interleukin 6 (IL-6; Fig. 5A, 5D), tumor necrosis factor-α (TNF-a; Fig. 5B, 5E), and suppressor of cytokine signaling 3 (SOCS3; Fig. 5C, 5F). IL-6—a key cytokine in acute inflammatory responses—can directly activate the STAT3–SOCS3 pathway in the liver, upregulate the expression of phospho-STAT3, and increase SOCS3 expression through negative feedback. Taken together with the results shown in Fig. 4, we found that JB regulates JAK/STAT3 signaling, preventing inflammatory responses.
Fig. 5.
Jolkinolide B suppresses inflammatory responses. (A-F) Pro-inflammatory cytokines were measured using qRT-PCR. Expression of inflammatory cytokines IL-6, TNF-α, and SOCS3 determined by qRT-PCR shown as fold change compared with the control. Relative mRNA expression levels were normalized to mouse GAPDH levels. The data are expressed as means ± sem. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
DISCUSSION
Our approach to develop MASLD therapeutics was focused on two representative processes. The first involves hepatocyte lipid accumulation and lipotoxicity, which is associated with mitochondrial abnormalities (Geng et al., 2021). The second is a multifactorial process that includes inflammation, ROS production, and lipid peroxidation (Leyane et al., 2022). As a strategy to prevent the above, JB extracted from Euphorbia fischeriana Steud has received attention (Shen et al., 2017). Here, we describe the regulatory mechanism of JB in the inhibition of lipid accumulation, mitochondrial dysfunction, and inflammatory responses in hepatocytes.
To construct the MASLD model, hepatocyte steatosis and lipotoxicity were induced by treatment with the saturated fatty acid PA. Treatment with JB mitigated cytotoxicity in a concentration-dependent manner and reduced PA-induced LDH release. LDH is a cytoplasmic enzyme released into the cell culture medium upon plasma membrane damage and is associated with liver damage via apoptosis. Based on these results, the expression of Bax/Bcl-2, an apoptotic marker, was confirmed. When PA-damaged hepatocytes were treated with JB, the expression of anti-apoptotic markers decreased, suggesting that mitochondrial function was reversed (Fig. 1). Mechanistically, PA induces mitochondrial dysfunction, and has received considerable attention as a major producer of mtROS. Particularly in hepatocytes, PA induces mitochondrial dysfunction, further triggering a cascade of events leading to MASH and fibrosis. The reduction of mtROS in JB improves mitochondrial function and increases mtDNA production, helping to maintain homeostasis. However, when predicting the signal transduction mechanism of PA, we focused on the possibility of upregulation by reducing lipid accumulation, rather than direct functional regulation (Fig. 2). Primary hepatocytes were stained using Oil Red O and BODIPY, which can visually determine lipid droplet content. Quantification of the stained area for each group confirmed that lipid accumulation was reduced when treated with JB compared to the amount of lipid droplets treated with PA. Additionally, PA treatment suppressed the increased expression of the lipogenic genes ACC and SCD1 (Fig. 3). Taken together, JB affected lipid accumulation and de-novo lipogenesis through certain signaling pathways, and we determined the exact molecular mechanisms underlying these links.
Signal transducers and STAT3 play important roles in cellular processes such as cell growth, apoptosis, and lipid metabolism. Plant-derived compounds are known to regulate key cell signaling pathways involved in lipid homeostasis and metabolism, including the STAT3 signaling pathway. Therefore, we examined whether JB inhibited PA-induced STAT3 signaling and found that it significantly inhibited its activation. However, to clearly prove that the STAT3 pathway is active, it was additionally confirmed that the lipid content was reduced to a level similar to that of Stattic, a strong STAT3 inhibitor (Fig. 4). Finally, as a result of confirming the inflammatory response control through qRT-PCR, we found that JB significantly reduced the pro-inflammatory cytokines IL-6 and TNF-a compared to the PA group. JB also inhibited additional inflammatory responses via negative feedback from the cytokine signaling inhibitor SOCS3 (Fig. 5). Collectively, these results indicate that JB prevents liver damage by suppressing lipid accumulation, mitochondrial dysfunction, and inflammatory responses in the lipotoxicity model.
The current study showed that JB exerts anti-steatosis and anti-inflammatory effects in hepatocytes by suppressing lipid accumulation and de-novo lipogenesis through regulating STAT3 signaling and improving mitochondrial function. These findings support the notion that JB is a novel protective compound against MASLD. Further in vivo preclinical studies are required to validate the effects of JB on MASLD. Our study highlights a novel molecular mechanism underlying the protective effect of JB against hepatic steatosis and provides a basis for the potential repurposing of JB for MASLD treatment.
ACKNOWLEDGMENTS
This research was supported by National Research Foundation of Korea (2017R1A5A2015541), Korean Food and Drug Administration (20182MFDS425), Regional Innovation Strategy (RIS) of National Research Foundation of Korea (2021RIS-001), Chungbuk National University BK21 program (2023).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- Abd El-Kader S. M., El-Den Ashmawy E. M. Non-alcoholic fatty liver disease: the diagnosis and management. World J. Hepatol. 2015;7:846–858. doi: 10.4254/wjh.v7.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Li L., Liu X., Luo R., Liao G., Li L., Liu J., Cheng J., Lu Y., Chen Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018;203:291–304. doi: 10.1016/j.lfs.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Flowers M. T., Ntambi J. M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin .Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Faber K. N., de Meijer V. E., Blokzijl H., Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021;15:21–35. doi: 10.1007/s12072-020-10121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z. F., Liu Z. L., Wang C. F., Liu Q. Z., Shen S. M., Liu Z. M., Du S. S., Deng Z. W. Feeding deterrents against two grain storage insects from Euphorbia fischeriana. Molecules. 2011;16:466–476. doi: 10.3390/molecules16010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Li J., Fu M., Zhao X., Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Target. Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojta I., Chacinska M., Blachnio-Zabielska A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. 2020;12:32375231. doi: 10.3390/nu12051305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyane T. S., Jere S. W., Houreld N. N. Oxidative stress in ageing and chronic degenerative pathologies: molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int J. Mol. Sci. 2022;23:35806275. doi: 10.3390/ijms23137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Zhang Z., Tu J., Wang Z., Gao X., Deng K., El-Samahy M. A., You P., Fan Y., Wang F. gamma-Linolenic acid prevents lipid metabolism disorder in palmitic acid-treated alpha mouse liver-12 cells by balancing autophagy and apoptosis via the LKB1-AMPK-mTOR pathway. J. Agric. Food Chem. 2021;69:8257–8267. doi: 10.1021/acs.jafc.1c02596. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li D., Wang S., Peng Z., Tan Q., He Q., Wang J. 6-Gingerol ameliorates hepatic steatosis, inflammation and oxidative stress in high-fat diet-fed mice through activating LKB1/AMPK signaling. Int. J. Mol. Sci. 2023;24:37047258. doi: 10.3390/ijms24076285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makri E. S., Makri E., Polyzos S. A. Combination therapies for nonalcoholic fatty liver disease. J. Pers. Med. 2022;12:35887662. doi: 10.3390/jpm12071166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Y., Xin Y., Tian G., Zhou J., Liu X. Mitochondrial ROS-modulated mtDNA: a potential target for cardiac aging. Oxid. Med. Cell. Longev. 2020;2020:9423593. doi: 10.1155/2020/9423593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros P. M., Goyal A., Jha P., Auwerx J. Analysis of mtDNA/nDNA ratio in mice. Curr. Protoc. Mouse Biol. 2017;7:47–54. doi: 10.1002/cpmo.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Zhang S. Q., Liu L., Sun Y., Wu Y. X., Xie L. P., Liu J. C. Jolkinolide A and Jolkinolide B inhibit proliferation of A549 cells and activity of human umbilical vein endothelial cells. Med. Sci. Monit. 2017;23:223–237. doi: 10.12659/MSM.902704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M. L., Ng C. H., Huang D. Q., Chan K. E., Tan D. J., Lim W. H., Yang J. D., Tan E., Muthiah M. D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023;29:S32–S42. doi: 10.3350/cmh.2022.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi R., Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. B., Chu W. J., Wang Y., Ji P., Wang Y. B., Yu Q., Qin G. W. Diterpenoids from the roots of Euphorbia fischeriana. J. Asian Nat. Prod. Res. 2010;12:1038–1043. doi: 10.1080/10286020.2010.532490. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang Z., Xu M., Tu W., Hsin I. F., Stotland A., Kim J. H., Liu P., Naiki M., Gottlieb R. A., Seki E. Neurotropin inhibits lipid accumulation by maintaining mitochondrial function in hepatocytes via AMPK activation. Front. Physiol. 2020;11:950. doi: 10.3389/fphys.2020.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Shen S. Y., Liu L., Zhang X. D., Liu D. Y., Liu N., Liu B. H., Shen L. Jolkinolide B inhibits proliferation or migration and promotes apoptosis of MCF-7 or BT-474 breast cancer cells by downregulating the PI3K-Akt pathway. J. Ethnopharmacol. 2022;282:114581. doi: 10.1016/j.jep.2021.114581. [DOI] [PubMed] [Google Scholar]
- Yang W., Zhu L., Lai S., Ding Q., Xu T., Guo R., Dou X., Chai H., Yu Z., Li S. Cimifugin ameliorates lipotoxicity-induced hepatocyte damage and steatosis through TLR4/p38 MAPK- and SIRT1-involved pathways. Oxid. Med. Cell. Longev. 2022;2022:4557532. doi: 10.1155/2022/4557532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Deng Z. T., Huang S., Ning M., Feng Y., Shen Y., Zhao Q. S., Leng Y. Alisol B alleviates hepatocyte lipid accumulation and lipotoxicity via regulating RARalpha-PPARgamma-CD36 cascade and attenuates non-alcoholic steatohepatitis in mice. Nutrients. 2022;14:35745142. doi: 10.3390/nu14122411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.