Abstract
Background
Inadequate representation of women and racial minorities in heart failure (HF) clinical trials continues to limit the generalizability of the results. This could create a disparity in treatment for future heart failure therapies and devices. The study aims to assess the representation of women and racial minorities in recent heart failure studies involving sodium-glucose cotransporter-2 (SGLT-2) inhibitors.
Methods
PubMed was used to search randomized controlled trials (RCTs) looking at SGLT-2 inhibitors and heart failure, which were published from inception to August 2024.
Results
A total of 43 RCTs with 27,703 participants were identified. The studies were published between 2018 and 2024. Seven studies (41 %) were multi-country, with 45 countries represented. The overall proportion of women enrolled in the studies was 35.6 %. The proportion of women was 24.06 % in studies that recruited only patients with HFrEF, 44.33 % in those that recruited only patients with HFpEF, and 41.4 % in those that recruited both HFrEF and HFpEF. Data on race was partially reported in 25 studies (58 %). 76 % of the pharmaceutical industry-funded studies reported race data. However, only 33.3 % of the unfunded or non-industry-funded studies reported race data. In the studies that reported race data, 72.91 % were Caucasians, 15.48 % were Asians, 5.62 % were African-American and 4.1 % were mixed race or others.
In the bivariate analysis, race was more likely to be reported in studies done in the US (p < 0.001), multi-country studies (p = 0.013), and studies sponsored by pharmaceutical companies. More than a third of the study participants were more likely to be women in more recently published studies than older studies (p < 0.001). Additionally, more than a third of the study participants were more likely to be women in studies done in the US (p = 0.055). The multivariate analysis showed an increased odds of having more than a third of the study participants being women in more recently published studies (OR 1.83, 95 % CI 1.06–3.17, p = 0.031) and in studies done in the US (OR 7.69, 95 % CI 1.53–38.59, p = 0.013).
Conclusion
Our study found that women and racial minority individuals have remained underrepresented in recent heart failure studies. Although some progress has been made over the years, more work is needed to improve data reporting and address barriers to enrollment for women and racial minority individuals in clinical trials.
Keywords: Heart failure, Racial minorities, Gender
1. Introduction
Heart failure (HF) remains a leading cause of death and mortality, affecting nearly 6.2 million US adults. [1] By 2030, HF total costs are anticipated to top $69.8 billion, representing a vast and pressing public health concern. [1] It is well known that African-American, Native American, and Asian populations bear a disproportionate burden of modifiable risk factors, such as hypertension, obesity, and diabetes, which can elevate the risk of heart failure. [1] The development of guideline-directed medical therapy (GDMT) for HF with reduced ejection fraction remains one of the most important therapeutic advancements in cardiovascular medicine. [2], [3] However, underutilization of effective GDMT has been observed across racial and ethnic groups and between males and females. [4], [5].
Clinical trials play a crucial role in elucidating disease prevalence and the effects of pharmaceutical therapies and interventions on diverse populations. For instance, the Coronary Artery Risk Development in Young Adults (CARDIA) study revealed a significant twenty-fold higher incidence of heart failure in young African-American women and men under the age of 50, with cumulative incidences of 1.1 % and 0.9 %, respectively, compared to Caucasian women and men in the same cohort, with incidences of 0.08 % and 0 %, respectively. [6] Additionally, studies have shown racial variations in response to heart failure medications. [7] Thus, heart failure medications that work in one group at a particular dose might not be effective in another group. Furthermore, sex-related differences have been reported in the pharmacokinetics, pharmacodynamics, and safety profile of some GDMT for HF. [5] Thus, it is crucial in medical research to have diverse participant demographics to ensure that findings apply to a broader population.
While most clinical trials randomize the study participants to reduce selection bias and confounding, adequate representation by gender and race may not always occur. The objective of our study was to assess the representation of women and racial minorities in recent heart failure studies of sodium-glucose cotransporter 2 (SGLT-2) inhibitors. This is important because gender and racial disparity in the representation of study participants may limit the generalizability of the results, creating a disparity in future heart failure treatment.
2. Methods
We searched the PubMed database and identified RCTs that included SGLT-2 inhibitors and heart failure from inception to August 8, 2024. We included clinical trials that involved SGLT2 inhibitors in heart failure patients. The search was restricted to clinical trials, but no restrictions were based on country, patient age, or type of heart failure. We excluded studies that were not primarily for HF and only reported a sub-group analysis of heart failure patients. Other exclusion criteria include studies with duplicate or overlapping data, conference abstracts, articles without available full text, case reports, case series, and review studies.
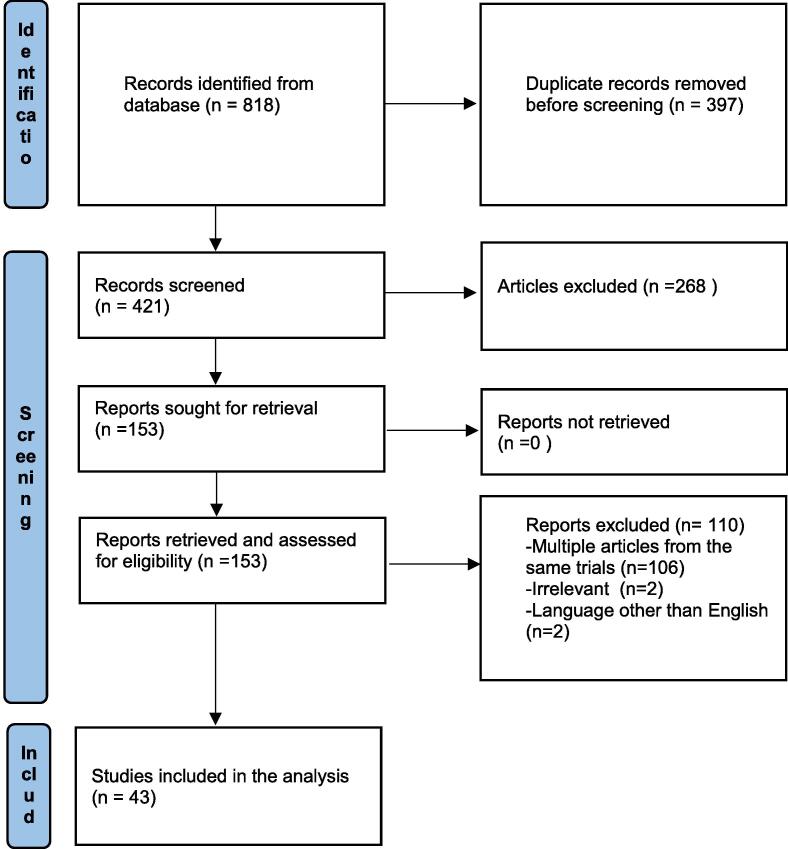
We searched for heart failure and SGLT2 inhibitors, empagliflozin and heart failure, dapagliflozin and heart failure, canagliflozin and heart failure, sotagliflozin and heart failure, and ertugliflozin and heart failure. Our search produced 818 studies, of which 397 were duplicates. All search records were collected into one Endnote library to delete duplicates. We reviewed the remaining 421 studies and found 43 studies that met our inclusion and exclusion criteria (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines (PRISMA) flowchart of the selection process.
Descriptive statistics were done using means, medians, and percentages. For studies where electronic and paper publication dates differed, we used the date that came first as the date of publication. Bivariate analysis was done using the Chi-square and Fisher's exact tests, and a p-value of less than 0.05 was considered significant. Multivariate analysis was done using backward selection logistic regression with statistically significant variables from the bivariate analysis included in the initial model. Statistical analyses were performed using IBM SPSS version 27.
3. Results
A total of 43 RCTs were identified, which included 27,703 participants. These studies were published between 2018 and 2024. Eight studies (18.6 %) were multicountry, with 45 countries represented. 41.9 % of the studies recruited only patients with heart failure with reduced ejection fraction (HFrEF), 7 % recruited only patients with heart failure with preserved ejection fraction (HFpEF); and 51.2 % recruited patients with both HFrEF and HFpEF. The overall proportion of women enrolled in the studies was 35.64 %. The proportion of women was 24.06 % in studies that recruited only patients with HFrEF, 44.33 % in those that recruited only patients with HFpEF, and 41.4 % in those that recruited both HFrEF and HFpEF. The range of women participants in the 43 RCTs was between 10.00 % and 56.79 %. Four studies (9.3 %) had female participation above 50 %, while in 20 studies (46.5 %) at least one-third of the study participants were female. (Table 1).
Table 1.
Descriptive statistics of studies included.
| Number of studies | 43 | |
| Total number of participants | 27,703 | |
| Mean study participants (standard deviation) | 644 (sd: 1518) | |
| Median study participants | 102 | |
| Frequency |
Percentage |
|
| Year Published | (N = 43) | |
| 2018 | 1 | 2.3 % |
| 2019 | 3 | 7.0 % |
| 2020 | 10 | 23.3 % |
| 2021 | 8 | 18.6 % |
| 2022 | 10 | 23.3 % |
| 2023 | 7 | 16.3 % |
| 2024 | 4 | 9.3 % |
| Type of heart failure patients in the studies | ||
| HFrEF | 18 | 41.9 % |
| HFpEF | 3 | 7.0 % |
| Both | 22 | 51.2 % |
| Multi-country study | ||
| No | 35 | 81.4 % |
| Yes | 8 | 18.6 % |
| Pharmaceutical industry-funded studies | ||
| No | 18 | 41.9 % |
| Yes | 25 | 58.1 % |
| Race data reported | ||
| No | 18 | 41.9 % |
| Yes | 25 | 58.1 % |
| Females were at least a third of the participants. | ||
| No | 23 | 53.5 % |
| Yes | 20 | 46.5 % |
Data on race was partially reported in 25 studies (58 %) with a total of 25,707 participants. 76 % of the pharmaceutical industry-funded studies reported race data. However, only 33.3 % of the unfunded or non-industry-funded studies reported race data. In the studies that reported race data, 72.91 % were Caucasians, 15.48 % were Asians, 5.62 % were African-American, and 4.1 % were others.
In the bivariate analysis, race was more likely to be reported in studies done in the US (p < 0.001), multi-country studies (p = 0.013), and studies sponsored by pharmaceutical companies. There was no association between the year of publication and inclusion of race data. Conversely, the year of publication was associated with female participation in the studies. More than a third of the study participants were more likely to be women in more recently published studies than older studies (p < 0.001). Studies done in the US have a higher tendency to include more than a third female participants (p = 0.055) (Table 2 and Table 3).
Table 2.
Bivariate analysis of factors associated with race data reporting.
| Race Information Reported |
p-value | ||
|---|---|---|---|
| No | Yes | ||
| Year Published | |||
| 2018 | 1 (100 %) | 0 (0 %) | 0.355 |
| 2019 | 1 (33.3 %) | 2 (66.7 %) | |
| 2020 | 2 (20 %) | 8 (80 %) | |
| 2021 | 2 (25 %) | 6 (75 %) | |
| 2022 | 6 (60 %) | 4 (40 %) | |
| 2023 | 4 (57.1 %) | 3 (42.9 %) | |
| 2024 | 2 (50 %) | 2 (50 %) | |
| USA study | |||
| No | 18 (69.2 %) | 8 (30.8 %) | <0.001 |
| Yes | 0 (0 %) | 16 (100 %) | |
| Multi-country study | |||
| No | 18 (51.4 %) | 17 (48.6 %) | 0.013 |
| Yes | 0 (0 %) | 8 (100 %) | |
| Pharmaceutical industry-sponsored study | |||
| No | 12 (66.7 %) | 6 (33.3 %) | 0.011 |
| Yes | 6 (24 %) | 19 (76 %) | |
| Recruited > 100 patients in the study | |||
| No | 11 (52.4 %) | 10 (47.6 %) | 0.223 |
| Yes | 7 (31.8 %) | 15 (68.2 %) | |
Table 3.
Bivariate analysis of factors associated with more than one-third of the participants being females.
| More than one-third of the participants are females |
p-value | ||
|---|---|---|---|
| No | Yes | ||
| Year Published | |||
| 2018 | 0 (0 %) | 1 (100 %) | <0.001 |
| 2019 | 3 (100 %) | 0 (0 %) | |
| 2020 | 10 (100 %) | 0 (0 %) | |
| 2021 | 2 (25 %) | 6 (75 %) | |
| 2022 | 3 (30 %) | 7 (70 %) | |
| 2023 | 2 (28.6 %) | 5 (71.4 %) | |
| 2024 | 3 (75 %) | 1 (25 %) | |
| USA study | |||
| No | 17 (65.4 %) | 9 (34.6 %) | 0.055 |
| Yes | 5 (31.3 %) | 11 (68.7 %) | |
| Multi-country study | |||
| No | 20 (57.1 %) | 15 (42.9 %) | 0.270 |
| Yes | 3 (37.5 %) | 5 (62.5 %) | |
| Pharmaceutical industry-sponsored study | |||
| No | 11 (61.1 %) | 7 (38.9 %) | 0.537 |
| Yes | 12 (48 %) | 13 (52 %) | |
| Recruited > 100 patients in the study | |||
| No | 13 (61.9 %) | 8 (38.1 %) | 0.219 |
| Yes | 10 (45.5 %) | 12 (54.5 %) | |
| Race information included | |||
| No | 11 (61.1 %) | 7 (38.9 %) | 0.537 |
| Yes | 12 (48 %) | 13 (52 %) | |
The multivariate analysis showed an increased odds of having more than a third of the study participants being women in more recently published studies (OR 1.83, 95 % CI 1.06–3.17, p = 0.031) and in studies done in the US (OR 7.69, 95 % CI 1.53–38.59, p = 0.013). A one-year increase in the year of publication is associated with an 83 % increased odds of having more than a third of the study participants being women (Table 4).
Table 4.
Studies Included in the Analysis.
| Study | Year published | Number enrolled | Percentage of females | Type of heart failure | USA Study | Multi-country | Industry funded | Race data included |
|---|---|---|---|---|---|---|---|---|
| Voor et al. EMPULSE study [27] | 2022 | 530 | 33.77 | 3 | Yes | Yes | Yes | Yes |
| Nassif et al [28] | 2021 | 324 | 56.79 | 2 | Yes | No | Yes | Yes |
| Solomon et al. DELIVER Trial [29] | 2022 | 6263 | 43.86 | 3 | Yes | Yes | Yes | Yes |
| McMurray et al. DAPA-HF Trial [30] | 2019 | 4744 | 23.38 | 1 | Yes | Yes | Yes | Yes |
| Spertus CHIEF-HF trial [31] | 2022 | 448 | 44.87 | 3 | Yes | No | Yes | Yes |
| Packer et al. EMPEROR-Reduced Trial [32] | 2020 | 3730 | 23.94 | 1 | Yes | Yes | Yes | Yes |
| Bhatt et al. SOLOIST-WHF Trials [33] | 2021 | 1222 | 33.72 | 3 | Yes | Yes | Yes | Yes |
| Mordi et al. The RECEDE-CHF Trial [34] | 2020 | 23 | 26.09 | 3 | No | No | No | Yes |
| Santos-Gallego et al. EMPA-TROPISM [35] | 2021 | 84 | 35.71 | 1 | Yes | No | Yes | Yes |
| Damman et al. EMPA-RESPONSE-AHF [36] | 2020 | 79 | 32.91 | 3 | No | No | Yes | Yes |
| Nassif et al. EMBRACE-HF Trial [37] | 2021 | 65 | 36.92 | 3 | Yes | No | Yes | Yes |
| Kolwelter et al. [38] | 2022 | 74 | 16.22 | 1 | No | No | Yes | No |
| Thiele et al. [39] | 2022 | 19 | 52.63 | 3 | No | No | Yes | No |
| Tanaka et al. CANDLE Trial [40] | 2020 | 233 | 25.32 | 3 | No | No | Yes | No |
| Pietschner et al [41] | 2021 | 53 | 15.09 | 1 | No | No | Yes | No |
| Omar et al. EMPIRE HF [42] | 2022 | 187 | 14.97 | 1 | No | No | No | Yes |
| Omar et al. [43] | 2020 | 70 | 10.00 | 1 | No | No | No | Yes |
| Nassif et al. DEFINE-HF Trial [44] | 2019 | 263 | 26.62 | 1 | Yes | No | Yes | Yes |
| Carbone et al. CANA-HF Trial [45] | 2020 | 36 | 22.22 | 1 | Yes | No | Yes | Yes |
| Anker et al. EMPEROR-Preserved Trial [46] | 2021 | 5988 | 44.69 | 2 | Yes | Yes | Yes | Yes |
| Lee et al. SUGAR-DM-HF [47] | 2020 | 105 | 26.67 | 1 | No | No | No | No |
| Abraham et al. EMPERIAL Trial [48] | 2021 | 627 | 34.45 | 2 | Yes | Yes | Yes | Yes |
| Jensen et al [49] | 2020 | 190 | 14.74 | 1 | No | No | No | Yes |
| Hao et al [50] | 2022 | 100 | 41.00 | 1 | No | No | No | No |
| De Boer et al [51] | 2020 | 125 | 28.00 | 3 | Yes | Yes | Yes | |
| Charaya et al [52] | 2022 | 102 | 45.10 | 3 | No | No | No | No |
| Griffin et al. EMPA [53] | 2020 | 20 | 25.00 | 3 | Yes | No | Yes | Yes |
| Schulze et al. EMPAG-HF [54] | 2022 | 59 | 38.98 | 3 | No | No | Yes | No |
| Hundertmark MJ et al [55] | 2023 | 72 | 41.67 | 3 | No | No | Yes | Yes |
| Tamaki S et al [56] | 2021 | 79 | 29.11 | 3 | No | No | No | No |
| Hao et al. [57] | 2023 | 300 | 45.33 | 1 | No | No | No | No |
| Sezai et al. [58] | 2019 | 35 | 20.00 | 3 | No | No | No | No |
| Cox ZL et al. DICTATE-AHF [59] | 2024 | 238 | 39.08 | 3 | Yes | No | Yes | Yes |
| Yeoh et al [60] | 2023 | 61 | 54.10 | 3 | No | No | No | Yes |
| Palau et al. DAPA-VO2 [61] | 2022 | 90 | 23.33 | 1 | No | No | Yes | No |
| Fatima Gilani SF et al. [62] | 2024 | 150 | 18.67 | 1 | No | No | No | No |
| Lewis et al. PRESERVED-HF [63] | 2023 | 289 | 56.40 | 3 | Yes | No | No | Yes |
| Charaya K et al. [64] | 2023 | 285 | 47.02 | 3 | No | No | No | No |
| Xie L et al. [65] | 2024 | 107 | 27.10 | 1 | No | No | No | No |
| Emara et al. DAPA-RESPONSE-AHF [66] | 2023 | 87 | 28.74 | 3 | No | No | No | No |
| Fu et al. [67] | 2023 | 60 | 28.33 | 1 | No | No | No | No |
| Marton et al. DAPA-Shuttle1 [68] | 2024 | 29 | 10.34 | 1 | No | No | Yes | Yes |
| Soga et al.[69] | 2018 | 58 | 36.21 | 3 | No | No | No | No |
4. Discussion
Disparities in the enrollment of women and racial minorities in HF trials have been a challenge that remains unresolved. Our search revealed approximately 36 % of women enrolled in HF RCTs and studies. Similar trends were reported in prior studies from the National Health and Nutrition Examination Survey, where only two trials had approximately 50 % women representation. The same was true across surveys from the European Subcontinent. [8], [9], [10] It is important to note the historical context of gender representation in clinical trials, especially in heart failure research. In the 1980 s–1990 s, women were indeed underrepresented in clinical trials investigating treatments for heart failure with reduced ejection fraction (HFrEF) (20–30 %). This underrepresentation was observed across various landmark trials investigating medications such as beta-blockers, renin-angiotensin-aldosterone inhibitors, vasodilators, and digoxin. [11] In 2018, Scott et al. examined women's participation in 57 trials involving 35 drugs across six areas of cardiovascular disease, three of them in HFrEF. The proportion of women among trial participants was lowest in HFrEF trials at 24 %. [12] This is similar to our study, which showed only 24.06 % were females in HFrEF trials, compared to 44.33 % in HFpEF trials and 41.4 % in trials that recruited HFrEF and HFpEF patients. This disparity might be because women are more likely to have HFpEF than men [13], [14] and, thus, more likely to be recruited in HFpEF studies than HFrEF studies.
A 2014 study examined the enrollment of 230 RCTs cited in the American College of Cardiology and compared women's representation in the trials. It suggested that although the inclusion of women in HF trials had improved from the 1980 s to the 2000 s, women's representation was lowest at 29 % in HF, despite an estimated population prevalence of 47 % [15] This improvement in female enrollment is consistent with our study that showed that studies in which at least a third of the participants were women increased between 2018 and 2024.
The causes of the gender disparity in the enrollment of participants in HF studies have been studied, though not extensively. Harrison et al. conducted a retrospective analysis of survey data from 97 women who were offered to participate in at least one of four heart failure studies but declined. They identified lack of interest, lack of time, poor health, and travel burdens as the primary deterrents to participation. [16] Many trials exclude women who are pregnant or of childbearing age. Knowledge of these barriers could help design and implement personalized and focused interventions at both the trial and patient levels to improve recruitment and retention of women as clinical trial participants. Novel approaches for recruiting and involving women in cardiovascular clinical trials are advancing. For instance, Sisk et al. showcased a culturally sensitive recruitment approach that was effective in the enrollment of a gender- and racially/ethnically diverse patient population into an RCT. [17] It involved using bilingual, African-American, or Latino recruiters trained to give patients simple and clear information. They welcomed patients to bring a relative or friend to the recruitment session and were flexible in scheduling times for telephone and in-person conversations, including evenings. They also arranged for a taxi if the patient needed one. [17] Additionally, the United States Food and Drug Administration Office of Women's Health offers abundant resources concerning the recruitment and retention of women in clinical research. [18] In addition, the National Institute of Health (NIH) and US Office of Research on Women's Health, in a joint guideline, mandated that all NIH-funded clinical research must include women, that trials must be designed to measure sex-based differences, and that dedicated outreach programs be created to recruit and retain women as clinical trial participants. [19] The leading efforts by the NIH and the US Office of Research on Women's Health could partly be responsible for our result that showed that at least one-third of the study participants were more likely to be females in US studies than elsewhere.
Furthermore, associations have been reported between the composition of HF trial leadership and the inclusion of women as trial participants. In a systematic review of 317 HF RCTs published between January 2000 and May 2019, Whitelaw et al. found that sex-related eligibility criteria such as recruiting women who are not of childbearing age or who are on contraceptives; ambulatory recruitment; drug and device/surgery interventions; and trials with men in first and last authorship positions were each independently associated with under-enrollment of women participants. [20].
Conversly, an analysis of 118 phase 2–4 clinical trials published between 2001 and 2016 by Reza et al. revealed that HF trial publications with a woman as the first author were associated with a higher proportion of women trial participants, and trial publications authored by a larger proportion of women enrolled higher proportions of women participants (39 % versus 26 %, p < 0.001). [21] This association was also demonstrated by Gong et al. [22] Thus, having more women lead heart failure research may help reduce the disparity. Furthermore, Reza et al. found that heart failure trials conducted in North America were the most likely to have a woman as the first or senior author (24 %), compared with Western European (17 %) and multiregional trials (17 %). [21] While we do not know the proportion of the studies in our analysis that have female first or senior authors, the increased proportion of female first or senior authors in North America could have contributed to the increased likelihood of having more women in studies conducted in the US in our analysis. Additionally, Mehran et al. reviewed 8613 cardiology RCTs indexed in PubMed from 2011 to 2020 and discovered that the studies with women as first authors increased from 22 % in 2011 to 35 % in 2020. [23] The increased proportion of female first authors in recent years could partly explain the increased likelihood of having more women in more recent studies, which we saw in our analysis. However, this likely association is speculative and is an area for future research.
We found that race data was partially reported in 58 % of the studies and that study participants were predominantly Caucasians. A detailed analysis by Asefeh et al. of the representation of women, the elderly, and racial minorities in HF clinical trials suggested similar concerns. An analytic and epidemiologic study conducted and published by Vital and Health Statistics showed an underrepresentation of African-American and other racial minorities in HF trials when compared with the Caucasian population. [24] Though the racial composition in the United States was reported to be 59 % Caucasians and 13.6 African-Americans, as per the Census Bureau Population Estimate in 2020, [25] only 5.6 % of African-Americans participated in HF studies in our analysis. The reasons for the racial disparity in clinical trial participants are likely multifactorial. However, racial disparity among clinical investigators has been cited as a significant reason for the underrepresentation of racial minorities in clinical trials, especially among United States Food and Drug Administration-regulated clinical trials funded by the pharmaceutical industry. [26] The physician race has been shown to influence the race of the clinical trial volunteers, with physicians from racial minorities more likely to enroll racial minority participants. [26] Thus, addressing the significant racial disparity among clinical investigators might help address the racial disparity among clinical trial participants. [26].
5. Limitations
Our analysis included only studies published in English, and the findings may not be generalizable to studies published in other languages. Additionally, there could be potential errors in reporting gender and race, though gender reporting faults are much less likely than reporting of race. This could lead to a misclassification bias, although we do not have any evidence that this occurred in any of the papers.
6. Conclusion
Our study found that women and racial minority individuals have remained underrepresented in recent heart failure studies. More work is needed to improve race data reporting and address barriers to enrollment for women and racial minority individuals in clinical trials. Though barriers to enrolment and participation appear multifactorial, more inclusive trials will help to properly understand the disease burden, safe and effective treatment, and safe and effective medication dose in different racial and gender groups. Improving the number of female and racial minority clinical trial investigators may help to alleviate the disparity in study participant volunteers.
Ethical Disclosure
Since the study was done from data available in public domain, IRB approval was not required for the analysis.
CRediT authorship contribution statement
Rahul Gupta: Writing – review & editing, Writing – original draft, Validation, Supervision, Conceptualization. Chukwuemeka Umeh: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Tamanna Mohta: Writing – review & editing. Ajay Vaidya: Writing – review & editing, Visualization, Validation, Supervision. Aaron Wolfson: Writing – review & editing, Visualization, Validation, Supervision. Jonathan Nattiv: Writing – review & editing, Visualization, Validation, Supervision. Harpreet Bhatia: Writing – review & editing, Visualization, Validation, Supervision. Gagan Kaur: Writing – review & editing, Validation. Raghav Dhawan: Writing – review & editing, Visualization, Validation. Puja Darji: Writing – review & editing, Validation. Benson Eghreriniovo: Writing – review & editing, Validation. Eseosa Sanwo: Writing – review & editing, Validation. Priya Hotwani: Writing – review & editing, Visualization, Validation. Payaam Mahdavian: Writing – review & editing, Validation. Sabina Kumar: Writing – review & editing, Validation. Bhoodev Tiwari: Visualization, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report From the American Heart Association. Circulation. March 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Dupre M.E., Gu D., Xu H., Willis J., Curtis L.H., Peterson E.D. Racial and ethnic differences in trajectories of hospitalization in US men and women with heart failure. J. Am. Heart. Assoc. November 2017;6(11) doi: 10.1161/JAHA.117.006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.E. Akintoye, A. Briasoulis, A. Egbe, et al., National Trends in Admission and In-Hospital Mortality of Patients With Heart Failure in the United States (2001–2014). J. Am. Heart Assoc. November, 6(12), 2017. [DOI] [PMC free article] [PubMed]
- 4.Bagchi A.D., Stewart K., McLaughlin C., Higgins P., Croghan T. Treatment and outcomes for congestive heart failure by race/ethnicity in TRICARE. Med. Care. 2011 May 1;49(5):489–495. doi: 10.1097/MLR.0b013e318207ef87. [DOI] [PubMed] [Google Scholar]
- 5.Tamargo J., Caballero R., Delpon E. Sex-related differences in the pharmacological treatment of heart failure. Pharmacol. Therap. 2022 Jan;1(229) doi: 10.1016/j.pharmthera.2021.107891. [DOI] [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K., Pletcher M.J., Lin F., et al. Racial differences in incident heart failure among young adults. N. Engl. J. Med. March 2009;360(12):1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor M.R., Sun A.Y., Davis G., Fiuzat M., Liggett S.B., Bristow M.R. Race, common genetic variation, and therapeutic response disparities in heart failure. JACC. Heart. Failure. 2014 Dec;2(6):561–572. doi: 10.1016/j.jchf.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowie M.R., Wood D.A., Coats A.J., et al. Incidence and aetiology of heart failure: a population-based study. Eur. Heart. J. 1999:20421–20428. doi: 10.1053/euhj.1998.1280. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Solal ADesnos MDelahaye FEmeriau JPHanania G A national survey of heart failure in French hospitals: the Myocardiopathy and Heart Failure Working Group of the French Society of Cardiology, the National College of General Hospital Cardiologists and the French Geriatrics Society. Eur Heart J. 21763- 769, 2000. [DOI] [PubMed]
- 10.National Heart, Lung, and Blood Institute/NIH data fact sheet. Congestive heart failure in the United States: a new epidemic, 1996.
- 11.Lindenfeld J.O., Krause-Steinrauf H., Salerno J.U. Where are all the women with heart failure? J. Am. College. Cardiol. 1997 Nov 15;30(6):1417–1419. doi: 10.1016/s0735-1097(97)00343-4. [DOI] [PubMed] [Google Scholar]
- 12.Scott P.E., et al. participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J. Am. Coll. Cardiol. 2018;71(18):1960–1969. doi: 10.1016/j.jacc.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 13.V. Regitz-Zagrosek, Sex and gender differences in heart failure. Int. J. Heart Failure. 2020 Jul;2(3):157. 10.36628/ijhf.2020.0004. [DOI] [PMC free article] [PubMed]
- 14.Strömberg A., Mårtensson J. Gender differences in patients with heart failure. Eur. J. Cardiovasc. Nurs. 2003 Apr;2(1):7–18. doi: 10.1016/S1474-5151(03)00002. [DOI] [PubMed] [Google Scholar]
- 15.Sardar M.R., Badri M., Prince C.T., Seltzer J., Kowey P.R. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA. Int. Med. 2014 Nov 1;174(11):1868–1870. doi: 10.1001/jamainternmed.2014.4758. [DOI] [PubMed] [Google Scholar]
- 16.Harrison J.M., et al. Refusal to participate in heart failure studies: do age and gender matter? J. Clin. Nurs. 2016;25(7–8):983–991. doi: 10.1111/jocn.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisk J.E., Horowitz C.R., Wang J.J., McLaughlin M.A., Hebert P.L., Tuzzio L. The success of recruiting minorities, women, and elderly into a randomized controlled effectiveness trial. Mt. Sinai. J. Med. 2008;75(1):37–43. doi: 10.1002/msj.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA. Office of Women's Health. Accessed on April 7, 2024 from https://www.fda.gov/about-fda/office-commissioner/office-womens-health.
- 19.Reza N., Gruen J., Bozkurt B. Representation of women in heart failure clinical trials: barriers to enrollment and strategies to close the gap. American. Heart. J. Plus:. Cardiol. Res. Practice. 2022 Jan;1(13) doi: 10.1016/j.ahjo.2022.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitelaw S., Sullivan K., Eliya Y., Alruwayeh M., Thabane L., Yancy C.W., Mehran R., Mamas M.A., Van Spall H.G. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur. J. Heart. Failure. 2021 Jan;23(1):15–24. doi: 10.1002/ejhf.2034. [DOI] [PubMed] [Google Scholar]
- 21.Reza N., Tahhan A.S., Mahmud N., DeFilippis E.M., Alrohaibani A., Vaduganathan M., Greene S.J., Ho A.H., Fonarow G.C., Butler J., O'Connor C. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circulation:. Heart. Failure. 2020 Aug;13(8):e006605. doi: 10.1161/CIRCHEARTFAILURE.119.006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong I.Y., Tan N.S., Ali S.H., Lebovic G., Mamdani M., Goodman S.G., Ko D.T., Laupacis A., Yan A.T. Temporal trends of women enrollment in major cardiovascular randomized clinical trials. Can. J. Cardiol. 2019 May 1;35(5):653–660. doi: 10.1016/j.cjca.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Mehran R., Kumar A., Bansal A., Shariff M., Gulati M., Kalra A. Gender and disparity in first authorship in cardiology randomized clinical trials. JAMA. Netw. Open. 2021 Mar 1;4(3):e211043. doi: 10.1001/jamanetworkopen.2021.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis A.A., Ayers C.R., Selvin E., et al. Racial Differences in Malignant Left Ventricular Hypertrophy and Incidence of Heart Failure: A Multicohort Study. Circulation. March 2020;141(12):957–967. doi: 10.1161/CIRCULATIONAHA.119.043628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta H., Armstrong A., Swett K., et al. Burden of Systolic and Diastolic Left Ventricular Dysfunction Among Hispanics in the United States: Insights From the Echocardiographic Study of Latinos. Circ. Heart. Fail. April 2016;9(4):e002733. doi: 10.1161/CIRCHEARTFAILURE.115.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Getz K., Faden L. Racial disparities among clinical research investigators. Am. J. .Ther. 2008 Jan 1;15(1):3–11. doi: 10.1097/MJT.0b013e31815fa75a. [DOI] [PubMed] [Google Scholar]
- 27.Voors A.A., Angermann C.E., Teerlink J.R., et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat. Med. 2022;28(3):568–574. doi: 10.1038/s41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassif M.E., Windsor S.L., Borlaug B.A., et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat. Med. 2021;27(11):1954–1960. doi: 10.1038/s41591-021-01536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon S.D., McMurray J.J.V., Claggett B., et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022;387(12):1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 30.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 31.Spertus J.A., Birmingham M.C., Nassif M., et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat. Med. 2022;28(4):809–813. doi: 10.1038/s41591-022-01703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.M. Packer, S.D. Anker, J. Butler, et al., Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial [published correction appears in Circulation. 2021 Jan 26;143(4):e30]. Circulation. 2021;143(4):326-336. doi:10.1161/CIRCULATIONAHA.120.051783. [DOI] [PMC free article] [PubMed]
- 33.Bhatt D.L., Szarek M., Steg P.G., et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 34.N.A. Mordi, I.R. Mordi, J.S. Singh, R.J. McCrimmon, A.D. Struthers, C.C. Lang, Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial [published correction appears in Circulation. 2020 Nov 3;142(18):e316]. Circulation. 2020;142(18):1713-1724. doi:10.1161/CIRCULATIONAHA.120.048739. [DOI] [PMC free article] [PubMed]
- 35.Santos-Gallego C.G., Vargas-Delgado A.P., Requena-Ibanez J.A., et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 2021;77(3):243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Damman K., Beusekamp J.C., Boorsma E.M., et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF) Eur. J. Heart. Fail. 2020;22(4):713–722. doi: 10.1002/ejhf.1713. [DOI] [PubMed] [Google Scholar]
- 37.Nassif M.E., Qintar M., Windsor S.L., et al. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation. 2021;143(17):1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503. [DOI] [PubMed] [Google Scholar]
- 38.Kolwelter J., Kannenkeril D., Linz P., et al. The SGLT2 inhibitor empagliflozin reduces tissue sodium content in patients with chronic heart failure: results from a placebo-controlled randomised trial. Clin. Res. Cardiol. 2023;112(1):134–144. doi: 10.1007/s00392-022-02119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiele K., Rau M., Hartmann N.K., et al. Empagliflozin reduces markers of acute kidney injury in patients with acute decompensated heart failure. ESC. Heart. Fail. 2022;9(4):2233–2238. doi: 10.1002/ehf2.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka A., Hisauchi I., Taguchi I., et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE) ESC. Heart. Fail. 2020;7(4):1585–1594. doi: 10.1002/ehf2.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R. Pietschner, J. Kolwelter, A. Bosch, et al., Effect of empagliflozin on ketone bodies in patients with stable chronic heart failure. Cardiovasc Diabetol. 2021;20(1):219. Published 2021 Nov 9. doi:10.1186/s12933-021-01410-7. [DOI] [PMC free article] [PubMed]
- 42.Omar M., Jensen J., Kistorp C., et al. The effect of empagliflozin on growth differentiation factor 15 in patients with heart failure: a randomized controlled trial (Empire HF Biomarker) Cardiovasc. Diabetol. 2022;21(1):34. doi: 10.1186/s12933-022-01463-2. Published 2022 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omar M., Jensen J., Frederiksen P.H., et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 2020;76(23):2740–2751. doi: 10.1016/j.jacc.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Nassif M.E., Windsor S.L., Tang F., et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140(18):1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929. [DOI] [PubMed] [Google Scholar]
- 45.Carbone S., Billingsley H.E., Canada J.M., et al. The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: The CANA-HF study. DiabetesMetab. Res. Rev. 2020;36(8):e3335. doi: 10.1002/dmrr.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.S.D. Anker, J. Butler, G. Filippatos, J.P. Ferreira, E. Bocchi, M. Böhm, H.-P. Brunner-La Rocca, D.-J. Choi, V. Chopra, E. Chuquiure, et al., Empagliflozin in heart failure with a preserved ejection fraction [published online August 27, 2021]. N Engl J Med. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed]
- 47.Lee M.M.Y., Brooksbank K.J.M., Wetherall K., et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF) Circulation. 2021;143(6):516–525. doi: 10.1161/CIRCULATIONAHA.120.052186. Epub 2020 Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham W.T., Lindenfeld J., Ponikowski P., et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur. Heart. J. 2021;42(6):700–710. doi: 10.1093/eurheartj/ehaa943. [DOI] [PubMed] [Google Scholar]
- 49.Jensen J., Omar M., Kistorp C., et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial. Am. Heart. J. 2020;228:47–56. doi: 10.1016/j.ahj.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Hao Z., Zhang Y. Different Doses of Empagliflozin in Patients with Heart Failure with Reduced Ejection Fraction. Int. Heart. J. 2022;63(5):852–856. doi: 10.1536/ihj.22-151. [DOI] [PubMed] [Google Scholar]
- 51.De Boer R.A., Núñez J., Kozlovski P., Wang Y., Proot P., Keefe D. Effects of the dual sodium-glucose linked transporter inhibitor, licogliflozin vs placebo or empagliflozin in patients with type 2 diabetes and heart failure. Br. J. Clin. Pharmacol. 2020;86(7):1346–1356. doi: 10.1111/bcp.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charaya K., Shchekochikhin D., Andreev D., et al. Impact of dapagliflozin treatment on renal function and diuretics use in acute heart failure: a pilot study. Open. Heart. 2022;9(1):e001936. doi: 10.1136/openhrt-2021-001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin M., Rao V.S., Ivey-Miranda J., Fleming J., Mahoney D., Maulion C., Suda N., Siwakoti K., Ahmad T., Jacoby D., Riello R. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020 Sep 15;142(11):1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691. Epub 2020 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulze P.C., Bogoviku J., Westphal J., Aftanski P., Haertel F., Grund S., von Haehling S., Schumacher U., Möbius-Winkler S., Busch M. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF) Circulation. 2022 Jul 26;146(4):289–298. doi: 10.1161/CIRCULATIONAHA.122.059038. Epub 2022 Jun 29. [DOI] [PubMed] [Google Scholar]
- 55.Hundertmark M.J., Adler A., Antoniades C., Coleman R., Griffin J.L., Holman R.R., Lamlum H., Lee J., Massey D., Miller J.J., Milton J.E. Assessment of cardiac energy metabolism, function, and physiology in patients with heart failure taking empagliflozin: the randomized, controlled EMPA-VISION trial. Circulation. 2023 May 30;147(22):1654–1669. doi: 10.1161/CIRCULATIONAHA.122.062021. Epub 2023 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamaki S., Yamada T., Watanabe T., Morita T., Furukawa Y., Kawasaki M., Kikuchi A., Kawai T., Seo M., Abe M., Nakamura J. Effect of empagliflozin as an add-on therapy on decongestion and renal function in patients with diabetes hospitalized for acute decompensated heart failure: a prospective randomized controlled study. Circ. Heart. Fail. 2021 Mar;14(3):e007048. doi: 10.1161/CIRCHEARTFAILURE.120.007048. Epub 2021 Mar 5. [DOI] [PubMed] [Google Scholar]
- 57.Z. Hao, Y. Li, Y. Zhang, Randomized, double-blind, pilot study comparison of 10 mg and 25 mg of empagliflozin in patients with heart failure with preserved ejection fraction. doi: 10.1016/j.cjca.2023.01.019. Epub 2023 Jan 25. [DOI] [PubMed]
- 58.Sezai A., Sekino H., Unosawa S., Taoka M., Osaka S., Tanaka M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc. Diabetol. 2019 Dec;18:1–3. doi: 10.1186/s12933-019-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox Z.L., Collins S.P., Hernandez G.A., McRae A.T., III, Davidson B.T., Adams K., Aaron M., Cunningham L., Jenkins C.A., Lindsell C.J., Harrell F.E., Jr Efficacy and safety of dapagliflozin in patients with acute heart failure. J. Am. Coll. Cardiol. 2024 Apr 9;83(14):1295–1306. doi: 10.1016/j.jacc.2024.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Yeoh S.E., Osmanska J., Petrie M.C., Brooksbank K.J., Clark A.L., Docherty K.F., Foley P.W., Guha K., Halliday C.A., Jhund P.S., Kalra P.R. Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics. Eur. Heart. J. 2023 Aug 14;44(31):2966–2977. doi: 10.1093/eurheartj/ehad341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palau P., Amiguet M., Domínguez E., Sastre C., Mollar A., Seller J., Garcia Pinilla J.M., Larumbe A., Valle A., Gómez Doblas J.J., De la Espriella R. Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction (DAPA-VO2): A randomized clinical trial. Eur. J. Heart. Fail. 2022 Oct;24(10):1816–1826. doi: 10.1002/ejhf.2560. Epub 2022 Jun 6. [DOI] [PubMed] [Google Scholar]
- 62.Fatima Gilani S.F., Ali S., Farhat K., Noor M., Siddiqui M.B., Waqar F. Early initiation of Dapagliflozin and its effect on health related quality of life in acute heart failure: a randomised controlled trial. JPMA. J. Pak. Med. Assoc. 2024 Apr 1;74(4):621–625. doi: 10.47391/JPMA.9813. [DOI] [PubMed] [Google Scholar]
- 63.G.D. Lewis, K. Gosch, L.P. Cohen, M.E. Nassif, S.L. Windsor, B.A. Borlaug, D.W. Kitzman, S.J. Shah, T. Khumri, G. Umpierrez, S. Lamba, Effect of dapagliflozin on 6-minute walk distance in heart failure with preserved ejection fraction: PRESERVED-HF. Circulation: Heart Failure. 2023 Nov;16(11):e010633. doi: 10.1161/CIRCHEARTFAILURE.123.010633. Epub 2023 Oct 23. [DOI] [PMC free article] [PubMed]
- 64.Charaya K., Shchekochikhin D., Agadzhanyan A., Vashkevich M., Chashkina M., Kulikov V., Andreev D. Impact of dapagliflozin treatment on serum sodium concentrations in acute heart failure. Cardiorenal. Med. 2023 Feb 10;13(1):101–108. doi: 10.1159/000529614. Epub 2023 Feb 20. [DOI] [PubMed] [Google Scholar]
- 65.Xie L., Li S., Yu X., Wei Q., Yu F., Tong J. DAHOS Study: Efficacy of dapagliflozin in treating heart failure with reduced ejection fraction and obstructive sleep apnea syndrome—A 3-month, multicenter, randomized controlled clinical trial. Eur. J. Clin. Pharmacol. 2024 May;80(5):771–780. doi: 10.1007/s00228-024-03643-3. Epub 2024 Feb 22. [DOI] [PubMed] [Google Scholar]
- 66.A.N. Emara, M. Wadie, N.O. Mansour, M.E. Shams, The clinical outcomes of dapagliflozin in patients with acute heart failure: A randomized controlled trial (DAPA-RESPONSE-AHF). European Journal of Pharmacology. 2023 Dec 15;961:176179. doi: 10.1016/j.ejphar.2023.176179. Epub 2023 Nov 2. [DOI] [PubMed]
- 67.Fu Q., Zhou L., Fan Y., Liu F., Fan Y., Zhang X., Wang L., Cheng L. Effect of SGLT-2 inhibitor, dapagliflozin, on left ventricular remodeling in patients with type 2 diabetes and HFrEF. BMC. Cardiovasc. Disord. 2023 Nov 8;23(1):544. doi: 10.1186/s12872-023-03591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marton A., Saffari S.E., Rauh M., Sun R.N., Nagel A.M., Linz P., Lim T.T., Takase-Minegishi K., Pajarillaga A., Saw S., Morisawa N. Water conservation overrides osmotic diuresis during SGLT2 inhibition in patients with heart failure. J. Am. Coll. Cardiol. 2024 Apr 16;83(15):1386–1398. doi: 10.1016/j.jacc.2024.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Soga F., Tanaka H., Tatsumi K., Mochizuki Y., Sano H., Toki H., Matsumoto K., Shite J., Takaoka H., Doi T., Hirata K.I. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc. Diabetol. 2018 Dec;17:1 -8. doi: 10.1186/s12933-018-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]