Highlights
-
•
There are currently limited suitable therapies available for treating fine motor disorders in Parkinson’s disease (PD).
-
•
Baduanjin Qigong exercise has been shown to be effective in improving hand dexterity and quality of life in PD patients.
-
•
Baduanjin could be a valuable complementary therapy and could potentially be extended to the community or home setting.
Keywords: Baduanjin, Qigong, Parkinson’s disease, Fine motor, Dexterity
Abstract
Background
Fine motor impairment is common in Parkinson’s disease (PD), which reduces patients’ quality of life. There are few suitable targeted treatments. We conducted a clinical trial to determine whether Baduanjin Qigong exercise would increase fine motor skills in PD patients.
Methods
Sixty PD patients (Hoehn-Yahr stage 1–4) with hand fine motor impairment were randomly assigned to the Baduanjin group and the physical activity group. Baduanjin group practiced Baduanjin exercise five times weekly for 40 min (warm-up 5 min, Baduanjin 30 min, cool-down 5 min). The usual physical activity groups maintained their habit of usual physical activities. The participants underwent assessments in the “ON” medication state at baseline and 4-week follow-up time points. The Purdue Pegboard Test (PPT) was used as the primary outcome to assess manual dexterity. The secondary outcomes included the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale, part III (MDS-UPDRS III), and the Parkinson’s disease questionnaire (PDQ-39).
Results
The results of PPT revealed the Baduanjin group showed statistically significant improvement in the “non-dominant hand” and “assembly” scores compared to the usual physical activity group (P < 0.05), but with no significant difference in “dominant hand” and “both hands” (P > 0.05). Additionally, the Baduanjin group showed better performance in the PDQ-39 (P < 0.05).
Conclusion
Our study concludes that a 4-week Baduanjin exercise is effective in improving fine motor function and quality of life in patients with mild and moderate PD. The results suggest a promising intervention to be implemented in community or home settings for managing fine motor impairment in PD.
1. Introduction
Fine motor impairment is a common symptom of Parkinson’s disease (PD). Fine motor skills affect daily living activities of PD patients, such as dressing, writing, or feeding, from early to advanced stages. Coordination of finger movement plays an important role in many daily tasks involving fine motor skills. Medication is the primary treatment for motor symptoms, but the effectiveness of pharmacological treatment may decrease over time, which can lead to severe motor fluctuations and dyskinesia. There are multiple and complex mechanisms involved in fine motor impairment in PD, leading to a poor response to pharmacological treatment [1]. Therefore, it is important to consider non-pharmacological approaches to address fine motor impairment in patients with PD. Targeted exercise programs developed by researchers have shown the most promising results, but comprehensive physical exercise may be more suitable and easier to generalize to the wider PD population [2], [3].
Mind-body exercises like Baduanjin Qigong have gained attention as effective non-pharmacological interventions for improving motor symptoms in PD patients [4]. Baduanjin pays more attention to the upper limbs, may be more accessible for those with lower fitness levels compared to other mind–body exercises with its simple movements [3]. However, there is a lack of effective targeted therapies for widespread use in the PD population. Therefore, this study aimed to evaluate the effects of a 4-week Baduanjin intervention on the fine finger movements function in PD patients.
2. Material and methods
2.1. Participants
Sixty patients who were diagnosed with idiopathic Parkinson's disease (PD) and hand fine motor impairment recruited from The First Affiliated Hospital of Anhui University of Chinese Medicine between March 2022 and September 2023. Idiopathic PD was diagnosed according to the 2015 Movement Disorder Society (MDS) clinical diagnostic criteria. The inclusion criteria were: (a) aged 18–80 years; (b) Hoehn and Yahr stages I- Ⅳ; (c) Ability to stand unaided and follow simple commands; (d) Participants should receive stable anti-Parkinsonian therapy for at least 2 weeks without changing their medications during the trial. The exclusion criteria were: (a) currently enrolled in any behavioral or pharmacological intervention study or instructor-led exercise training program; (b) Other conditions that may cause fine motor impairment, such as brain tumor or hand trauma.
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of The First Affiliated Hospital of Anhui University of Chinese Medicine. Written informed consent was obtained from all participants, and the trial was registered at Clinical Trials.gov (identifier: ChiCTR2200061891).
2.2. Study design and randomization
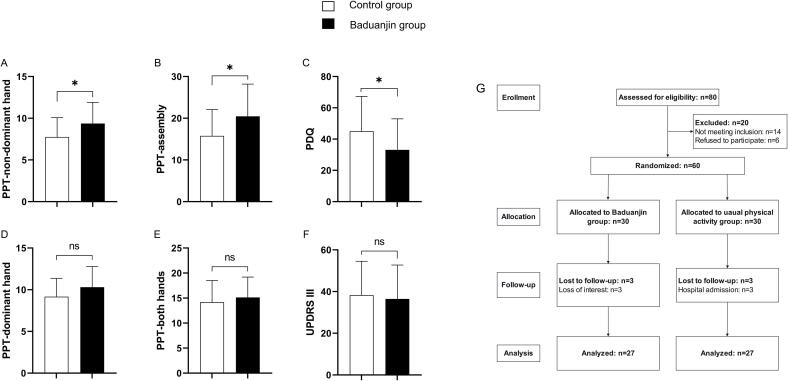
This was a randomized, assessor-blinded, parallel design trial adhered to the CONSORT (Consolidated Standards of Reporting Trials). The participants were allocated in a 1:1 ratio by the computer-generated random numbers table (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). Participants were randomly assigned to either a 4-week Baduanjin exercise group or a control group that maintained their usual physical activities. The randomization sequence was only revealed to the Baduanjin teacher, and the others, including the outcome assessors and data analysts, were unaware of group assignments. Flow chart is shown in Fig. 1G.
Fig. 1.
ABCDEF: The results of PPT, MDS-UPDRS III, PDQ-39 after intervention. PPT, Purdue pegboard test; MDS-UPDRS III, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale, Part III (motor examination); PDQ-39, Parkinson’s Disease Questionnaire-39. White: Baduanjin group; Black: Usual physical activity group. *P < 0.05. Fig. 1. G: Flow chart of trial design.
2.3. Baduanjin
Participants in the Baduanjin group received 4-week training based on the standards set by the General Administration of Sport in China. The whole Baduanjin exercise usually takes about 15 min to complete at the usual pace. This protocol included a 5 min warm-up, 30 min of Baduanjin training, and a 5 min cool-down. The training was conducted 5 times per week for 40 min each session by a professional coach. The training regime of 5 sessions per week and 40 min per session will be taught by a professional coach. Participants received on-site guidance and video demonstrations to help with daily practice. Adjustments to the difficulty of movements were allowed based on each individual's physical condition.
2.4. Clinical assessments
Participants were assessed the week before initiation of Baduanjin training and the week after the 4-week training. Assessments were carried out in the “ON” medication state, i.e. the time of optimal medication effect as defined by the participants. All assessments occurred at approximately the same time of day to minimize the effects of motor fluctuations.
Changes in Purdue Pegboard Test (PPT; Lafayette Instrument Company, USA) scores between follow-up and baseline served as the primary outcome measures. Compared to other testing methods, PPT is more sensitive and accurate for fine motor skills, especially in the early stage of PD [5]. PPT was evaluated for participants with PD in the “ON” medication state (within 1 h after taking the anti-PD drug) [5]. Four subtests were included: dominant hand, non-dominant hand, both hands, and assembly task. In the assembly task, patients had 60 s to assemble the parts onto the board in the order of pegs, washers, collars, and washers. The final score was the total number of parts assembled. The both-hands task required participants to simultaneously and independently insert pegs into the holes. Inserting one pair of pegs counted for two points, while inserting alone counted for no points.
Secondary outcome measures included the motor section of the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III) and the Parkinson’s disease questionnaire (PDQ-39). The severity of motor symptoms of PD was evaluated using the MDS-UPDRS III, while quality of life was measured using the PDQ- 39. Higher scores on these scales indicate greater symptom severity.
2.5. Statistics analysis and sample size
Demographic data were presented as mean ± SD for continuous variables. The t-test was used to compare continuous variables, and the χ2 test was used to compare categorical variables. The independent two samples t-test was used to test for group differences in mean change from baseline at the 4-week follow-up. Statistical analysis was performed using IBM SPSS 27.0 and GraphPad Prism 9 software. A P-value of less than 0.05 was considered significant for all tests.
The sample size was determined as a priori using G*Power 3 and calculated using PPT scores (the primary clinical outcome) obtained from a previous study [6]. The estimated minimum sample size was 52 participants based on a power of 0.80, and an α error probability of 0.05. With an estimated dropout rate of 10 %, a final sample size of 58 was required.
3. Results
The Baduanjin group and usual physical activity group both initially had 30 members, but each group lost 3 members to follow-up. The Baduanjin and usual physical activity groups were similar in terms of baseline characteristics. The participants’ demographic and clinical characteristics are shown in Table 1. The results of intervention, along with statistical significance, are presented in detail in Table 1 and Fig. 1. Additionally, there were no reported significant adverse events in either group.
Table 1.
Baseline characteristics and outcomes after intervention.
| Variables | Baduanjin group(n = 27) | Usual physical activity group(n = 27) | T value (χ2) | p value |
|---|---|---|---|---|
| Gender (male/female) | 13/14 | 10/17 | 0.682 | 0.409 |
| Age (years) | 65.59 ± 9.16 | 60.48 ± 11.52 | −1.805 | 0.077 |
| Years of education (years) | 7.07 ± 4.67 | 7.52 ± 3.84 | 0.382 | 0.704 |
| Disease duration (years) | 4.59 ± 3.70 | 7.56 ± 7.95 | 1.757 | 0.087 |
| H-Y stage(pre-test) | 2.57 ± 0.57 | 2.56 ± 0.56 | −0.121 | 0.904 |
| LEDDs(mg) | 387.63 ± 193.25 | 553.13 ± 397.58 | 1.945 | 0.057 |
| PPT (pre-test) | ||||
| Dominant hand | 9.15 ± 2.501 | 9.25 ± 2.048 | 0.158 | 0.875 |
| Non-dominant hand | 8.15 ± 2.556 | 7.77 ± 2.259 | −0.583 | 0.562 |
| Both hand | 13.04 ± 4.148 | 13.95 ± 4.336 | 0.791 | 0.432 |
| Assembly | 18.23 ± 8.232 | 15.78 ± 6.315 | −1.231 | 0.224 |
| MDS-UPDRS III (pre-test) | 40.67 ± 16.964 | 38.52 ± 16.023 | −0.478 | 0.634 |
| PDQ-39 (pre-test) | 37.07 ± 20.147 | 44.93 ± 22.518 | 1.350 | 0.183 |
| PPT (post-test) | ||||
| Dominant hand | 10.31 ± 2.456 | 9.17 ± 2.192 | −1.792 | 0.079 |
| Non-dominant hand | 9.37 ± 2.532 | 7.75 ± 2.310 | −2.449 | 0.018* |
| Both hand | 15.14 ± 4.055 | 14.17 ± 4.346 | −0.841 | 0.404 |
| Assembly | 20.47 ± 7.714 | 15.76 ± 6.316 | −2.452 | 0.018* |
| MDS-UPDRS III (post-test) | 36.48 ± 16.225 | 38.30 ± 16.231 | 0.411 | 0.683 |
| PDQ-39(post-test) | 33.07 ± 19.873 | 45.04 ± 22.243 | 2.084 | 0.042* |
Means and standard deviations are shown for continuous variables.
Abbreviations: H-Y stage, Hoehn and Yahr stage; LEDDs, levodopa-equivalent daily doses; PPT, Purdue pegboard test; MDS-UPDRS III, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale, Part III (motor examination); PDQ-39, Parkinson’s Disease Questionnaire-39. *P < 0.05.
3.1. Primary outcomes
At post-intervention, the comparison of the PPT performance of the Baduanjin group with baseline revealed that scores were increased in four tasks (Table 1). Participants in Baduanjin exercise training group performed significantly better in the non-dominant-hand task (p = 0.018) (Fig. 1A) and assembly task (p = 0.018) (Fig. 1B) compared to those who maintained their usual physical activities. However, there was no significant difference between the two groups in the dominant hand task (p = 0.079) and both-hands task (p = 0.404).
3.2. Secondary outcomes
Baduanjin exercise training also significantly improved the quality of life, as measured by the PDQ-39 (p = 0.042) (Fig. 1C), in PD patients compared to control group. However, there were no significant differences between the two groups for the MDS-UPDRS III (p = 0.404) after the intervention.
4. Discussion
To our knowledge, this is the first controlled trial to specifically examine the efficacy of Baduanjin exercise as a treatment for fine finger movement disorder in mild to moderate PD. In this study, we found that Baduanjin exercise had a positive impact on fine motor performance of fingers and quality of life in patients with mild to moderate PD, without significant adverse events. The Baduanjin intervention was will-received, with high acceptance, adherence, and safety, particularly in terms of reducing the risk of falls [7]. This suggests that Baduanjin may be a promising treatment option for fine finger movement disorder in the mild to moderate PD patient population, and could potentially be integrated into clinical guidelines and community- or home-based exercise programs [8]. This model of integration between clinical settings and lifestyle could be potential beneficial for rehabilitation and social benefits. Cultural differences and acceptance of the Baduanjin exercise may limit its application. However, some studies [8], [9] show that Baduanjin is readily accepted by patients, indicating that it is potential to be implemented in various cultures.
Some studies have investigated the clinical utility of targeted exercise interventions as a treatment for fine motor impairment in PD. For example, Valentina Varalta et al. [10] tested the effects of modified vibratory stimulation training in 20 patients with mild and moderate PD over a period of 3 weeks. They found that this therapy produced reductions in fine motor impairment severity at 1 month follow-up after the intervention. Similarly, our study showed greater improvements in fine motor skills of fingers in the Baduanjin group compared to the control group after a 4-week intervention. However, there was only a trend towards significance for the dominant hand task (p = 0.079). It should be noted that only one of our participants was left-handed, and previous studies have reported differences in fine motor function between dominant and non-dominant hands [11]. This suggests that there may be more room for improvement on the non-dominant side. Additionally, all participants in our study were in the mild to moderate stages of PD, which may have limited our ability to detect subtle changes in fine motor function in the dominant hand due to a ceiling effect. This is consistent with previous research, which also found a significant improvement in an assembly task but not in a both-hands task [12]. A possible explanation for this discrepancy is that the scoring rules used in the both-hands task are not sensitive enough to capture changes, since only pairs of pegs are recorded.
In terms of secondary outcomes, the subjectivity may limit sensitivity of PDQ-39 (p = 0.042) in this 4-week intervention, especially compared with the PPT. Nonetheless, our results are consistent with previous studies, showing a significant improvement in quality of life along with an increase in fine motor skills [13]. This further supports the close relationship between fine motor function and quality of life in PD patients. However, similar to a previous 4-week exercise intervention program, we did not find a significant improvement in the MDS-UPDRS III, which may be due to the subjectivity of this measure, and the fact that a longer training period (more than 12 weeks) may be needed to see clinically meaningful changes [13], [14].
This study also has some limitations. First, it is a single-center clinical trial, and the sample size was determined based on a single previous study, which may have underestimated the necessary sample size. Therefore, multicenter larger-size cohort studies are warranted. Second, the follow-up duration is relatively too short to determine the long-term effects of the Baduanjin intervention. Long-term follow-up should be carried out in subsequent research. Third, we included a relatively small sample of patients with mild to moderate PD. Therefore, we cannot generalize our findings to more severe PD patients. Finally, all participants were tested during the “ON” medication state, which may have masked underlying changes induced by the training interventions and could be addressed in future studies.
5. Conclusion
In conclusion, our study demonstrated that a 4-week Baduanjin program is effective in improving fine motor impairment and quality of life in mild to moderate PD patients. These findings suggest that Baduanjin could be a valuable complementary therapy for managing fine motor impairment of fingers in PD, and could potentially be extended to the community or home setting.
6. Ethical compliance statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The authors confirm that the approval of an institutional review board was not required for this work. The patient gave written informed consent for all treatments and the publication of this case report. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding sources
This study was funded by the Anhui University Science Research Project(grant numbers: KJ2021A058), the Clinical research project of the Anhui University of Traditional Chinese Medicine(grant numbers: 2021yfylc06), the Outstanding Young Talents Project of Universities in Anhui Province Study Abroad (grant number: gxgwfx2021028), the Anhui Provincial Health Research Project (No: AHWJ2022b015), the Anhui key R & D plan(NO:2022e07020025), Nature Fund of the Department of Education of Anhui Province (No: 2024 AH050998),Anhui Province Natural Resources Fund (No: 2408085MH228).
CRediT authorship contribution statement
Ke-Fan Li: Writing – original draft, Project administration, Methodology. Jun Li: Writing – review & editing, Formal analysis, Conceptualization. A-Long Xia: Software, Project administration. Xiao-Wei Wang: Validation, Formal analysis. Ai-Ling Wang: Methodology, Formal analysis. Ying Shi: Validation, Software. Huai-Zhen Chen: Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank the whole patients for their cooperation with treatments and tests.
References
- 1.De Vleeschhauwer J., Broeder S., Janssens L., Heremans E., Nieuwboer A., Nackaerts E. Impaired touchscreen skills in parkinson's disease and effects of medication. Mov. Disord. Clin. Pract. 2021;8(4):546–554. doi: 10.1002/mdc3.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai V., Gupta A., Wong M. Commentary: an interactive videogame for arm and hand exercise in people with Parkinson's disease: a randomized controlled trial. Front. Neurosci. 2018;12:328. doi: 10.3389/fnins.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proud E.L., Miller K.J., Morris M.E., McGinley J.L., Blennerhassett J.M. Effects of upper limb exercise or training on hand dexterity and function in people with parkinson disease: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2023 doi: 10.1016/j.apmr.2023.11.009. [DOI] [PubMed] [Google Scholar]
- 4.He S., Fang W., Wu J., et al. Whether mindfulness-guided therapy can be a new direction for the rehabilitation of patients with Parkinson's disease: a network meta-analysis of non-pharmacological alternative motor-/sensory-based interventions. Front. Psychol. 2023;14:1162574. doi: 10.3389/fpsyg.2023.1162574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proud E.L., Miller K.J., Bilney B., Morris M.E., McGinley J.L. Construct validity of the 9-hole peg test and purdue pegboard test in people with mild to moderately severe Parkinson's disease. Physiotherapy. 2020;107:202–208. doi: 10.1016/j.physio.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Chen C.Y., Wang W.N., Lu M.K., et al. The rehabilitative effect of archery exercise intervention in patients with parkinson's disease. Parkinsons Dis. 2023;2023:9175129. doi: 10.1155/2023/9175129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai J., Cai Y., Yang L., Xia M., Cheng X., Chen Y. Effects of Baduanjin exercise on motor function, balance and gait in Parkinson's disease: a systematic review and meta-analysis. BMJ Open. 2022;12(11) doi: 10.1136/bmjopen-2022-067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho L.P., Décary S., Beaulieu-Boire I., et al. Baduanjin qigong intervention by telerehabilitation (teleparkinson): a proof-of-concept study in Parkinson's Disease. Int. J. Environ. Res. Public Health. 2021;18(13) doi: 10.3390/ijerph18136990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira A., Matos L.C., Conceição A.M. Does Qigong Practice Have Benefits on the Management of Parkinson’s Disease? Multidiscip. Res. J. 2019;2(3):352–363. [Google Scholar]
- 10.Varalta V., Righetti A., Evangelista E., et al. Effects of upper limb vibratory stimulation training on motor symptoms in Parkinson's disease: an observational study. J. Rehabil. Med. 2024;56:jrm19495. doi: 10.2340/jrm.v56.19495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Beek J.J.W., van Wegen E.E.H., Bohlhalter S., Vanbellingen T. Exergaming-based dexterity training in persons with Parkinson disease: a pilot feasibility study. J. Neurol. Phys. Ther. 2019;43(3):168–174. doi: 10.1097/npt.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 12.Kalyani H.H., Sullivan K.A., Moyle G.M., Brauer S.G., Jeffrey E.R., Kerr G.K. Dance improves symptoms, functional mobility and fine manual dexterity in people with Parkinson disease: a quasi-experimental controlled efficacy study. Eur. J. Phys. Rehabil. Med. 2020;56(5):563–574. doi: 10.23736/s1973-9087.20.06069-4. [DOI] [PubMed] [Google Scholar]
- 13.Vanbellingen T., Nyffeler T., Nigg J., et al. Home based training for dexterity in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat. Disord. 2017;41:92–98. doi: 10.1016/j.parkreldis.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Radder D.L.M., Silva L., de Lima A., Domingos J., et al. Physiotherapy in Parkinson's disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair. 2020;34(10):871–880. doi: 10.1177/1545968320952799. [DOI] [PMC free article] [PubMed] [Google Scholar]