Abstract
Major trade-offs often manifest as axes of diversity in organismal functional systems. Overarching trade-offs may result in high trait integration and restrict the trajectory of diversification to be along a single axis. Here, we explore the diversification of the feeding mechanism in coral reef fishes to establish the role of trade-offs and complexity in a spectacular ecological radiation. We show that the primary axis of variation in the measured musculo-skeletal traits is aligned with a trade-off between mobility and force transmission, spanning species that capture prey with suction and those that bite attached prey. We found weak or no covariation between about half the traits, reflecting deviations from the trade-off axis. The dramatic trophic range found among reef fishes occurs along the primary trade-off axis, with numerous departures that use a mosaic of trait combinations to adapt the feeding mechanism to diverse challenges. We suggest that morphological evolution both along and independent of a major axis of variation is a widespread mechanism of diversification in complex systems where a global trade-off shapes major patterns of diversity. Significant additional diversity emerges as systems use weak integration and complexity to assemble functional units with many trait combinations that meet varying ecological demands.
Subject terms: Evolution, Ecology
The diversity of coral reef fishes’ feeding apparatus aligns with the trade-off between mobility and force transmission, but weak trait covariation and complexity allow jaw traits to evolve independently to adapt to diverse ecological challenges.
Introduction
The diversification of complex biomechanical and physiological systems is often shaped by important trade-offs1–4 where changes to a component improve performance in one function but necessarily decrease performance in a second function. Major trade-offs may be substantial organizing forces in evolution, ultimately manifesting as overarching axes of diversification, such as is seen in the wings of birds5, the legs of frogs6, the carapace of turtles7, and the fins of fishes8. Trade-offs can have substantial macroevolutionary consequences as they can constrain evolution by limiting the compatibility of certain trait values2,3,9–11 and the resulting high trait covariation may promote phenotypic macroevolution along an adaptive line of least resistance10,11. In this way, trade-offs may induce such strong trait covariations as to limit phenotypes independent of the major trade-off10,11.
Dominant trade-offs have established primary axes of diversity in many functional systems, yet for many major radiations, there is extensive ecological and morphological diversity that extends well beyond the trend of the primary traits involved in the trade-off. Here, the increasing number and diversity of traits contributing to performance allows for expanded diversification as unique trait combinations can satisfy different functional demands12,13. This may manifest as weakened covariation among traits and expand the range of ecomorphological diversity observed, independent of the major trade-off, by allowing traits to respond more independently to natural selection14–18. On macroevolutionary timescales, increased independence of traits within complex mechanical systems may result in a variety of evolutionary pathways not limited by the dominant trade-off axis11,18–21. In other words, the greater complexity of a functional system may weaken integration, thereby allowing diversity to be generated in different directions beyond the major defining trade-off.
Multiple complex vertebrate functional systems appear to follow a general model of diversification where a global trade-off combines with relatively weak integration to assemble high functional diversity21–24. This model includes an overarching trade-off defining a primary axis of trait variation, such as the axis of elongation in the mammalian skull related to bite force and jaw closure speed25–27. Considerable functional and ecological diversity evolves along this relatively integrated axis, but additional diversity also emerges independent of the major trade-off, made possible by morphological complexity and the increased evolutionary independence of traits. For instance, in carnivoran mammals, the decoupled evolution of the cranium and mandible led to lineages adapting to multiple dietary regimes while keeping clade-specific adaptations needed for sensory structures28. Many studies have identified an association between ecological shifts and changes in patterns and magnitudes of evolutionary integration, where ecological diversity and novel trait combinations result in varying degrees of integration and trait independence11,22,29,30. We explore whether this potentially general model explains the exceptional diversity of the feeding system of coral reef fishes, a highly diverse functional system that supports extensive ecological diversity26–29.
Reef fishes are a promising candidate for this model as they are thought to show a dominant functional trade-off in their feeding mechanism. This trade-off is associated with the contrast between capturing prey using suction and biting prey attached to the substrate, the two most common feeding modes in reef fishes. Suction feeding involves the rapid expansion of the mouth and buccal cavity to generate a flow of water that pulls in prey31–33, and it is often paired with a variable amount of forward swimming (i.e., ‘ram’)34,35. To increase suction performance, suction feeders often evolve elongate and highly kinetic oral jaw elements, including lengthened upper and lower jaw elements. Further, suction feeders often show considerable protrusion of the upper jaw, extensive expansion of the mouth cavity, and the formation of a circular mouth aperture31,33,36–39. Conversely, biting involves removing resources directly from the substrate40–45. Biters depend to varying degrees on forces exerted by the oral jaws to dislodge food from the substrate39,46–49. In contrast to suction feeders, biters are expected to have smaller and more robust oral jaw elements, thus increasing the force transmission, strength in resisting forces, and mechanical stability within the oral jaw lever systems39,46–49. This contrast in key functional attributes between skeletal mobility in suction feeding vs. strength in biting reflects the classic mechanical trade-off in skeletal linkage systems. This trade-off between the transmission of motion and force has long been thought to represent the major axis of diversity in the feeding mechanism across fishes50,51.
Despite its importance in the fish feeding system, this trade-off cannot fully account for the astonishing trophic diversity found among coral reef fishes52–55. Coral reef fishes feed on virtually all reef animals, plants, and some microbial organisms54,56–58, suggesting the potential for extensive fine-tuning of feeding morphology extending beyond the force vs. mobility trade-off. Within biters, there are lineages of herbivores that crop algal turfs59; detritivores that scrape detritus from the substrate60; and invertivores that remove sessile invertebrates44,61,62 or that feed on structurally defended prey like urchins and mollusks56. Suction feeders include many general carnivores that feed on large mobile prey like other fish and cephalopods; mobile invertivores that capture invertebrates residing within the reef such as polychaetes and many crustaceans; and planktivores that feed on a diversity of organisms in the water column63–66. Such ecological diversity highlights the potential for the evolution of resource-specific, morphological, and functional specialization of feeding structures, resulting in enhanced feeding performance.
Coral reef fishes are a highly polyphyletic group of over 70 families, reflecting a long history of transitions on and off of reef habitats67. Most modern reef fishes belong to the large teleost radiation of spiny-rayed fishes, Acanthomorpha, and represent lineages that have been associated with reefs across such varied timescales as 20–150 Mya68. Species accumulation and ecological diversification of modern reef lineages have occurred in tandem with the ecological restructuring of reefs following the end-Cretaceous mass extinction59,69–72. Benthic biting lineages, in particular, have been growing continuously in number and ecological importance since at least the early Eocene73,74. This complex history has contributed substantially to the morphological and functional diversity of reef fishes75,76.
To understand how reef fish feeding mechanisms have evolved to support their extensive ecological diversity, we applied phylogenetic comparative methods to morphological traits capturing the diversity of fish feeding mechanisms, measured from 110 species representing 43 major coral reef fish families. Our study aims to explore (1) whether the primary axis of variation in the feeding mechanism reflects a trade-off between suction and biting, (2) how these two feeding modes relate to morphological diversity and evolutionary patterns, and (3) whether adaptation to seven major diet categories is better explained by moving along the major trade-off, or through independent trait diversification. Our study demonstrates that diversification of the feeding apparatus across broad lineages of coral reef fishes involves the presence of the well-established mechanical trade-off between craniofacial mobility and biting performance, combined with extensive, diet-specific, trait evolution that has capitalized on the complexity and weak integration of feeding structures to support one of the most ecologically diverse assemblages of vertebrates on Earth.
Results
A functional morphospace for the feeding mechanism of reef fishes
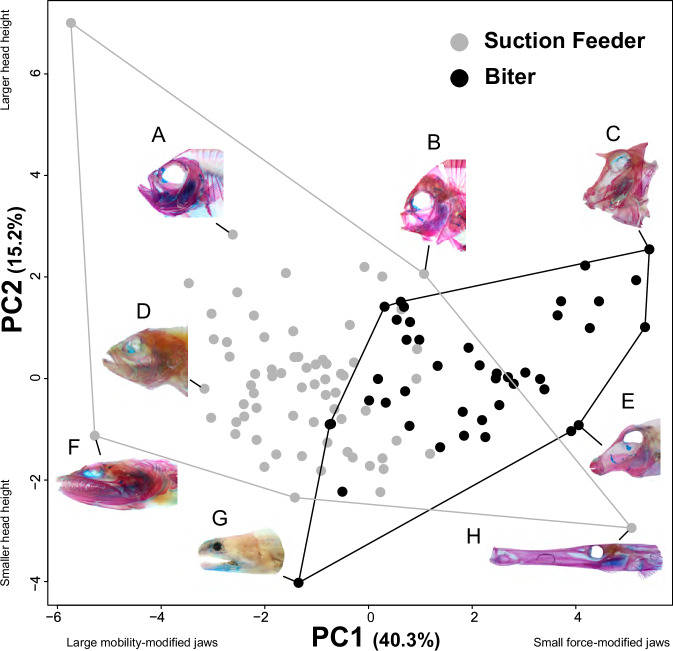
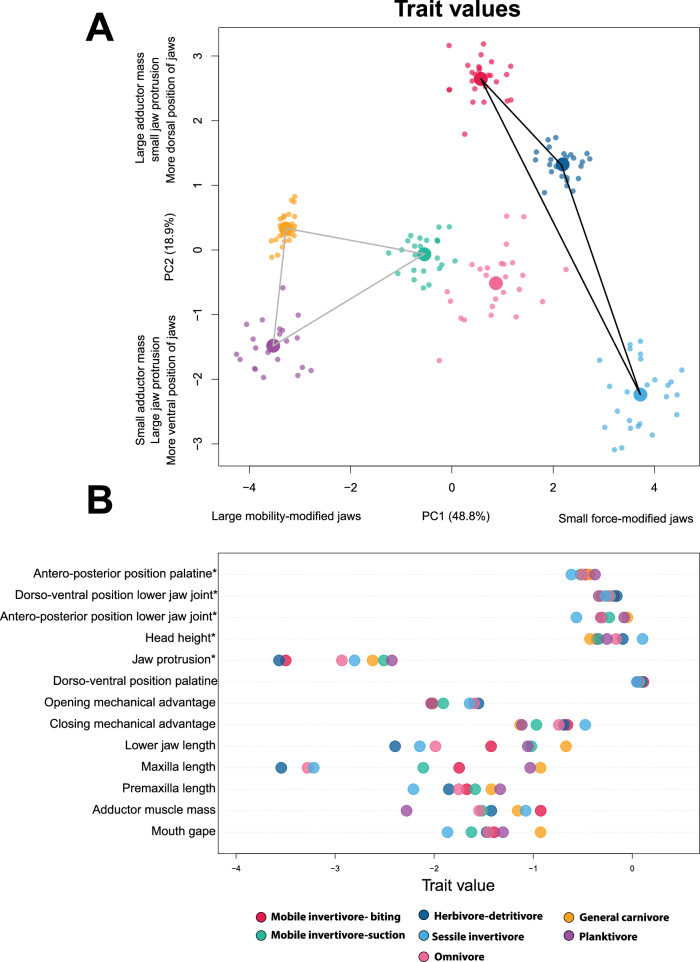
First, we explored the major axes of variability in the reef fish feeding apparatus to determine if the largest axis of morphological diversity in measured traits aligns with the mechanical trade-off in musculo-skeletal morphology between suction feeding and biting. A principal components analysis (PCA) of 13 variables (Fig. 1) describing the linear dimensions of the feeding apparatus in 110 species from 43 coral reef fish families shows that the major axis of variation, PC1, largely separates species that feed by suction from species that feed by biting (Fig. 2 and Supplementary Fig. 1). The species examined showed considerable variation in oral jaw and craniofacial morphology (Fig. 2 & Supplementary Fig. 1). Taxa varied widely in the shape, size, and positioning of the oral jaw across PC1. For example, species with higher values of PC1 (e.g., triggerfish and boxfish) have small, anteriorly placed oral jaws, while species with smaller values of PC1 (e.g., lizardfish and frogfish) have large, posteriorly placed oral jaws. Species also varied considerably in head morphology across PC2. Those with lower values of PC2 (e.g., moray eel and trumpetfish) have a smaller head height and larger head length. In contrast, species with high values of PC2 (e.g., frogfish) have larger head heights and smaller head lengths.
Fig. 1. The 13 craniofacial traits measured for each species.
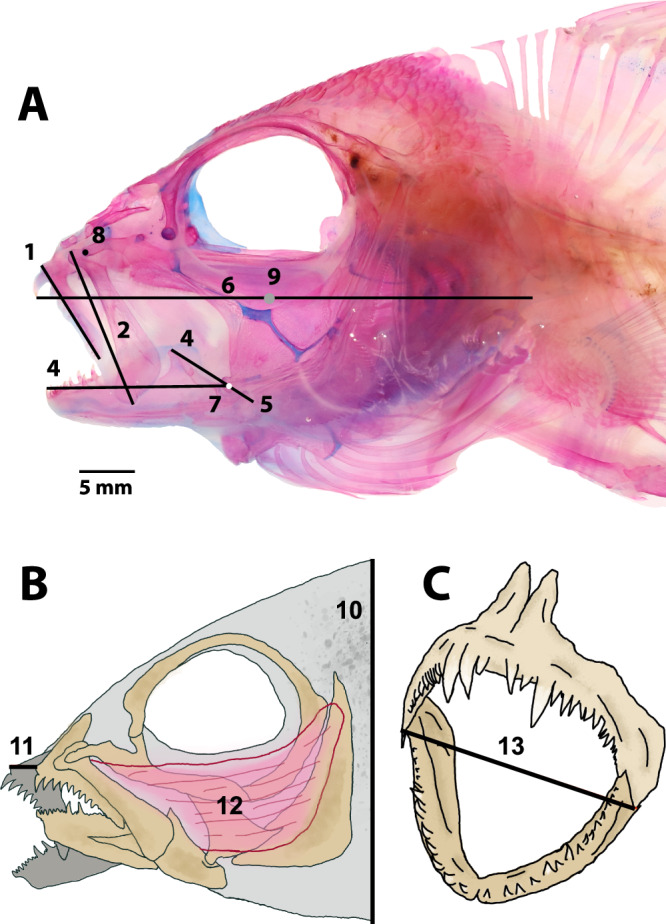
A A lateral view of the head of a cleared and stained coral reef fish (Lutjanus kasmira) illustrating 9 of the 13 traits measured and the location of the horizontal and vertical body axis used for position measurements. (1) length of the dentigerous arm of the premaxilla, (2) length of the maxilla, (3) lower jaw length, (4) jaw closing in-lever length (used to calculate mechanical advantage) (5) jaw opening in-lever length (used to calculate mechanical advantage), (6) head length, (7) antero-posterior and dorso-ventral position of the lower jaw joint, (8) antero-posterior and dorso-ventral position of the anteriormost portion of the palatine, (9) location of the intersection of a horizontal body axis that passed through the tip of the first tooth in the premaxilla and the last vertebrate centra and a vertical axis, orthogonal to the first axis that passed through the center of the orbit of the eye. B Illustration of the head region showing (10) head height, (11) jaw protrusion, and (12) the adductor mandibulae. C Illustration of the jaws showing the (13) mouth gape measurement.
Fig. 2. Principal component analysis showing major axes of craniofacial variation in 110 species of coral reef fishes for each functional feeding mode.
Each point corresponds to a species and is colored by feeding mode. Photos show representative species illustrating morphological variation across the plot including (A) Pristigenys serrula, (B) Dascyllus trimaculatus, (C) Acanthostracion quadricornis, (D) Cephalopholis baenck, (E) Canthigaster solandri, (F) Synodus saurus, (G) Echidna nebulosa, and (H) Aulostomus maculatus.
PC1 was negatively correlated with lengths of the premaxilla, maxilla, lower jaw length, and the relative position of the palatine (palatine-maxilla joint) and lower jaw joint (quadrate-articular joint; Supplementary Table 1). This axis reflects the tendency for biters to have short oral jaws, anteriorly placed in the skull, versus the longer jaws and more posterior jaw joints of suction feeders. PC2 was correlated with head height (Supplementary Table 1), where species with higher PC2 scores had larger head heights, greater jaw opening mechanical advantage, and a more ventral position of the lower jaw joint than species with lower PC2 scores.
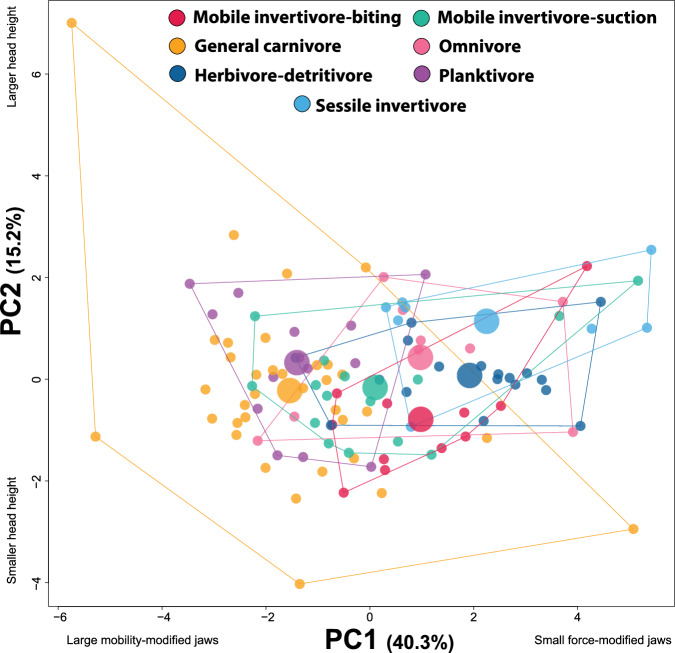
A plot of PC scores for each of the seven diet categories showed substantial overlap among the groups amidst some general differences (Fig. 3). Species that primarily feed by biting, including herbivore-detritivores, biting mobile invertivores, and sessile invertivores, tended to have more positive PC1 scores than species depending largely on suction to capture prey, including planktivores, suction-feeding mobile invertivores, and general carnivores. General carnivores are unique amongst the diet categories in that they represent almost all the extreme phenotypes in the morphospace and are all the species of suction feeders that tend to have more positive PC1 scores indicative of biters. Omnivores, which are almost equally split between biters and suction feeders, have more positive PC1 scores, though a few species of omnivores have negative PC1 scores. There was substantial overlap amongst all the diet groups along PC1, and diet groups tended to overlap completely along principal component 2, with no clear effect of diet on PC2 score.
Fig. 3. Principal component analysis showing major axes of craniofacial variation in 110 species of coral reef fishes for each diet.
Each point is the average shape of a species, colored by diet. Large circles denote the location of the centroid for each diet.
Differences in oral jaw trait evolution between feeding modes
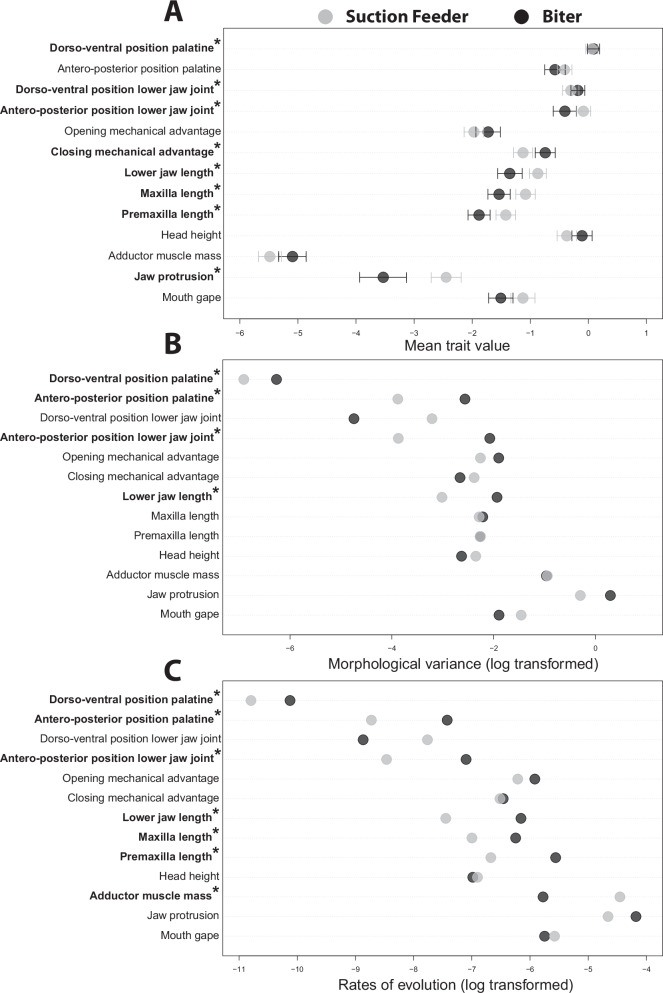
To further explore the morphological differences among suction feeders and biters, we used a phylogenetic MANOVA and individual phylogenetic ANOVAs that determined if and how feeding mode affects oral jaw and craniofacial morphology. The patterns within the morphospace were confirmed by the phylogenetic MANOVA, which found that suction feeders and biters differed significantly in average trophic morphology (F = 4.01; p = 0.004; Fig. 2 and Supplementary Fig. 1). Suction feeders had significantly higher jaw protrusion and longer premaxillae, maxillae, and lower jaws than biters (Fig. 4A). Suction feeders also had more posteriorly positioned lower jaw joints than biters, with the palatine and lower jaw joints also more ventral (Fig. 4A). In contrast, biters had significantly greater jaw closing mechanical advantage than suction feeders (Fig. 4A and Supplementary Table 2).
Fig. 4. Dot plots showing differences between biters and suction feeders for all 13 oral jaw and craniofacial traits.
Dot plots of (A) the mean trait value for each trait with confidence intervals around the mean, (B) log transformed morphological variance, and (C) log transformed rates of evolution. Traits that differed significantly between the functional feeding groups (p < 0.05) are bolded and have an asterisk.
To compare which feeding mode had the most diversity in oral jaw traits, we used multivariate and univariate disparity and rate analyses. There was no significant difference in multivariate oral jaw disparity between biters and suction feeders (biters: 2.73; suction feeders: 1.99; p = 0.2). Biters did, however, have significantly higher disparity in four univariate traits related to the length and positioning of the oral jaw: the lower jaw length, the antero-posterior position of the lower jaw joint, and the antero-posterior and dorso-ventral positions of the palatine (Fig. 4B and Supplementary Table 2). Patterns of oral jaw evolutionary rate were similar to disparity, with no significant difference in the rate of multivariate oral jaw evolution between biters and suction feeders (biters: 0.0027; suction feeders: 0.0025; p = 0.6). However, biters have higher rates of evolution across six of 13 individual traits, including in the antero-posterior and dorso-ventral position of the palatine, antero-posterior position of the lower jaw joint, lower jaw length, maxilla length, and premaxilla length. Suction feeders had a faster rate of evolution in just one trait: adductor muscle mass (Fig. 4C and Supplementary Table 3).
Evolutionary correlation among oral jaw traits
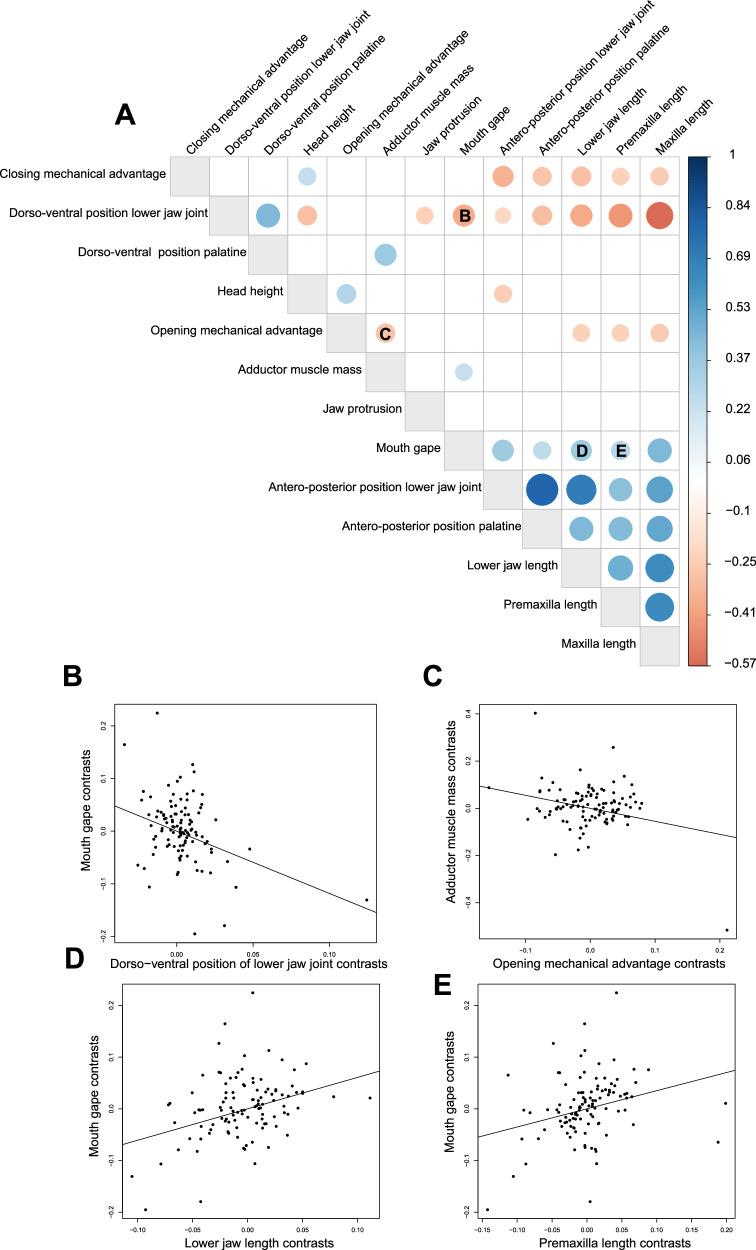
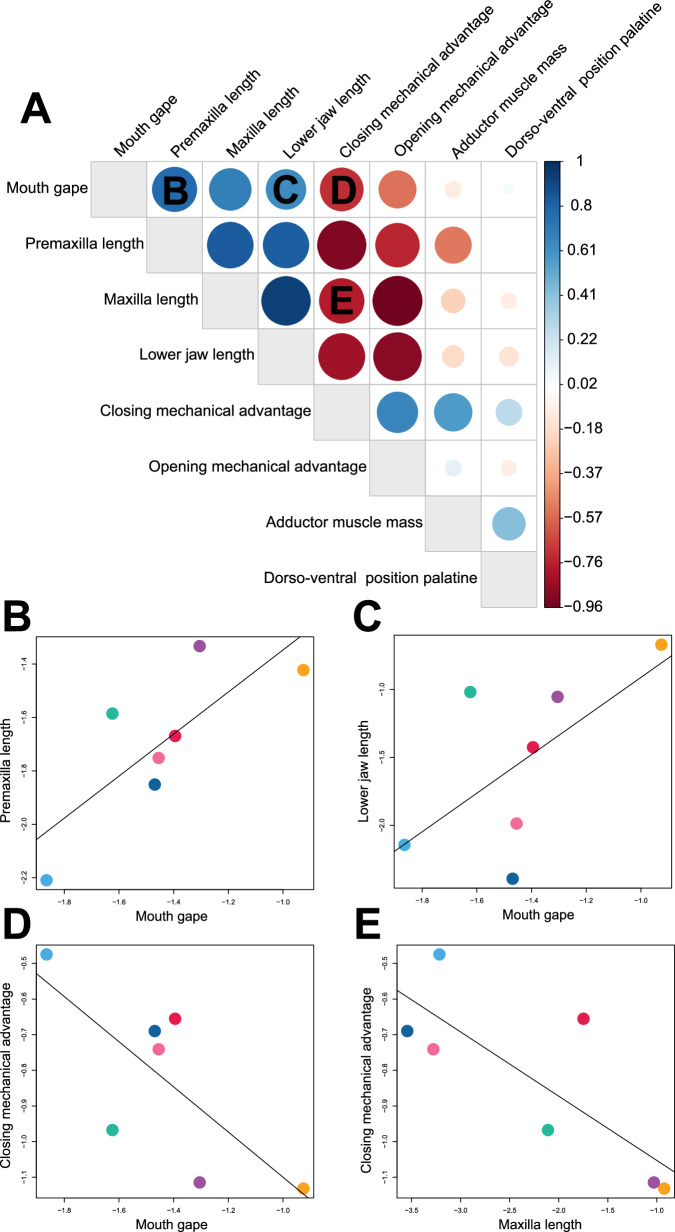
To determine whether oral jaw and craniofacial traits were correlated, we tested whether oral jaw traits were significantly more correlated than expected under simulated uncorrelated Brownian motion. About half of the pairwise correlations between oral jaw traits (38 of 78) were significantly greater than expected if they were evolving under uncorrelated Brownian motion (Fig. 5A). Many of the significant correlations reflected intimately linked elements of the feeding apparatus, such as, positive correlations among mouth gape, premaxilla length, maxilla length, lower jaw length with the antero-posterior positionings of the lower jaw joint and palatine (Fig. 5A). However, 31 of the 38 significant evolutionary correlations were moderate or weak (r < 0.5) and exhibit many outliers (Fig. 5B). In all, 40 of 78 combinations were not significant, indicating that many oral jaw and craniofacial traits within coral reef fishes exhibit a high degree of independent evolution.
Fig. 5. Heatmap of trait correlations and bivariate plots showing weak correlations between most traits.
A Heatmap of trait correlations. Blank boxes are trait correlation values that were not larger than the correlations from the Brownian motion simulation. Darker blue colors indicate a stronger positive correlation, while darker red colors indicate a stronger negative correlation. The size of the circle indicates the strength of the correlation. Letters B–E correspond to the bivariate plots. B–E Evolutionary correlations of the independent contrasts for a sampling of oral jaw traits that show weak, but significant, correlations.
Effects of diet on patterns of trait evolution
We used multi-peak Ornstein–Uhlenbeck (OU) models, estimates of disparity, and evolutionary rates to determine whether adaptation to the different diets resulted from diversification along the major trade-off or if evolution occurred independently, resulting in unique trait combinations and patterns of diversification. However, five traits, including the antero-posterior position of the palatine, the dorso-ventral and antero-posterior positions of the lower jaw joint, head height, and jaw protrusion, were based on the mean value as the trait optima could not be reliably recovered. We also used principal components analyses of these different metrics to visualize whether a variety of trait combinations, rates of evolution, and morphological variances, combined to differentiate the diets. To quantify whether different metrics of diversification adhere to the primary trade-off, we performed a linear regression of each pair of trait values to estimate the consistency of trait order across diets. A strong relationship between traits indicates similar patterns of evolution, while a weak correlation suggests mosaic evolution for different traits.
These analyses demonstrated that the trade-off between mobility and force is the main axis along which the seven diets are aligned in our dataset (Fig. 6A, B—PC1), but there is additional variation in trait combinations associated with diet diversity that occurs independently of the major trade-off (Fig. 6A, B— PC2). Optimal adductor muscle mass and the dorso-ventral position of the palatine show very little relationship with the other optimal trait values (Fig. 7A), indicating that diets can have either large or small mouth gapes with large adductor muscles and more ventrally placed jaws (Figs. 6 and 7 and Supplementary Table 3). Furthermore, even when oral jaw traits exhibit a strong relationship across most diets, some diets deviate strongly from the pattern. For instance, optimal mouth gape shows a strong relationship with optimal premaxilla length and lower jaw length, so when species in a diet category tend to have a large mouth gape, they also have a large premaxilla and lower jaw length (Fig. 7B, C). However, mobile invertivores break this pattern and have one of the smallest optimal mouth gapes and among the largest optimal premaxilla and lower jaw lengths (Fig. 7B, C). In another departure from the overall pattern, optimal mouth gape and maxilla length are strongly negatively correlated with optimal closing mechanical advantage, meaning that diets with large mouth gapes and maxilla lengths tend to have small closing mechanical advantage (Fig. 7D, E). However, mobile invertivore biters deviate from this pattern, as they have some of the largest optimal mouth gapes and maxilla lengths and the second-highest optimal closing mechanical advantage (Fig. 7D, E). We found similar results using a dataset that only consisted of mean trait values (Supplementary Fig. 2). Thus, while the species in the different diets clearly diversify along the trade-off between mobility and force, further diversification in the oral jaws occurs independently of this trade-off.
Fig. 6. Principal components analysis and dot plots showing differences in the optimal or mean trait values for each diet.
A Principal component analysis of optimal or mean trait values for all 13 oral jaw traits showing that, while trait combinations associated with diet are largely aligned with an overarching trade-off between jaw strength and mobility, there is variation in trait combinations beyond this trend. The black polygon represents diets that feed primarily through biting. The gray polygon represents diets that feed primarily through suction. Omnivores (pink circle) are represented by an almost equal split of biters and suction feeders. B Dotplot showing the variation in trait values between the diet groups for each trait. Asterisks denote traits in which the mean trait value was used instead of the optimal trait value as the multi-peak OU models could not converge.
Fig. 7. Heatmap of correlation coefficients and bivariate plots showing the relationships between optimal trait values for the different diets.
A Heatmap of correlation coefficients from the regression analysis of diets between the optimal trait values. Darker blue colors indicate a stronger positive correlation, while darker red colors indicate a stronger negative correlation. The size of the circle indicates the strength of the correlation. Letters B–E correspond to the bivariate plots. B–E Bivariate plots of the diets for a sampling of oral jaw trait optima that exhibit deviations from the overall relationship trend despite a significant correlation.
Different combinations exist for rates of evolution and morphological variance. No single diet consistently showed the fastest rate or highest disparity for all 13 traits (Supplementary Figs. 3 and 4 and Supplementary Tables 4 and 5). Instead, there is high variation in the rate at which traits evolve and their contributions to generating disparity within each dietary category. Thus, our results show that the evolution of the trophic apparatus in reef fish is multidimensional, where diet-specific modifications exploit the complex nature of fish feeding systems to generate unique and varying combinations of trait dynamics.
Discussion
The evolution of the feeding mechanism in coral reef fishes is dominated by a primary axis of diversification related to the mechanical trade-off between biting force and jaw mobility, which mirrors an ecological axis of feeding on attached vs. non-attached prey. Adaptation to different diets is achieved by a combination of evolution along this mechanical trade-off and adopting diverse secondary trait combinations. Thus, while the primary axis of diversity accounts for about 40% of trait variation (Fig. 2), generally weak evolutionary integration among a subset of traits allows diverse trait combinations to be associated with different diet categories (Figs. 5 and 6). Some oral jaw traits, such as adductor muscle mass and the relative position of the palatine joint, show weak or no correlation with other traits and yet exhibit strong diet-specific patterns of evolution (Figs. 5 and 6). Such patterns suggest that the complexity of traits and evolutionary independence was crucial to adapting oral jaw elements to distinct ecological niches. The combination of this major trade-off, and the further diversification of specific morphological elements independent of this trade-off, appears to be a key component to the exceptional morphological and ecological diversity of coral reef fishes.
Force vs. mobility underlies the primary axis of diversity
The dominant axis of variation in measured oral jaw morphology between suction feeders and biters reflects significant evolutionary correlations between gape size, the length of jaw elements, jaw closing mechanical advantage, and the position of key jaw joints in the skull. Species with large gapes, long jaw elements, low mechanical advantage, and a posteriorly placed jaw joint are exclusively taxa that feed using amplified suction. At the other extreme, species with small gapes, short jaw elements, high jaw closing mechanical advantage, and anteriorly placed jaw joints are mostly taxa that feed by directly biting prey attached to the reef. This overarching trade-off in oral jaw morphology strongly reflects the difference in functional demands of removing attached prey from the substrate versus capturing free-swimming prey from the water column. The small, robust, and more anteriorly placed jaws observed in biters more efficiently transmit bite force because of higher mechanical advantage, reflecting capacity for removing attached prey39–45. The longer skeletal components of the oral jaw of suction feeders create more cranial kinesis30 and are often associated with more jaw protrusion and increased gape size, reflecting the capacity for suction mechanisms to capture elusive prey in the water column31,33,36–39. Thus, in our study, the well-examined mechanical trade-off in musculo-skeletal systems24,36,39,40 between force and mobility produces the primary axis of morphological variation in the feeding apparatus of reef fishes.
Feeding mode (suction feeding vs. biting) did not significantly affect the multivariate rate of evolution or total disparity of the trophic apparatus, but a significant effect was observed on several individual traits (Fig. 4), underscoring that trait independence has played a prominent role in diversification of feeding mechanisms. These variable patterns of evolution in oral jaw traits suggest that a system with weak integration enhances evolvability by allowing evolution to occur along and independent of the integrated axis, potentially resulting in a variety of trait values, trait disparity, and evolutionary rates77–79. While the impact of feeding mode on craniofacial diversification is not universal, it is clear that feeding mode impacts disparity, rates of evolution, and co-evolution among oral jaw traits.
Diet generates a variety of trophic trait combinations
Beyond the effect of the dominant feeding mode, the trophic apparatus shows further refinement associated with different prey. Although diet categories overlap in morphospace, the distribution of the diet centroids for each group shows a strong imprint of the overarching trade-off between biting and suction feeding in that they are spread across PC1 in a sequence that reflects their reliance on either biting or suction for prey capture (Fig. 3). Diet centroids are distributed from low-to-high values on PC1, in order of generalized carnivores, planktivores, mobile invertivore suction feeders, biting mobile invertivores, omnivores, herbivore/detritivores, and sessile invertivores.
While the morphology of species in these diet categories largely follows the suction-biting axis, there are several trait-specific departures from the main axis of variation. For instance, general carnivores use ram and suction to feed on fishes, cephalopods, and large mobile crustaceans and have among the largest optimal jaw adductor muscle size across all seven diet categories. In contrast, suction-feeding planktivores are adjacent to general carnivores in morphospace, but have the smallest adductor muscle optimum among all diets (Fig. 6). This difference in adductor muscle mass occurs without influencing other aspects of the oral jaw morphology and may reflect the higher demands associated with restraining the large captured prey by some generalized carnivores, compared to minimal such demands in planktivores50,80,81. Biting mobile invertivores have larger optimal oral jaw elements, including mouth gape, premaxilla, maxilla, and lower jaw length, compared to herbivores-detritivores, omnivores, and sessile invertivores, indicating diversification along the major trade-off that potentially reflects differences in prey size80. Despite biting mobile invertivores and sessile invertivores evolving different oral jaw sizes, both diets evolve towards larger optimal adductor muscle masses than herbivore-detritivores, indicating evolution away from the trade-off that potentially reflects increased force demands of feeding on hard invertebrates as opposed to algae47,50. In other words, while a mechanical trade-off for suction and biting induces an overarching axis of diversity in reef fishes, adaptation to major diet categories involves fine-tuning the feeding apparatus in a way that manifests as combinations of trophic traits independent of the major trade-off. About half of the evolutionary correlations among trophic traits are insignificant (Fig. 5), suggesting that the lack of strong integration has relaxed evolutionary constraints within the feeding apparatus. It appears that evolutionary trait independence has been instrumental in facilitating the modification of the feeding apparatus in response to diet within the overarching patterns associated with suction and biting. In addition to diverse trait combinations, we observe diversity across diets in trait disparity and rates of evolution. Our results support previous suggestions that variable levels of evolutionary independence of traits support diversification82–85 and can increase the potential for lineages to evolve novel morphologies with unique trait combinations11,14,15,17,18. For instance, the avian skull is comprised of a mosaic of traits with different strengths of integration, facilitating this hyperdiverse evolutionary radiation21,24 and allowing birds to adapt to various dietary niches24,86. Furthermore, variable levels of integration in aquatic snake kinematic jaw traits allowed lineages adequate biomechanical solutions to a wide range of feeding ecologies and behaviors22. The overall pattern that emerges is that the evolution of the feeding apparatus in response to diet produces a variety of trait combinations and dynamics of trait evolution. Overall, our findings, coupled with the extensive literature on trait covariation, suggest that this pattern of weak integration between traits is a key component of complex and biologically diverse systems.
Conclusions
The feeding apparatus of coral reef fishes is structurally and mechanically complex, phenotypically diverse, and supports exceptional ecological diversity. Like essential functional systems in other groups, understanding how they diversify is central to understanding organismal evolution and ecomorphological diversification. Our study indicates that diversification of the reef fish feeding apparatus involves (1) evolution along anaxis of diversity associated with a functional trade-off and (2) secondary trait modifications independent of this trade-off that result in variable trait combinations associated with ecological diversity. We expect this general model can be used to understand how the evolution of biomechanical complexity facilitates diversification in many groups of organisms across the tree of life.
Methods
Morphological Trait Data
We generated a morphological dataset comprising 13 traits that characterize many features of oral jaw functional morphology in reef fishes. Traits were measured on 362 specimens representing 110 species from 43 families of coral reef fishes (Supplementary Table 7). Specimens were obtained through the aquarium fish trade. All animal work in this study was approved by the University of California, Davis Institutional Animal Care and Use Committee (protocol #22206). We have complied with all relevant ethical regulations for animal use. All live animals were of adult age and unknown sex (see Supplementary Table 7 for the species included in the study). Once in the lab, specimens were euthanized with overexposure to a solution of MS-222 and several measurements were immediately taken with dial calipers and a scale while the fish were still flexible. These measurements included mouth gape, jaw protrusion, and body mass. Fish were then fixed in 10% buffered formalin solution, after which one muscle, the adductor mandibulae, was removed by dissection and weighed. Specimens were then cleared and stained for bone and cartilage before being photographed. Photos were taken with scale bars in lateral view on a copy stand, using supports of modeling clay under slender parts of the head to position the lateral perspective of the head as close to perpendicular to the camera axis as possible. All remaining measurements were made on these photos in ImageJ (Fig. 1).
Four of the oral jaw traits were measured to characterize the position of two important joints within the head. These included the center of the joint between the anterior-most portion of the palatine and the head of the maxilla, and the antero-posterior and dorso-ventral position of the joint between the quadrate of the suspensorium and articular of the mandible (Fig. 1). To describe the position of these joints in the head, we established a 2D space centered at the intersection of a horizontal body axis that passed through the tip of the first tooth in the premaxilla and the last vertebrate centra and a vertical axis, orthogonal to the first axis, that passed through the center of the orbit. The antero-posterior and dorso-ventral positions were calculated as the x-y position away from the center of this coordinate space.
Species means were calculated for each trait and were then size-corrected using ratios. Mouth gape, jaw protrusion, head height, dentigerous premaxilla length, maxilla length, lower jaw length, antero-posterior and dorso-ventral position of the lower jaw joint, and antero-posterior and dorso-ventral position of the joint between the palatine and maxilla were divided by head length. Maximum body depth and width were divided by standard length. Adductor mandibulae mass was divided by body mass. The closing and opening in-levers lengths were divided by the jaw length, resulting in estimates of mechanical advantage. All ratios were then log-transformed to normalize the data for the different statistical analyses. Since the antero-posterior and dorso-ventral positions of the anteriormost portion of the palatine and lower jaw joint contained negative numbers, we added a standard value of 1 to each measurement to make them positive before log transforming.
Phylogenetic Tree and Ecological Data
We pruned a large, time-calibrated phylogeny of ray-finned fishes87 to the species in our data set. For species that were not present in the phylogeny, we used the closest related species in the tree as a proxy (see Supplementary Table 6 for substitutions). We categorized the species in the study as either biters, which includes species that use both biting and some suction, or suction feeders based on published information about their feeding habits in natural populations73. Biting and suction feeding is a continuum in fishes35 and we based our classification on the primary feeding mode used to capture the dominant prey taxa in the diet. We identified 68 suction feeders and 42 biters in our dataset (see Supplementary Table 7 for classifications). We defined suction feeders as species that capture free-moving prey using some combination of suction and swimming, often referred to as ‘ram’, and biters as species that remove attached prey by directly biting or that must break up an armored prey to consume it, such as when triggerfishes feed on large crustaceans or urchins. We further classified each species by one of seven trophic categories modified from a previous study88 that characterized trophic ecology across reef fishes. The trophic categories defined by Siqueira et al. (2020) were as follows: GC (general carnivore), MI (mobile invertivore), OM (omnivore), PK (planktivore), SI (sessile invertivore), and HD (herbivores-detritivores). However, we separated the mobile invertivores category into two categories, suction feeding and biting mobile invertivores, to better reflect differences in the functional challenges associated with specific prey. Suction-feeding mobile invertivores were species that fed primarily on soft-bodied invertebrates, such as polychaetes and a variety of smaller crustaceans, where the mechanical defense of the prey is minimal. Biting mobile invertivores are those species that feed primarily on armored invertebrates, like echinoderms and large crustaceans, that require extensive mechanical processing (see Supplementary Table 7 for classifications).
Differences in oral jaw trait evolution between feeding modes and diets
We used a principal components analysis (PCA) of the 13 log-transformed trait ratios to determine the primary axes of diversity in craniofacial morphology among reef fishes. We used a PCA on the correlation matrix so that differences in measurement scale between traits would not impact our ability to visualize the variation in all traits using a PCA. We tested whether the overall construction of the oral jaw feeding apparatus (all traits) and each individual trait differed by functional and trophic group using phylogenetic MANOVA and ANOVAs, respectively. To do this, we used the procD.pgls function in geomorph and the pairwise function in rrpp89. Analyses were based on 10,000 permutations.
We compared morphological disparity among feeding modes and diets. We calculated oral jaw disparity for each functional group and trophic category as morphological variance using the function morphol.disparity in the R package geomorph89. We calculated the multivariate (all 13 morphological traits) morphological variance and a series of univariate variance estimates for the two feeding modes and seven diets. Each analysis was assessed for significance using 10,000 permutations.
We used state-dependent multivariate and univariate Brownian Motion models to understand whether feeding mode and diet influenced the rate of oral jaw evolution. Both multivariate and univariate Brownian Motion models were implemented in geomorph to estimate sigma, the evolutionary rate parameter89,90. We used feeding mode and diet as discrete traits in separate analyses. To test for significant differences in evolutionary rates between the feeding modes and diet categories, we used the permutation procedure for 10,000 iterations.
Correlation among oral jaw traits
We calculated evolutionary correlations among the 13 oral jaw traits and tested them for significance. First, we calculated phylogenetic independent contrasts91 for each trait using the pic function in ape92 and subsequently estimated the correlation coefficient for all pairwise combinations of traits from regressions through the origin following Garland et al. 93 To determine if the correlations were greater than expected under Brownian motion evolution, we generated a null distribution of evolutionary correlations from a Brownian motion process by simulating a set of uncorrelated traits across the phylogeny using the fastBM function in phytools94. We bounded the Brownian motion simulations by each empirical trait’s minimum and maximum values. We then estimated pairwise correlations of simulated traits to build a distribution of expected correlations under uncorrelated Brownian evolution. The simulations were performed 1000 times. Empirical correlations greater than the maximum correlation from the simulated dataset were deemed significant.
Quantifying trait combinations associated with diet
We inferred the evolutionary history of the seven trophic categories using stochastic character mapping implemented in the R package phytools94,95. We generated a distribution of 1000 stochastic character maps and fit a continuous-time Markov model for the evolution of each diet using a fixed Q matrix, and we estimated the stationary distribution from the Q matrix96,97.
We used the R package OUwie to fit multi-peak OU models (OUM) to diet to determine how theoretical optimal trait combinations differed between the diet groups if they were evolving under a multi-peak OU model. After estimating optimal trait values (thetas) across the entire simmap distribution, we took average theta values for each trait from across the 1000 estimates. To summarize how feeding modes and diets differed in optimal trait space, we performed a principal components analysis on a subset of the estimated thetas for each diet and feeding mode. However, we used the mean trait value in lieu of the optima in the case that the multi-peak OU model could not converge. Since the number of observations, i.e., diets, was smaller than the number of traits, which can cause erroneous patterns during a principal component analysis, we randomly sampled 25 theta values for each diet category, giving us a total of 175 observations. Since we did not have a distribution of mean trait values to sample from, we randomly sampled 75% of the individuals in each diet category and then calculated the mean for the subsample, for a total of 25 randomly sampled means per diet. We acknowledge that traditional model-based approaches test different models of evolution to determine which model best fits the data98. However, we opted not to take that approach here because we were not interested in the model of evolution but rather in how the optimal trait values would combine across diet categories if they were evolving under a multi-peak OU process. To determine whether our optimal trait estimates and PCA interpretations were robust to possible model bias, we also performed a principal component analysis on mean trait values only, following the random sampling approach above. This approach allowed us to compare patterns using a dataset consisting of both optimal and mean trait values or mean trait values alone.
To determine whether each diet was associated with a unique combination of trait patterns, we performed a linear regression of the estimates of trait optima, morphological variance, and rate of evolution for each trait. This strength of the relationship between the trait combinations, rates of evolution, and morphological variances provided insight into whether the pattern of evolution is similar across traits for each diet. A strong relationship between traits in each metric indicates that the diets exhibit similar patterns of morphological evolution for each trait. In contrast, a weak relationship between traits in each metric indicates a pattern of mosaic evolution for the different traits.
Statistics and reproducibility
All the above statistical analyses are reproducible by following the procedures in “Methods” section where the sample size and number of replicates are defined for each analysis. The Data availability and Code availability sections provide all the data and R scripts for the above analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank A. Adams and S. Liu for helpful comments on the figures. Two anonymous reviewers’ comments greatly improved the manuscript. This research was conducted in accordance with the University of California, Davis Institutional Animal Care and Use Committee (protocol #22206). MLY was supported by a Center for Population Biology postdoctoral fellowship. ASRH was supported by the Center for Population Biology at UC Davis, the Graduate Research Fellowship under Grant No. 1650042 from the National Science Foundation, and a Dissertation Fellowship from the Ford Foundation. KTR was supported by the Graduate Research Fellowship under Grant No. 2036201 from the National Science Foundation. DKW was supported by an NSF postdoctoral fellowship (DBI-1907156). KAC was supported by an American Dissertation Fellowship from the American Association of University Women and a fellowship from the Achievement Rewards for College Scientists Foundation. Additional support and funds for the research were provided by NSF grant DEB-1061981 and by the UC Davis College of Biological Sciences to PCW.
Author contributions
M.D.B., D.K.W., and P.C.W. designed the study. M.D.B., D.R.S., N.P., H.C., A.J.B., A.S.R., K.T.R, M.H, S.L.W. K.A.C., M.M., and D.K.W. collected the data. M.D.B. analyzed the data and wrote the manuscript. All authors contributed substantially to editing and the preparation of the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Johannes Stortz. [A peer review file is available.]
Data availability
All data supporting the results of this study are available as Supplementary Information and archived on Figshare (10.6084/m9.figshare.27245268.v1).
Code availability
The code used to perform the analysis is archived on Zenodo (10.5281/zenodo.13941776).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07148-8.
References
- 1.Wilson, D. S. & Yoshimura, J. On the Coexistence of Specialists and Generalists. Am. Nat.144, 692–707 (1994). [Google Scholar]
- 2.Koehl, M. A. R. When does morphology matter? Annu. Rev. Ecol. Syst.27, 501–542 (1996). [Google Scholar]
- 3.Wainwright, P. C. Functional versus morphological diversity in macroevolution. Annu. Rev. Ecol. Evol. Syst.38, 381–401 (2007). [Google Scholar]
- 4.Garland, T., Downs, C. J. & Ives, A. R. Trade-offs (and constraints) in organismal biology. Physiol. Biochem. Zool.95, 82–112 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Wang, X. & Clarke, J. A. The evolution of avian wing shape and previously unrecognized trends in covert feathering. Proc. R. Soc. B Biol. Sci.282, 20151935 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moen, D. S. What determines the distinct morphology of species with a particular ecology? The roles of many-to-one mapping and trade-offs in the evolution of frog ecomorphology and performance. Am. Nat.194, E81–E95 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Stayton, C. T. Performance in three shell functions predicts the phenotypic distribution of hard-shelled turtles. Evolution73, 720–734 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Aiello, B. R., Westneat, M. W. & Hale, M. E. Mechanosensation is evolutionarily tuned to locomotor mechanics. Proc. Natl Acad. Sci. USA114, 4459–4464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker, J. A. A general model of functional constraints on phenotypic evolution. Am. Nat.170, 681–689 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Felice, R. N., Randau, M. & Goswami, A. A fly in a tube: macroevolutionary expectations for integrated phenotypes. Evolution72, 2580–2594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns, M. D., Collyer, M. L. & Sidlauskas, B. L. Simultaneous integration and modularity underlie the exceptional body shape diversification of characiform fishes. Evolution77, 746–762 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Alfaro, M. E., Bolnick, D. I. & Wainwright, P. C. Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am. Nat.165, E140–E154 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Holzman, R., Collar, D. C., Mehta, R. S. & Wainwright, P. C. Functional complexity can mitigate performance trade-offs. Am. Nat.177, E69–E83 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Olsen, E. C. & Miller, R. J. Morphological Integration (University of Chicago Press, 1958).
- 15.Mayr, E. The Growth of Biological Thought: Diversity, Evolution, and Inheritance (Harvard University Press, 1982).
- 16.Klingenberg, C. P. Integration, Modules, and Development: Molecules to Morphology to Evolution. In Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes (eds. Pigliucci, M. & Preston, K.) (Oxford University Press, 2004).
- 17.Klingenberg, C. P. Variation: A Central Concept In Biology (eds. Hallgriımsson, B. & Hall, B. K.) 219–247 (Elsevier Academic Press, 2005).
- 18.Jablonski, D. Approaches to macroevolution: 1. General concepts and origin of variation. Evol. Biol.44, 427–450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larouche, O., Zelditch, M. L. & Cloutier, R. Modularity promotes morphological divergence in ray-finned fishes. Sci. Rep.8, 7278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larouche, O., Gartner, S. M., Westneat, M. W. & Evans, K. M. Mosaic evolution of the skull in labrid fishes involves differences in both tempo and mode of morphological change. Syst. Biol. 10.1093/sysbio/syac061 (2022). [DOI] [PubMed]
- 21.Felice, R. N. & Goswami, A. Developmental origins of mosaic evolution in the avian cranium. Proc. Natl Acad. Sci. USA115, 555–560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhoda, D., Polly, P. D., Raxworthy, C. & Segall, M. Morphological integration and modularity in the hyperkinetic feeding system of aquatic-foraging snakes. Evolution75, 56–72 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Conith, A. J., Kidd, M. R., Kocher, T. D. & Albertson, R. C. Ecomorphological divergence and habitat lability in the context of robust patterns of modularity in the cichlid feeding apparatus. BMC Evol. Biol.20, 95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orkney, A., Bjarnason, A., Tronrud, B. C. & Benson, R. B. J. Patterns of skeletal integration in birds reveal that adaptation of element shapes enables coordinated evolution between anatomical modules. Nat. Ecol. Evol.5, 1250–1258 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Goswami, A., Milne, N. & Wroe, S. Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proc. R. Soc. B Biol. Sci.278, 1831–1839 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slater, G. J., Dumont, E. R. & Van Valkenburgh, B. Implications of predatory specialization for cranial form and function in canids. J. Zool.278, 181–188 (2009). [Google Scholar]
- 27.Mitchell, D. R., Sherratt, E. & Weisbecker, V. Facing the facts: adaptive trade-offs along body size ranges determine mammalian craniofacial scaling. Biol. Rev.99, 496–524 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Law, C. J. et al. Decoupled evolution of the cranium and mandible in carnivoran mammals. Evolution76, 2959–2974 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Yuan, M. L., Wake, M. H. & Wang, I. J. Phenotypic integration between claw and toepad traits promotes microhabitat specialization in the Anolis adaptive radiation. Evolution73, 231–244 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Randau, M., Sanfelice, D. & Goswami, A. Shifts in cranial integration associated with ecological specialization in pinnipeds (Mammalia, Carnivora). R. Soc. Open Sci.6, 190201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauder, G. V. Evolution of the feeding mechanism in primitive actionopterygian fishes: a functional anatomical analysis of Polypterus, Lepisosteus, and Amia. J. Morphol.163, 283–317 (1980). [DOI] [PubMed] [Google Scholar]
- 32.Sanford, C. P. J. & Wainwright, P. C. Use of sonomicrometry demonstrates the link between prey capture kinematics and suction pressure in largemouth bass. J. Exp. Biol.205, 3445–3457 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Westneat, M. W. Fish Physiology. Vol. 23. p. 29–75 (Academic Press, 2005).
- 34.Norton, S. F. & Brainerd, E. L. Convergence in the feeding mechanics of ecomorphologically similar species in the centrarchidae and cichlidae. J. Exp. Biol.176, 11–29 (1993). [Google Scholar]
- 35.Longo, S. J., McGee, M. D., Oufiero, C. E., Waltzek, T. B. & Wainwright, P. C. Body ram, not suction, is the primary axis of suction-feeding diversity in spiny-rayed fishes. J. Exp. Biol.219, 119–128 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Schaeffer, B. & Rosen, D. E. Major adaptive levels in the evolution of the actinopterygian feeding mechanism. Am. Zool.1, 187–204 (1961). [Google Scholar]
- 37.Anker, G. Ch. Morphology and kinetics of the head of the stickleback, gasterosteus aculeatus. Trans. Zool. Soc. Lond.32, 311–416 (1974). [Google Scholar]
- 38.Elshoud-Oldenhave, M. J. W. Prey capture in the pike-perch,Stizostedion lucioperca (teleostei, percidae): a structural and functional analysis. Zoomorphologie93, 1–32 (1979). [Google Scholar]
- 39.Corn, K. A., Martinez, C. M., Burress, E. D. & Wainwright, P. C. A multifunction trade-off has contrasting effects on the evolution of form and function. Syst. Biol.70, 681–693 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Liem, K. F. Adaptive significance of intra- and interspecific differences in the feeding repertoires of cichlid fishes1. Am. Zool.20, 295–314 (1980). [Google Scholar]
- 41.McKaye, K. R. & Marsh, A. Food switching by two specialized algae-scraping cichlid fishes in Lake Malawi, Africa. Oecologia56, 245–248 (1983). [DOI] [PubMed] [Google Scholar]
- 42.Bellwood, D. R. & Choat, J. H. Alternative Life-history Styles Of Fishes (ed. Bruton, M. N.) 189–214 (Springer Netherlands, 1990).
- 43.Konow, N. & Bellwood, D. R. Prey-capture in Pomacanthus semicirculatus (Teleostei, Pomacanthidae): functional implications of intramandibular joints in marine angelfishes. J. Exp. Biol.208, 1421–1433 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Konow, N., Bellwood, D. R., Wainwright, P. C. & Kerr, A. M. Evolution of novel jaw joints promote trophic diversity in coral reef fishes. Biol. J. Linn. Soc.93, 545–555 (2008). [Google Scholar]
- 45.Gibb, A. C., Staab, K., Moran, C. & Ferry, L. A. The teleost intramandibular joint: a mechanism that allows fish to obtain prey unavailable to suction feeders. Integr. Comp. Biol.55, 85–96 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Kotrschal, B. K. Evolutionary patterns in tropical marine reef fish feeding. J. Zool. Syst. Evol. Res.26, 51–64 (1988). [Google Scholar]
- 47.Westneat, M. W. Transmission of force and velocity in the feeding mechanisms of labrid fishes (Teleostei, Perciformes). Zoomorphology114, 103–118 (1994). [Google Scholar]
- 48.Ferry-Graham, L. A. & Konow, N. The intramandibular joint in Girella: a mechanism for increased force production? J. Morphol.271, 271–279 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Martinez, C. M., McGee, M. D., Borstein, S. R. & Wainwright, P. C. Feeding ecology underlies the evolution of cichlid jaw mobility. Evolution72, 1645–1655 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Westneat, M. W. Evolution of levers and linkages in the feeding mechanisms of Fishes1. Integr. Comp. Biol.44, 378–389 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Westneat, M. W. Feeding, function, and phylogeny: analysis of historical biomechanics in labrid fishes using comparative methods. Syst. Biol.44, 361–383 (1995). [Google Scholar]
- 52.Spalding, M. D. & Grenfell, A. M. New estimates of global and regional coral reef areas. Coral Reefs16, 225–230 (1997). [Google Scholar]
- 53.Roberts, C. M. et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science295, 1280–1284 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Bellwood, D. R., Wainwright, P. C., Fulton, C. J. & Hoey, A. S. Functional versatility supports coral reef biodiversity. Proc. R. Soc. B Biol. Sci.273, 101–107 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spalding, M. D. & Brown, B. E. Warm-water coral reefs and climate change. Science350, 769–771 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Wainwright, P. C. & Bellwood, D. R. Coral Reef Fishes: Dynamics And Diversity In A Complex Ecosystem (Elsevier, 2002).
- 57.Randall, J. E. Food habits of reef fishes of the West Indies. Stud. Trop. Oceanogr.66, 665–847 (1967). [Google Scholar]
- 58.Clements, K. D., German, D. P., Piché, J., Tribollet, A. & Choat, J. H. Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc.120, 729–751 (2017). [Google Scholar]
- 59.Bellwood, D. R., Goatley, C. H. R., Brandl, S. J. & Bellwood, O. Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proc. R. Soc. B Biol. Sci.281, 20133046 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tebbett, S. B., Goatley, C. H. R. & Bellwood, D. R. Clarifying functional roles: algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs36, 803–813 (2017). [Google Scholar]
- 61.Bellwood, D. R. et al. Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J. Evol. Biol.23, 335–349 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Pratchett, M. S., Hoey, A. S., Cvitanovic, C., Hobbs, J.-P. A. & Fulton, C. J. Abundance, diversity, and feeding behavior of coral reef butterflyfishes at Lord Howe Island. Ecol. Evol.4, 3612–3625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mihalitsis, M. & Bellwood, D. R. Morphological and functional diversity of piscivorous fishes on coral reefs. Coral Reefs38, 945–954 (2019). [Google Scholar]
- 64.Grobecker, D. B. & Pietsch, T. W. High-speed cinematographic evidence for ultrafast feeding in antennariid anglerfishes. Science205, 1161–1162 (1979). [DOI] [PubMed] [Google Scholar]
- 65.Wainwright, P. C. & Richard, B. A. Predicting patterns of prey use from morphology of fishes. Environ. Biol. Fishes44, 97–113 (1995). [Google Scholar]
- 66.Mihalitsis, M., Morais, R. A. & Bellwood, D. R. Small predators dominate fish predation in coral reef communities. PLoS Biol.20, e3001898 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellwood, D. R. & Wainwright, P. C. Coral Reef Fishes. Dynamics and diversity in a complex ecosystem (ed. Sale, P. F.) p. 5–32 (Academic Press, 2002).
- 68.Ghezelayagh, A. et al. Prolonged morphological expansion of spiny-rayed fishes following the end-Cretaceous. Nat. Ecol. Evol.6, 1211–1220 (2022). [DOI] [PubMed] [Google Scholar]
- 69.Wood, R. The changing biology of reef-building. PALAIOS10, 517–529 (1995). [Google Scholar]
- 70.Wood, R. The ecological evolution of reefs. Annu. Rev. Ecol. Syst.29, 179–206 (1998). [Google Scholar]
- 71.Bellwood, D. R., Hoey, A. S., Bellwood, O. & Goatley, C. H. R. Evolution of long-toothed fishes and the changing nature of fish–benthos interactions on coral reefs. Nat. Commun.5, 3144 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Siqueira, A. C., Bellwood, D. R. & Cowman, P. F. Historical biogeography of herbivorous coral reef fishes: the formation of an Atlantic fauna. J. Biogeogr.46, 1611–1624 (2019). [Google Scholar]
- 73.Corn, K. A. et al. The rise of biting during the Cenozoic fueled reef fish body shape diversification. Proc. Natl Acad. Sci. USA119, e2119828119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellwood, D. R. Origins and escalation of herbivory in fishes: a functional perspective. Paleobiology29, 71–83 (2003). [Google Scholar]
- 75.Lobato, F. L. et al. Diet and diversification in the evolution of coral reef fishes. PLoS ONE9, e102094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Floeter, S. R., Bender, M. G., Siqueira, A. C. & Cowman, P. F. Phylogenetic perspectives on reef fish functional traits. Biol. Rev.93, 131–151 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Denton, J. S. S. & Adams, D. C. A new phylogenetic test for comparing multiple high-dimensional evolutionary rates suggests interplay of evolutionary rates and modularity in lanternfishes (Myctophiformes; Myctophidae). Evolution69, 2425–2440 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Zelditch, M. L. & Goswami, A. What does modularity mean? Evol. Dev.23, 377–403 (2021). [DOI] [PubMed] [Google Scholar]
- 79.Larouche, O., Gartner, S. M., Westneat, M. W. & Evans, K. M. Mosaic evolution of the skull in labrid fishes involves differences in both tempo and mode of morphological change. Syst. Biol.72, 419–432 (2023). [DOI] [PubMed] [Google Scholar]
- 80.Mihalitsis, M. & Bellwood, D. R. A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS ONE12, e0184679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Westneat, M. W. A biomechanical model for analysis of muscle force, power output and lower jaw motion in fishes. J. Theor. Biol.223, 269–281 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Wagner, G. P. & Altenberg, L. Perspective: complex adaptations and the evolution of evolvability. Evolution50, 967–976 (1996). [DOI] [PubMed] [Google Scholar]
- 83.Claverie, T. & Patek, S. N. Modularity and rates of evolutionary change in a power‐amplified prey capture system. Evolution67, 3191–3207 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Goswami, A., Binder, W. J., Meachen, J. & O’Keefe, F. R. The fossil record of phenotypic integration and modularity: a deep-time perspective on developmental and evolutionary dynamics. Proc. Natl Acad. Sci. USA112, 4891–4896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberts-Hugghis, A. S., Martinez, C. M., Corn, K. A. & Wainwright, P. C. A classic key innovation constrains oral jaw functional diversification in fishes. Evol. Lett. qrae046. 10.1093/evlett/qrae046 (2024).
- 86.Felice, R. N., Tobias, J. A., Pigot, A. L. & Goswami, A. Dietary niche and the evolution of cranial morphology in birds. Proc. R. Soc. B Biol. Sci.286, 20182677 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rabosky, D. L. et al. An inverse latitudinal gradient in speciation rate for marine fishes. Nature559, 392 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Siqueira, A. C., Morais, R. A., Bellwood, D. R. & Cowman, P. F. Trophic innovations fuel reef fish diversification. Nat. Commun.11, 2669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baken, E. K., Collyer, M. L., Kaliontzopoulou, A. & Adams, D. C. geomorph v4.0 and gmShiny: Enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods Ecol. Evol.12, 2355–2363 (2021). [Google Scholar]
- 90.Adams, D. C. Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Syst. Biol.63, 166–177 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Felsenstein, J. Phylogenies and the comparative method. Am. Nat.125, 1–15 (1985). [Google Scholar]
- 92.Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Garland, T. Jr., Harvey, P. H. & Ives, A. R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol.41, 18–32 (1992). [Google Scholar]
- 94.Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol.3, 217–223 (2012). [Google Scholar]
- 95.Revell, L. J. A comment on the use of stochastic character maps to estimate evolutionary rate variation in a continuously valued trait. Syst. Biol.62, 339–345 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Huelsenbeck, J. P., Nielsen, R., Bollback, J. P. & Schultz, T. Stochastic Mapping of Morphological Characters. Syst. Biol.52, 131–158 (2003). [DOI] [PubMed] [Google Scholar]
- 97.Bollback, J. P. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinform.7, 88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beaulieu, J. M., Jhwueng, D.-C., Boettiger, C. & O’Meara, B. C. Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution66, 2369–2383 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results of this study are available as Supplementary Information and archived on Figshare (10.6084/m9.figshare.27245268.v1).
The code used to perform the analysis is archived on Zenodo (10.5281/zenodo.13941776).