Abstract
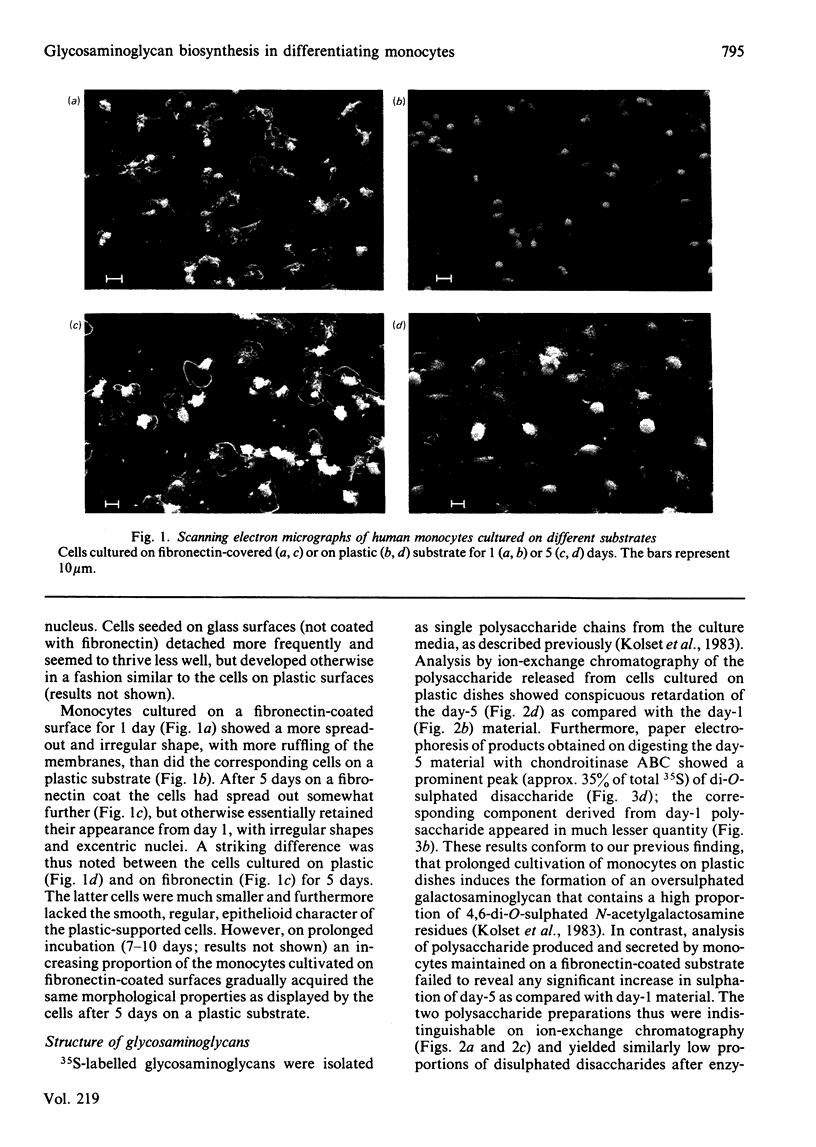
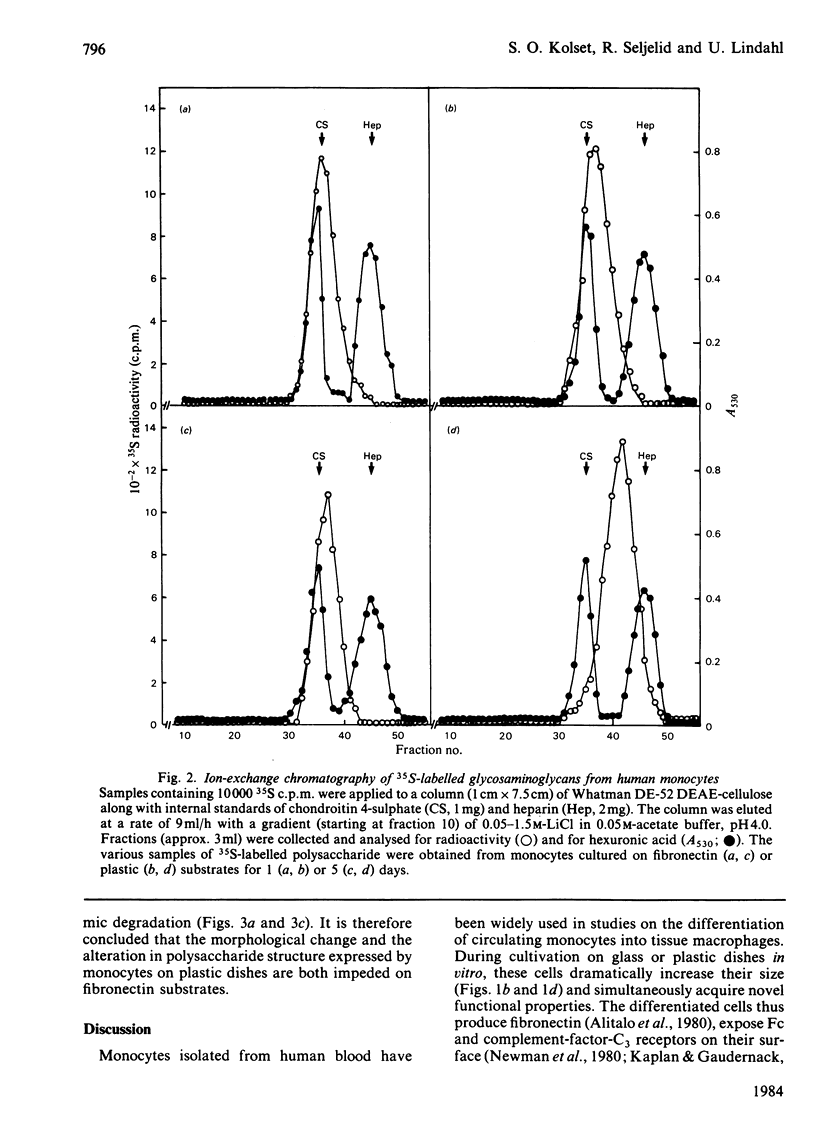
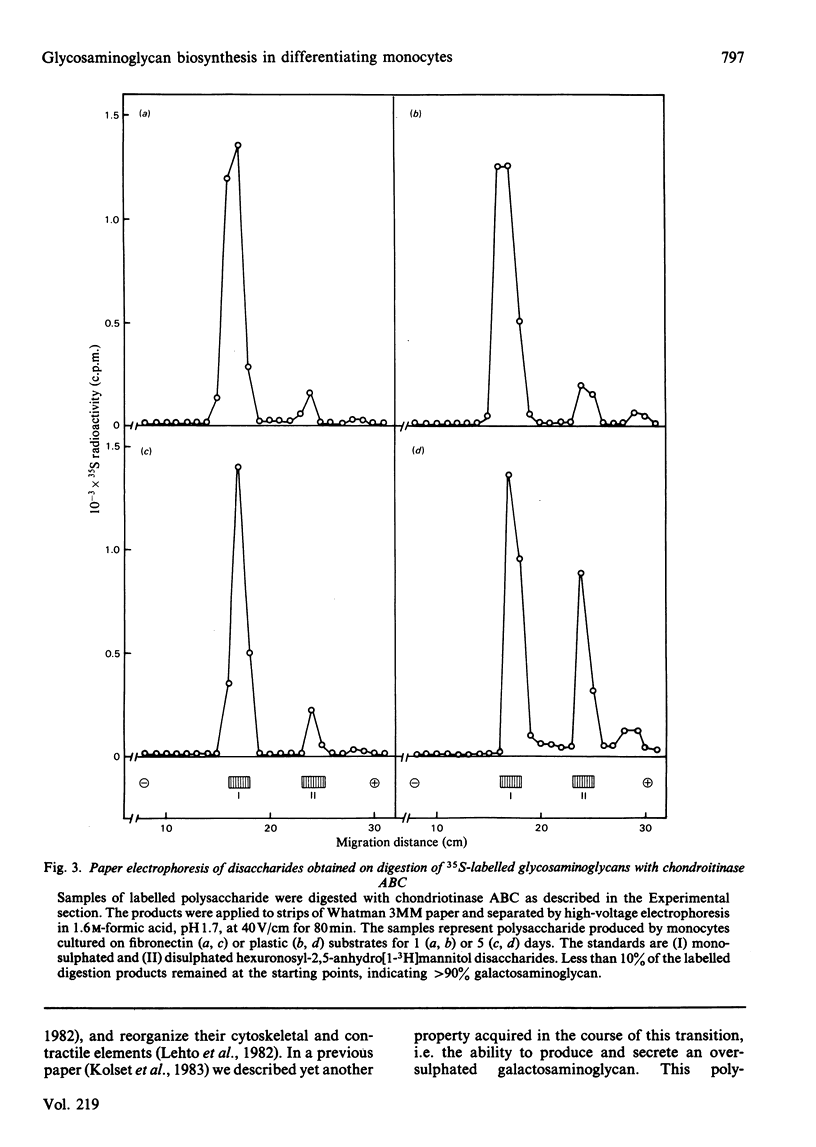
Monocytes were isolated from human blood and cultured in vitro on plastic culture dishes or on fibronectin-coated dishes. After 5 days in vitro, the cells on plastic dishes displayed marked morphological changes compared with day 1, with an epithelioid appearance resembling that of foreign-body cells. This transition was inhibited in cells cultured on fibronectin-coated dishes. 35S-labelled polysaccharides were isolated from the culture media after 24h incubation periods with inorganic [35S]sulphate. The cells cultured for 5 days on a plastic substrate synthesized, and secreted into the medium, an oversulphated galactosaminoglycan previously shown to contain 4,6-di-O-sulphated N-acetylgalactosamine units [Kolset, Kjellén, Seljelid & Lindahl (1983) Biochem. J. 210, 661-667]. In contrast, 35S-labelled polysaccharide produced by cells cultured on plastic for 1 day only, or on fibronectin for either 1 or 5 days, contained only minor amounts of such disulphated sugar units. These findings indicate that the formation of oversulphated chondroitin sulphate is coupled to the conversion of monocytes into epithelioid cells. Furthermore, they suggest that the overall process is induced by contact with artificial substrates, and that it may be regarded as the equivalent of a foreign-body reaction in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudewicz P. W., Molnar J., Lai M. Z., Beezhold D. W., Siefring G. E., Jr, Credo R. B., Lorand L. Fibronectin-mediated uptake of gelatin-coated latex particles by peritoneal macrophages. J Cell Biol. 1980 Nov;87(2 Pt 1):427–433. doi: 10.1083/jcb.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not. J Exp Med. 1983 Jun 1;157(6):2061–2072. doi: 10.1084/jem.157.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset S. O., Kjellén L., Seljelid R., Lindahl U. Changes in glycosaminoglycan biosynthesis during differentiation in vitro of human monocytes. Biochem J. 1983 Mar 15;210(3):661–667. doi: 10.1042/bj2100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lehto V. P., Hovi T., Vartio T., Badley R. A., Virtanen I. Reorganization of cytoskeletal and contractile elements during transition of human monocytes into adherent macrophages. Lab Invest. 1982 Oct;47(4):391–399. [PubMed] [Google Scholar]
- Levitt D., Dorfman A. Concepts and mechanisms of cartilage differentiation. Curr Top Dev Biol. 1974;8:103–149. doi: 10.1016/s0070-2153(08)60607-9. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- Odegaard A., Viken K. E., Lamvik J. Structural and functional properties of blood monocytes cultured in vitro. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):223–234. doi: 10.1111/j.1699-0463.1974.tb02316.x. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Goetinck P. F. Synthesis of proteochondroitin sulfate by normal, nanomelic, and 5-bromodeoxyuridine-treated chondrocytes in cell culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3385–3388. doi: 10.1073/pnas.69.11.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker J. P., Hassell J. R., Yamada K. M., Pratt R. M. The influence of an adhesive cell surface protein on chondrogenic expression in vitro. Exp Cell Res. 1979 Jul;121(2):411–415. doi: 10.1016/0014-4827(79)90022-3. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Johnsson A., Wärmegård B., Seljelid R. Separation of human monocytes on density gradients of Percoll. J Immunol Methods. 1980;33(3):221–229. doi: 10.1016/0022-1759(80)90209-4. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I., Schlessinger J., Yamada K. M. Fibronectin delays the fusion of L6 myoblasts. Exp Cell Res. 1979 Sep;122(2):317–326. doi: 10.1016/0014-4827(79)90308-2. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M., Sieber F., Yamada K. M. Cellular fibronectin promotes adrenergic differentiation of quail neural crest cells in vitro. Exp Cell Res. 1981 Jun;133(2):285–295. doi: 10.1016/0014-4827(81)90320-7. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Keller J. M. A transformation-dependent difference in the heparan sulfate associated with the cell surface. Biochem Biophys Res Commun. 1975 Mar 17;63(2):448–454. doi: 10.1016/0006-291x(75)90708-1. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., Lanza R., Rosenbloom J., Lowe M., Holtzer H., Avdalovic N. Fibronectin alters the phenotypic properties of cultured chick embryo chondroblasts. Cell. 1979 Jul;17(3):491–501. doi: 10.1016/0092-8674(79)90257-5. [DOI] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Altered metabolism of heparan sulfate in simian virus 40 transformed cloned mouse cells. J Biol Chem. 1978 Jul 25;253(14):5109–5120. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- van Ginkel C. J., van Aken W. G., Oh J. I., Vreeken J. Stimulation of monocyte procoagulant activity by adherence to different surfaces. Br J Haematol. 1977 Sep;37(1):35–45. [PubMed] [Google Scholar]




