Abstract
Plant species richness and composition are influenced by complex interactions between biotic and abiotic factors that operate on different spatial scales. Since spatial scales vary continuously in nature, it is expected that multiple factors simultaneously affect species richness and composition at an intermediate spatial scale (i.e., the mesoscale landscape level). Previous studies have shown that local topography and elevation are important factors for shaping intermediate spatial scale plant species richness; however, the relative importance of these factors has rarely been examined. Here, we used spatially explicit woody plant data to examine the factors that characterize the spatial pattern of primary evergreen forest biodiversity at the intermediate spatial scale. We found that the spatial pattern of species diversity in a predominantly warm temperate evergreen forest at the landscape level is mainly characterized by shifts in species composition along the elevation gradient. Our study also found that compositional shift along the elevational gradient was mainly caused by habitat specialization among congeneric species, suggesting that niche partitioning among closely-related species is a fundamentally important feature of the intermediate spatial scale species richness pattern. Furthermore, we found that specialization in a habitat of closely-related species can be established even within a limited environmental gradient. This suggests that biotic interactions among closely-related species may be an important factor driving habitat specialization, and biotic interactions may play an important role in shaping landscape-scale biodiversity patterns.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77438-y.
Subject terms: Plant ecology, Forest ecology, Biodiversity
Introduction
Plant species richness and composition are influenced by the complex interactions between biotic and abiotic factors. It is widely accepted that biotic and abiotic factors operate on different spatial scales. At the local or plot scale, patterns of plant diversity are influenced by biotic interactions1.
and/or disturbance regimes2. At larger spatial scales, habitat associations are strong and determine compositional shifts during changes in environmental conditions3,4. Since spatial scales vary continuously in nature, it is expected that biotic interactions and environmental factors simultaneously affect species richness and composition at intermediate spatial scales. Previous studies termed this intermediate spatial scale (1–100 km2) as the “mesoscale landscape”5,6. Since intermediate spatial scale forest processes link local gap dynamics to macroscale species distributions in a hierarchical manner, research at this scale is needed7.
Numerous studies have revealed that local topography and elevation are important factors in shaping intermediate spatial scale plant species richness. Since topographic features correlate with variables that are more directly related to plant resources, they are regarded as a good predictor of habitat. For example, topographic features such as slope inclination and aspect often correlate with the distribution of nutrients3 and soil moisture8. Additionally, topographic features often correlated with disturbance regimes. Accordingly, species diversity and composition change along topographic gradients, from ridge to valley and from hilltop to hollow (e.g., 9,10, but see11).
Changes in species richness along elevation gradients have also been documented in numerous studies12,13,14,15. Macroecological studies have shown that orographically enhanced precipitation, elevational and climatic gradients, and environmental heterogeneity are the key features linking high biodiversity to elevation16. Previous studies have also reported that plant species richness is positively skewed (hump shaped) or monotonically decreases along elevation gradients12,15,17. Furthermore, the determinant factors of community composition and diversity differ across distinct forest strata (e.g. overstorey, understorey;17,18). Unlike topographic gradients, elevational gradients often create biological patterns that are mediated by interspecific interactions (e.g. competition;19). Therefore, patterns of tree species distribution along elevations may reflect both climatic factors and biotic interactions20. These findings suggest that plant species diversity in heterogeneous forests is characterized by local or microscale environmental gradients that are mediated by elevational and topographic configurations21.
Lucidophyllous forests (warm-temperate evergreen forests) are widespread in the temperate and subtropical regions of East Asia22, and are a climax vegetation in southeastern Japan. This vegetation is generally covered by broad-leaved evergreen trees such as Castanopsis, Quercus, and Machilus. This vegetation supports more than 1000 plant species in Japan and at least 30% of them are considered endangered species23. Nowadays, temperate evergreen forest trees are being cut down and most of these areas are covered with substitute vegetation. The natural evergreen forests of the warm-temperate zone exist only as fragmented forests mainly in sanctuaries around shrines, secluded mountainous districts, and isolated islands. Therefore, relatively few studies have examined the relative importance of local topography and elevation in primary warm-temperate evergreen forests at the landscape level, and little is known about whether landscape structure determines species richness and community composition in primary evergreen forests of Japan.
In this context, we aimed to assess how landscape heterogeneity contribute to plant species richness and composition at the intermediate spatial scale in a primary warm-temperate evergreen forest. We specifically focus on the relative importance of topographic and elevational variables individually contribute to plant species richness at the intermediate spatial scale. Dearborn and Danby (2017)24 showed that plant communities varied more by slope than by elevation. However, other studies have shown that both elevation and local topography influence plant richness21,25. Additionally, the relative importance of these factors may differ between groups of plant life-forms18,21. We comprehensively investigated the diversity of intermediate spatial scale (1 km2) plant species in the Kasugayama primary forest, which is a primary warm-temperate evergreen forest located in central Japan. The Kasugayama primary forest encompasses diverse micro-habitats attributed to the topographical variation and elevational variation and displays variation in canopy gap size distribution. We addressed the following questions.
How does species richness change with local topography and elevation?
How does species composition change with local topography and elevation?
Does each species show a topographic-habitat association?
Materials and methods
Study area
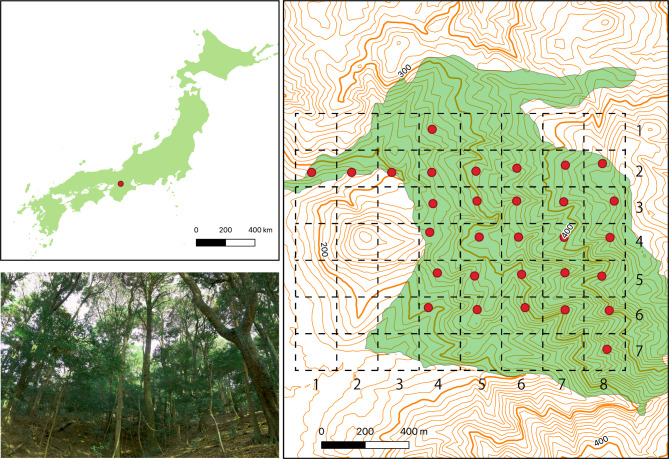
The Kasugayama primary forest has a total area of 250 ha and is in Nara City, western Japan (34° 41’ N, 135° 51’ E). The elevation in the region ranges from 100 to 496 m. Since the forest has been preserved as a holy site of the Kasuga Taisha Shrine, hunting and logging have been prohibited since 841 AD26. In 2019, the mean annual temperature was 16.3 °C, and the average annual precipitation was 1482.5 mm. The highest point in the forest is 498 m. The natural vegetation in the area consists of evergreen broadleaved forests27; however, the Sika deer population in the Kasugayama primary forest is believed to have increased in recent years, and Sika deer is probably affecting plant species composition and forest regeneration by feeding on ground vegetation28. For example, species of forest floor vegetation such as Tripterospermum japonicum or Scutellaria laeteviolacea disappeared by the year 200028, whereas deer inedible plants, such as Sapium sebiferum and Nagia nagi, have increased since the 1960s26.
Field survey
Field studies were conducted from June to September 2015. In the study area, 30 circular transect plots with a radius of 17 m (ca. 0.1 ha in size) were established within a radius of 1 km2 (Fig. 1). The individual plots were systematically arranged approximately 200 m apart (Fig. 1). Tree species richness was surveyed in each plot; all tree species with heights > 130 cm and numbers of individuals were recorded. We measured the stem girth at breast height (1.3 m above ground) and calculated the diameter at breast height (DBH) from the girth. Species were grouped into three life-form categories, namely trees, shrubs, and climbing plants, according to Satake (1981–1982)29.
Figure 1.
Location of the study site (~ 1 km2) at the Kasugayama primary forest, Nara prefecture, Western Japan. The specific locations of the study plots are denoted by dots. Green area in right panel indicates National Heritage area. Scale bar: 400 m.
Environmental variables
Five environmental variables were collected from each transect plot. The elevation was determined using a GPS device. The slope inclination and slope aspect were measured using a clinometer. Laplacian (i.e., an index of concavity and convexity of the ground) was obtained from a 20 m x 20 m digital elevation model. Tangent transformation was applied to slope inclination. Sine and cosine transformations are applied to the slope aspect. In the sine transform, the values range from − 1 (west-facing) to 1 (east-facing), whereas in the cosine transform, values range from − 1 (south-facing) to 1 (north-facing). Plot scale canopy openness (%) was determined using hemispherical photographs and used as a proxy for the extent of recent tree fall gap and light condition. In this study, we assumed a situation where certain species, such as early successional species, invade a community after a tree fall gap has been created, thereby affecting richness and species composition.
Hemispherical photographs were obtained using a Coolpix 8400 digital camera (Nikon, Tokyo, Japan) with an FC-E9 0.2x fisheye converter lens (Nikon, Tokyo, Japan). The camera was mounted on a tripod and oriented so that the top of each photograph faced the magnetic north. The lens was positioned at a height of 1.2 m, and photographs were taken using the open-sky reference method30,31. We analyzed the color hemispherical photos using the Gap Light Analyzer software ver. 2.0232.
Statistical analysis
Because statistically significant spatial autocorrelation existed in the species richness of all woody plant species, tree species and climbing plant species, we used Generalized Estimating Equations (GEE), which provide a highly efficient means of removing spatial autocorrelation in a generalized linear model framework33,34 to investigate the relationship between species richness and environmental values (elevation, slope inclination, slope aspect, Laplacian function and canopy openness).
The GEE is an extension of generalized linear models (GLM) and allows correlated responses. GEEs include an additional variance–covariance matrix which takes into account that observations are not independent to account for correlated data. Unlike GLMs, this matrix has non-zero off-diagonal values corresponding to the correlation between observations. We applied GEE models to spatial, two-dimensional datasets sampled in rectangular grids34.
We constructed a GEE model with species diversity as the response variable (Poisson error and log link) and elevation, openness, slope aspect, slope inclination and Laplacian as explanatory variables. We standardized all explanatory variables for analysis (Z-score). We introduced the location of transect plots in the 8 × 7 grid cells (Fig. 1) into the models and used an interchangeable correlation structure that assumes an exchangeable correlation between all observations in a subject also for the analysis of woody species, tree species and climbing plant species, as well as an independence correlation structure that assumes no spatial dependencies for shrub species. For each response variable, after constructing models for all combinations of environmental variables, we identified the model with the smallest Quasi Information Criterion (QIC) as the best model. We used the Spind package35 in R.
To identify differences in plant community composition among transect plots, we performed non-metric multidimensional scaling (NMDS) ordination using the vegan library36 in R. NMDS is a distance-based ordination technique that is robust to the nonlinearity of the data37. To determine whether elevation, topographic factors (slope aspect, slope inclination and Laplacian), and canopy openness were associated with differences in community composition, we used Vegan’s envfit function, which evaluates multidimensional correlations between NMDS site scores and independent environmental predictors. The significance of each environmental variable was determined based on a permutation test with 999 permutations.
To elucidate the relationship between species distribution and environmental variable, we categorized the species habitat association across the environmental gradient (i.e. elevation, openness, slope inclination, slope aspect and Laplacian) using a generalized linear model (GLM) that assumed a binomial distribution (logistic regression). We used the presence/absence of each species in each transect plot as a response variable. We categorized the distribution patterns of each species into four categories: (1) specialist species (species whose presence/absence patterns are significantly correlated with the elevation gradient), (2) generalist species (species whose presence/absence patterns are not significantly correlated with elevation), and (3) rare species (species present in less than three plots). Herein, we have used the term “specialist” to refer to a species that utilizes a narrow range of resources38 based on the distribution pattern along environmental gradient, for convenience.
To detect the distance decay relationship39, we investigated the relationships between community dissimilarity and geographical distance. The statistical significance of the relationships was tested via Mantel correlations with 1000 permutations using package vegan36 in R. All analyses were conducted using R version 4.3.240.
Results
Factors affecting species richness
We recorded 70 species at the study site, including 49 tree species, 13 shrub species, and eight climbing plant species. Castanopsis cuspidata was the most abundant species based on total basal area, and Neolitsea aciculate the most abundant species based on the number of stems (S1,S2). Cryptomeria japonica, Quercus sessilifolia, and Abies firma were also abundant in terms of total basal area. Cleyera japonica was also abundant based on the number of stems (S1,S2).
In the best models for the total species richness of woody plants, the height, slope aspect (north and east), and slope inclination (tan(slope)) were selected (Table 1), but these are not statistically significant (p > 0.05). Slope inclination was selected as an explanatory variable for the total woody plant, tree, and climbing plant species richness (Table 1). Slope inclination was significantly positively correlated with climbing plant species richness (p < 0.01, Table 1), indicating that climbing plant species richness tends to increase the slope inclination.
Table 1.
Top significant generalized estimating equation(GEE) species richness in subpolots.
| Response variables | Intercept | Standardized partial regression coefficient for selected explanatory variables | QIC | |||||
|---|---|---|---|---|---|---|---|---|
| Elevation | sin(aspect) | cos(aspect) | tan(slope) | Openness | lap | |||
| Total woody plant species richness | 2.870 | 0.074 | 0.042 | 0.048 | 168.786 | |||
| Tree species richness | 2.039 | 0.001 | -0.857 | 0.409 | 168.264 | |||
| Shrub species richness | 1.533 | 111.438 | ||||||
| Climbing plant species richness | 0.050 | 0.402* | -0.252 | 81.600 | ||||
QIC, Quasi Information Criterion; tan(slope), tangent transformed slope inclination; sin(aspect), sine transformed slope aspect; cos(aspect), cosine transformed slope aspect; Openness, relative openness; lap, convex/concavity.
Asterisk indicate statistically significant (p <0.01).
Factors affecting species composition and distribution
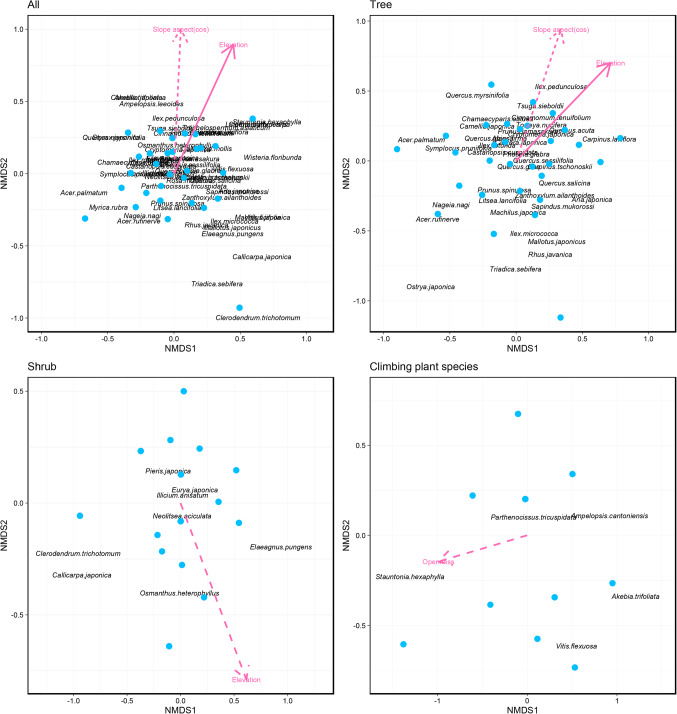
Differences in species composition were adequately represented by a two-dimensional NMDS ordination (stress = 0.11, 0.19, 0.03, and 0.02 for total species, tree species, shrub species, and climbing plant species). Three of the five environmental variables (i.e. elevation, slope aspect, canopy openness) tested were significantly correlated with species composition in the NMDS space (Fig. 2). Of these gradients, elevation was significantly correlated with species composition of all species, trees, and shrubs (Fig. 2), and it indicates that it is most strongly related to variation in plant composition.
Figure 2.
Differences in species composition of woody plant species determined using a non-metric multidimensional scaling (NMDS) and permutation test. Elevation, topographic factors (slope aspect, slope inclination and laplacian) and canopy openness were incorporated as explanate variables. Solid and dotted arrows indicate that the variables statistically significantly (p < 0.01 and p < 0.05 respectively) relate to community dissimilarity. Slope aspect (cos), cosine transformed slope aspect; Openness, plot scale canopy openness.
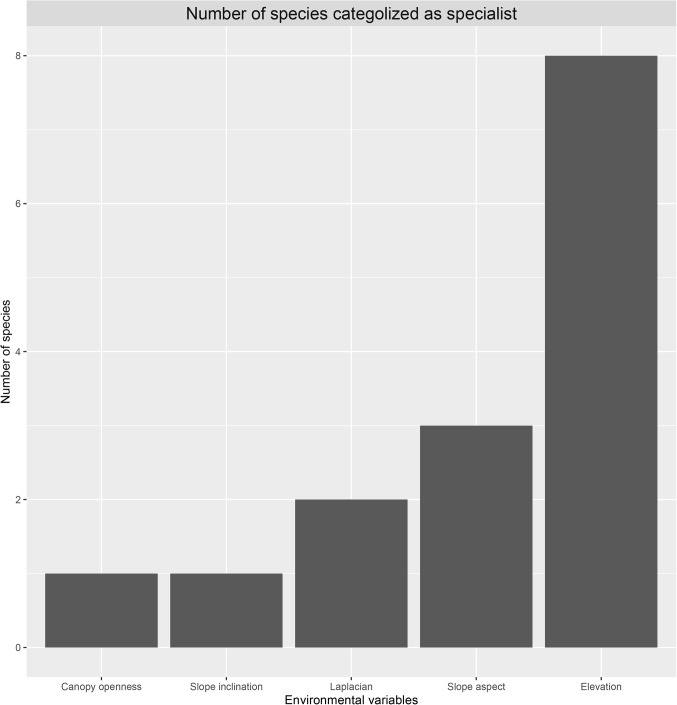
Significant distance decay relationships, i.e., increasing community dissimilarity with increasing geographical distance was found (Fig. 3). Logistic regression analysis revealed significant species-habitat association for 10 species. We found one specialist species for openness, one species for slope inclination, three species for slope aspect (two species positively correlated), two species for Laplacian, and eight species for elevation (Fig. 4), and suggest primary importance for elevation.
Figure 3.
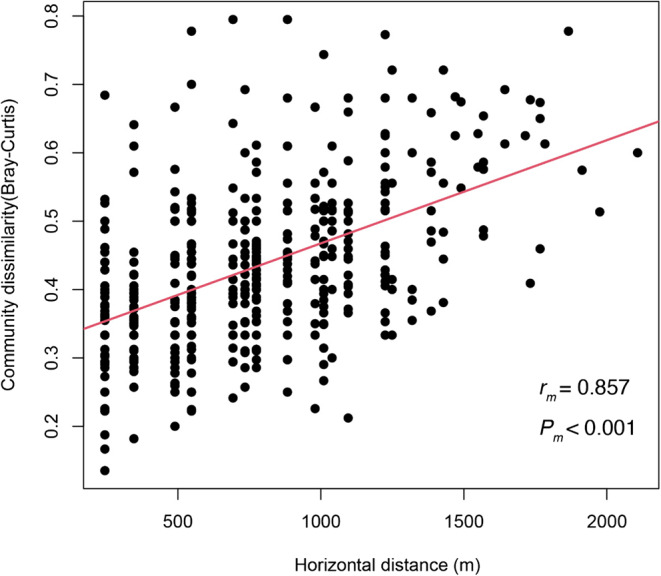
Distance decay relationships between increasing community dissimilarity (Bray-Curtis index) and geographic distance. Mantel correlations coefficients (rm) and P values (Pm) are also shown.
Figure 4.
Number of species significantly associated with a given environmental gradient.
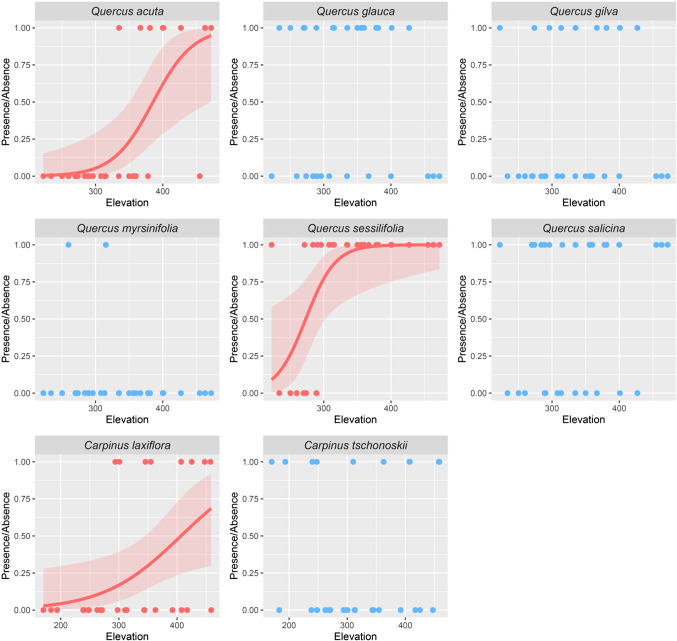
Specialist species at high elevation were seven species Abelia spathulata, Carpinus laxiflora, Cinnamomum tenuifolium, Cryptomeria japonica, Osmanthus heterophyllus, Quercus acuta and Quercus sessilifolia (p < 0.05 respectively; S3,S4). In this analysis, we also detected 1 low-elevation species and 32 generalist species (S3,S4). Three of the seven species categorized as high-elevation species had another species of the same genus categorized as generalists. For example, in Quercus, three of the six species were categorized as generalists and two as high-elevation species; in Carpinus, C. laxiflora was categorized as a high-elevation species and C. tschonoskii as a generalist species (Fig. 5). This suggests that niche partitioning with elevation occurs mainly among congeneric species. Tsuga sieboldii was the specialist species at the high openness site (S3,S4). Specialist species at the steep slope site was Quercus acuta (S3,S4). Specialist species at the west-, north-, and south-facing sites were Cleyera japonica, Litsea lancifolia, and Tsuga sieboldii, respectively (S3,S4). Cryptomeria japonica and Ilex rotunda were the specialist species at the valley site (S3,S4).
Figure 5.
Distribution patterns of the Quercus along elevations in the study area. Curves indicate estimates of generalized linear model (p < 0.05).
Discussion
Effects of microtopography on species richness and composition
The present results show that most environmental variables were not significantly correlated with species richness, and the number of species significantly associated with micro-topographic factors (such as slope inclination, laplacian function, and slope aspect) was limited (Table 1; Fig. 4). These results indicate that direct link of micro-topographic factors to tree species richness and composition were unclear and these are hard to explain the spatial variation of plant tree species diversity at the scale of this study. Generally, topographic variability is a possible factor in increasing local plant species richness of tree species, and this trend has been attributed to the differentiation of topographic niches. Topographic niche differentiation has been supported by studies that focused on the seedling or juvenile stage; however, these studies often failed to fully explain the distribution patterns of large trees by the identified species-specific responses at the seedling or juvenile stage41,42,43,44,45. Certain studies attribute this mismatch to dispersal limitations and density-dependent mortality11,46. Dispersal limitation generally has a greater impact on species distribution than on germination or survival47,48,49, and density-dependent mortality50,51 weakens spatial correlation between juveniles and adults52. These processes may have offset the pattern caused in the recruitment stage and resulted in weak associations between micro-topographic factors and species richness.
In the present study, slope inclination (tan(slope)) was selected as an explanatory variable for best model in total species richness, tree species richness and climbing plants species richness (Table 1). Additionally, elevation was significantly positively correlated with the species richness of climbing plant (Table 1). These results indicate that slope inclination is a relatively good predictor for species richness and climbing plants species richness seems to be more sensitive to microtopographic factor than tree or shrub species richness. Previous studies show that association between species distribution and topographic configuration being attributed to species-specific responses to disturbance frequency10. Additionally, Ledo and Schmezer (2014)53 reported that the intensity and frequency of disturbance determine the population density and species diversity of tropical lianas trough to their clonally increasing and becoming spatially coherent in response to disturbance. One or a few of the above factors might have played a role in habitat association detected in this study.
It is widely accepted that canopy gaps often facilitate the establishment and growth of many tree species54 and that the patchy distribution of canopy gaps of different ages across a wide range of areas considered plays an important role in determining the survival of intolerant and short-lived tree species such as Z. ailanthoides, S. prunifolia, and A. rufinerve in this study site27.
However, our results showed that canopy openness was not correlated with species richness and composition (Table 1). A likely explanation for this result is the influence of deer grazing. The population density of deer in the study area is estimated at 30 ~ 40 individual per square kilometer and it tends to be higher around forest canopy gaps55. Previous studies report that deer grazing strongly regulates the regeneration of pioneer tree species and studies comparing the effects of deer grazing in this study area between fenced and unfenced sites found an increase in pioneer tree species (e.g., Mallotus japonicus and Zanthoxylum ailanthoides) and a 1.7-fold increase in the total number of species in fenced plots under the gap compared to unfenced plots28,56. These facts suggest that gap dynamics, widely considered to be the major mechanism for maintaining species diversity, may have been regulated by deer grazing in this study area. However, it has been noted that some pioneer tree species, such as Z. ailanthoides, may have regenerated over several years even under the influence of deer grazing pressure56. Therefore, it is possible that severe disturbances, such as large typhoons, did not occur, and the invasion of pioneer species was thereby regulated.
Factors affecting species composition and habitat partition
The results of the NMDS analysis show that species composition at this study site changed mainly with elevation (Fig. 2), and suggest that such trend characterizes the intermediate spatial scale plant species diversity in this study area. The partitioning of beta diversity using spatial analysis method in previous research revealed that distributions of tree species richness and composition within a 24-ha plot were strongly affected by elevation and convexity57; our results were partially consistent with this previous finding. Compositional differences between sites are commonly explained by two major processes, dispersal limitation and species sorting39,58, which are not mutually exclusive59,60. In this study, we found significant distance decay relationship between community similarity and geographic distance (Fig. 3) suggesting that dispersal limitation possibly contributes to a compositional shift along elevational gradient. However, the results of the logistic regression analysis revealed that species habitat association frequently observed along elevational gradient and suggests that species-specific habitat partition could also contribute to the compositional shift along the elevational gradient.
We also found that habitat partitioning along the elevational gradient was frequently observed among congeneric species (Fig. 5, S3, S4). These findings suggest that habitat specialization especially among closely-related species, could be an important driver of changes in species composition with elevation. Habitat partitioning and specialization between closely-related taxa is generally considered to result from a complex interplay between biotic and abiotic factors. Numerous studies have reported that water-use strategies, edaphic conditions, and light conditions often shape divergent habitat associations between congeneric tree species pairs9,61,62,63; however, the interplay of edaphic, hydrological, and topographical factors is often complex64; moreover, the mechanism that induces habitat niche partitioning between closely-related taxa and the contribution of species-habitat association to the maintenance of species diversity remain inconclusive65.
In the study area, the distribution pattern along elevation for each species in our study area can be classified into two major types: generalist and high-elevation species (specialists). Notably, both the generalist and the specialist were often assigned to the same genus (Fig. 5, S2). This suggests that when multiple congeneric species are present, sites at lower elevations tend to be dominated by a particular species, whereas multiple closely-related species tend to coexist at higher elevation sites. The explanatory framework for the coexistence and evolution of generalist and specialist species has recently been provided by theoretical studies focusing specifically on insect host choice. According to this theory, habitat specialization is most likely when competition and negative reproductive interference (costly interspecific mating interactions)66 between closely-related species are at intermediate levels, and divergence of habitat specialist and generalist is likely caused by the combination of reproductive interference and weak interspecific resource competition67,68. Although many empirical studies showed that reproductive interference lead to species exclusion or niche partitioning in herbaceous plants69,70,71, less attention has been paid to tree species coexistence. Watanabe and Maesako (2021)72 report the local coexistence of Quercus and Carpinus in the study area and suggest that the effects of negative interactions among congeneric tree species may have been weaker than those reported in previous studies on herbaceous plants. The mechanism by which the effects of negative interactions between congeneric tree species arise in our study site is not well understood; however, it is very likely that the attenuation of negative interactions among congeneric species is responsible for the specialization of certain species into unsuitable environments (high elevation) at the intermediate spatial scale. Further theoretical and empirical investigations are required to determine how interspecific interactions are related to niche partitioning at the intermediate spatial scale.
Conclusion
We found that the spatial pattern of species diversity in a primarily warm temperate evergreen forest at the landscape level is mainly characterized by shifts in species composition along the elevation gradient. Our study also found that closely-related species coexisted along the elevation gradient, suggesting that niche partitioning among closely-related species is an important feature of intermediate spatial scale species richness patterns. Furthermore, we found that specialization in the habitat of closely-related species can be established even within a limited environmental gradient. This suggests that biotic interactions among closely-related species may be an important factor driving habitat specialization, and that biotic interactions may play an important role in shaping landscape-scale biodiversity patterns.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Minori Hikichi and Kayo Takasu for fieldwork assistance.
Author contributions
YM and SW designed research. SW, YM and TI conducted field survey. TI collected light condition data. SW analyzed data. SW wrote manuscript. All authors reviewed the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gross, N. et al. Uncovering multiscale effects of aridity and biotic interactions on the functional structure of Mediterranean shrublands. J. Ecol.101, 637–649 (2013). [Google Scholar]
- 2.Sipe, T. W. & Bazzaz, F. A. Gap partitioning among Maples (Acer) in Central New England: Survival and Growth. Ecology. 76, 1587–1602 (1995). [Google Scholar]
- 3.Wiens, J. J. & Donoghue, M. J. Historical biogeography, ecology and species richness. Trends Ecol. Evol.19, 639–644 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Chase, J. M. Spatial scale resolves the niche versus neutral theory debate. J. Veg. Sci.25, 319–322 (2014). [Google Scholar]
- 5.Heikkinen, R. K. Predicting patterns of vascular plant species richness with composite variables: a meso-scale study in Finnish Lapland. Vegetatio. 126, 151–165 (1996). [Google Scholar]
- 6.Clark, D. B., Clark, D. A. & Read, J. M. Edaphic variation and the mesoscale distribution of tree species in a neotropical rain forest. J. Ecol.86, 101–112 (1998). [Google Scholar]
- 7.Druckenbrod, D. L. et al. Redefining temperate forest responses to climate and disturbance in the eastern United States: new insights at the mesoscale. Global Ecol. Biogeogr.28, 557–575 (2019). [Google Scholar]
- 8.Daws, M. I., Mullins, C. E., Burslem, D. F. R. P., Paton, S. R. & Dalling, J. W. Topographic position affects the water regime in a semideciduous tropical forest in Panamá. Plant. Soil.238, 79–89 (2002). [Google Scholar]
- 9.Itoh, A., Yamakura, T., Ogino, K., Lee, H. S. & Ashton, P. S. Spatial distribution patterns of two predominant emergent trees in a tropical rainforest in Sarawak, Malaysia. Plant. Ecol.132, 121–136 (1997). [Google Scholar]
- 10.Enoki, T. Microtopography and distribution of canopy trees in a subtropical evergreen broad-leaved forest in the northern part of Okinawa Island, Japan. Ecol. Res.18, 103–113 (2003). [Google Scholar]
- 11.Suzuki, M. Effects of the topographic niche differentiation on the coexistence of major and minor species in a species-rich temperate forest. Ecol. Res.26, 317–326 (2011). [Google Scholar]
- 12.Aiba, S., Hill, D. A. & Agetsuma, N. Comparison between old-growth stands and secondary stands regenerating after clear-felling in warm-temperate forests of Yakushima, southern Japan. For. Ecol. Manag. 140, 163–175 (2001). [Google Scholar]
- 13.Grytnes & Vetaas. Species richness and Altitude: a comparison between null models and interpolated plant species richness along the Himalayan Altitudinal Gradient, Nepal. Am. Nat.159, 294 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Rahbek, C. The elevational gradient of species richness: a uniform pattern? Ecography18, 200–205 (1995).
- 15.Rahbek, C. The role of spatial scale and the perception of large-scale species‐richness patterns. Ecol. Lett.8, 224–239 (2005). [Google Scholar]
- 16.Antonelli, A. et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci.11, 718–725 (2018). [Google Scholar]
- 17.Bruun, H. H. et al. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci.17, 37–46 (2006). [Google Scholar]
- 18.Luo, Y. et al. Forest community assembly is driven by different strata-dependent mechanisms along an elevational gradient. J. Biogeogr.46, 2174–2187 (2019). [Google Scholar]
- 19.McCain, C. M. & Grytnes, J. Elevational gradients in species richness. Encyclopedia Life Sci. (ELS). 10.1002/9780470015902.a0022548 (2013). [Google Scholar]
- 20.Ettinger, A. K., Ford, K. R. & HilleRisLambers, J. Climate determines upper, but not lower, altitudinal range limits of Pacific Northwest conifers. Ecology92, 1323–1331 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Tsujino, R. & Yumoto, T. Vascular plant species richness along environmental gradients in a cool temperate to sub-alpine mountainous zone in central Japan. J. Plant. Res.126, 203–214 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Kira, T. Forest ecosystems of east and southeast Asia in a global perspective. Ecol. Res.6, 185–200 (1991). [Google Scholar]
- 23.Hattori, T., Minamiyama, N., Hashimoto, Y. & Ishida, H. Flora of the lucidophyllous forest in Japan. Nat. Hum. Act.8, 13–47 (2004). [Google Scholar]
- 24.Dearborn, K. D. & Danby, R. K. Aspect and slope influence plant community composition more than elevation across forest–tundra ecotones in subarctic Canada. J. Veg. Sci.28, 595–604 (2017). [Google Scholar]
- 25.Irl, S. D. H. et al. Climate vs. topography – spatial patterns of plant species diversity and endemism on a high-elevation island. J. Ecol.103, 1621–1633 (2015). [Google Scholar]
- 26.Maesako, Y., Nanami, S. & Kanzaki, M. Spatial distribution of two invasive alien species, Podocarpus nagi and Sapium sebiferum, spreading in a warm-temperate evergreen forest of the Kasugayama Forest Reserve,Japan. Veg. Sci.24, 103–112 (2007). [Google Scholar]
- 27.Naka, K. Community dynamics of evergreen broadleaf forests in southwestern Japan. I. wind damaged trees and canopy gaps in an evergreen oak forest. Bot. Mag Tokyo. 95, 385–399 (1982). [Google Scholar]
- 28.Maesako, Y. Kasugayama primary forest and Sika deer in Nara. in Threat of Deer-Damage and the Future of Forests - Effectiveness and limitations of vegetation conservation using fence- (eds. Maesako, Y. & Takatsuki, S.) 93–108 (Bun-ichi Sogo Shuppan., Tokyo, 2015).
- 29.Satake, Y., Ohwi, J., Kitamura, S., Watari, S. & Tominari, T. Wild flowers of Japan. Herbaceous plants (including dwarf subshrubs). I-III. (1981).
- 30.Tani, A., Ito, E., Tsujino, M., Araki, M. & Kanzaki, M. Threshold determination by reference to open sky overcomes photographic exposure error in indirect leaf area index estimation. Jpn J. Environ.53, 41–52 (2011). [Google Scholar]
- 31.Inada, T., Kitajima, K., Hardiwitono, S. & Kanzaki, M. The Effect of Logging and Strip cutting on Forest Floor Light Condition and following change. Forests. 8, 425 (2017). [Google Scholar]
- 32.Frazer, G. W., Canham, C. D. & Lertzman, K. P. Gap Light Analyzer (GLA), Version 2.0: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. (1999).
- 33.Zeger, S. L. & Liang, K. Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics42, 121–130 (1986). [PubMed] [Google Scholar]
- 34.Carl, G. & Kühn, I. Analyzing spatial autocorrelation in species distributions using Gaussian and logit models. Ecol. Model.207, 159–170 (2007). [Google Scholar]
- 35.Carl, G. & Kühn, I. Spind: a package for computing spatially corrected accuracy measures. Ecography. 40, 675–682 (2017). [Google Scholar]
- 36.Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.5-7. (2020).
- 37.Borcard, D., Gillet, F. & Legendre, P. Numerical ecology with R. R. 369-412 10.1007/978-3-319-71404-2_8 (2018).
- 38.Ferry-Graham, L. A., Bolnick, D. I. & Wainwright, P. C. Using functional morphology to examine the ecology and evolution of specialization1. Integr. Comp. Biol.42, 265–277 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Nekola, J. C. & White, P. S. The distance decay of similarity in biogeography and ecology. J. Biogeogr.26, 867–878 (1999). [Google Scholar]
- 40.Team, R. C. R: A Language and Environment for Statistical Computing (Vienna, 2023). [Google Scholar]
- 41.Webb, C. O. & Peart, D. R. Habitat associations of trees and seedlings in a bornean rain forest. J. Ecol.88, 464–478 (2000). [Google Scholar]
- 42.Nagamatsu, D., Seiwa, K. & Sakai, A. Seedling establishment of deciduous trees in various topographic positions. J. Veg. Sci.13, 35–44 (2002). [Google Scholar]
- 43.Harms, K. E., Condit, R., Hubbell, S. P. & Foster, R. B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol.89, 947–959 (2001). [Google Scholar]
- 44.Masaki, T. et al. Effects of microenvironmental heterogeneity on the seed-to-seedling process and tree coexistence in a riparian forest. Ecol. Res.22, 724–734 (2007). [Google Scholar]
- 45.Kanagaraj, R., Wiegand, T., Comita, L. S. & Huth, A. Tropical tree species assemblages in topographical habitats change in time and with life stage. J. Ecol.99, 1441–1452 (2011). [Google Scholar]
- 46.Bagchi, R. et al. Spatial patterns reveal negative density dependence and habitat associations in tropical trees. Ecology92, 1723–1729 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Stevens, M. H., Bunker, H., Schnitzer, D. E., Carson, W. P. & S. A. & Establishment limitation reduces species recruitment and species richness as soil resources rise. J. Ecol.92, 339–347 (2004). [Google Scholar]
- 48.Seidler, T. G. & Plotkin, J. B. Seed dispersal and spatial pattern in Tropical Trees. PLoS Biol.4, e344 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norden, N. et al. Is temporal variation of seedling communities determined by environment or by seed arrival? A test in a neotropical forest. J. Ecol.95, 507–516 (2007). [Google Scholar]
- 50.Janzen, D. H. Herbivores and number of tree species in tropical forests. Am. Nat.104, 501–528 (1970). [Google Scholar]
- 51.Connell, J. H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. in Dynamics of population (eds. Boer, P. J. D. & Gradwell, G. R.) 98–312 (PUDOC, Wageningen, 1971).
- 52.Gonzalez, M. A. et al. Shifts in species and phylogenetic diversity between sapling and tree communities indicate negative density dependence in a lowland rain forest. J. Ecol.98, 137–146 (2010). [Google Scholar]
- 53.Ledo, A. & Schnitzer, S. A. Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology95, 2169–2178 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Schnitzer, S. A. & Carson, W. P. Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology82, 913–919 (2001). [Google Scholar]
- 55.Maesako, Y., Koda, R., Sasaki, S., Sugiura, S. & Hanatani, Y. Forest use of Sika deer (Cervus nippon) in Kasugayama Forest Reserve, a warm temperate evergreen forest in Nara, western Japan. Bull. Kansai Organ. Nat. Conserv.2, 83–91 (2018). [Google Scholar]
- 56.Shimoda, K., Kimura, K., Kanzaki, M. & Yoda, K. The regeneration of pioneer tree species under browsing pressure of Sika deer in an evergreen oak forest. Ecol. Res.9, 85–92 (1994). [Google Scholar]
- 57.Legendre, P. et al. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology90, 663–674 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Soininen, J., McDonald, R. & Hillebrand, H. The distance decay of similarity in ecological communities. Ecography. 30, 3–12 (2007). [Google Scholar]
- 59.Gravel, D., Canham, C. D., Beaudet, M. & Messier, C. Reconciling niche and neutrality: the continuum hypothesis. Ecol. Lett.9, 399–409 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Martín-Devasa, R., Jiménez-Valverde, A., Leprieur, F., Baselga, A. & Gómez-Rodríguez, C. Dispersal limitation shapes distance-decay patterns of European spiders at the continental scale. Glob Ecol. Biogeogr.33, e13810 (2024). [Google Scholar]
- 61.Baltzer, J. L., Thomas, S. C., Nilus, R. & Burslem, D. F. R. P. Edaphic specialization in tropical trees: physiological correlates and responses to reciprocal transplantation. Ecology86, 3063–3077 (2005). [Google Scholar]
- 62.Yamasaki, N., Yamada, T. & Okuda, T. Coexistence of two congeneric tree species of Lauraceae in a secondary warm-temperate forest on Miyajima Island, south‐western Japan. Plant. Spec. Biol.28, 41–50 (2013). [Google Scholar]
- 63.Schmitt, S., Tysklind, N., Derroire, G., Heuertz, M. & Hérault, B. Topography shapes the local coexistence of tree species within species complexes of neotropical forests. Oecologia. 196, 389–398 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Gunatilleke, C. V. S. et al. Species–habitat associations in a Sri Lankan dipterocarp forest. J. Trop. Ecol.22, 371–384 (2006). [Google Scholar]
- 65.Queenborough, S. A., Burslem, D. F. R. P., Garwood, N. C. & Valencia, R. Habitat niche partitioning by 16 species of Myristicaceae in Amazonian Ecuador. Plant. Ecol.192, 193–207 (2007). [Google Scholar]
- 66.Gröning, J. & Hochkirch, A. Reproductive interference between animal species. Q. Rev. Biology83, 257–282 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Nishida, T., Takakura, K. & Iwao, K. Host specialization by reproductive interference between closely related herbivorous insects. Popul. Ecol.57, 273–281 (2015). [Google Scholar]
- 68.Kyogoku, D. Evolution of realized niche breadth diversity driven by community dynamics. Ecol. Lett.27, e14369 (2024). [DOI] [PubMed] [Google Scholar]
- 69.Takakura, K. I., Nishida, T., Matsumoto, T. & Nishida, S. Alien dandelion reduces the seed-set of a native congener through frequency-dependent and one-sided effects. Biol. Invasions11, 973–981 (2009). [Google Scholar]
- 70.Christie, K. & Strauss, S. Y. Frequency-dependent fitness and reproductive dynamics contribute to habitat segregation in sympatric jewelflowers. Proc. Royal Soc. B. 287, 20200559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishida, S., Takakura, K. I., Naiki, A. & Nishida, T. Habitat partitioning in native Geranium species through reproductive interference. Ann. Bot.125, 651–661 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe, S. & Maesako, Y. Co-occurrence pattern of congeneric tree species provides conflicting evidence for competition relatedness hypothesis. Peerj9, e12150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.