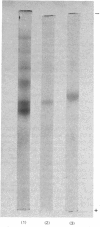
Abstract
An alpha-D-galactosidase (EC 3.2.1.22) and a beta-D-mannanase (EC 3.2.1.78), which were secreted into the growth medium when Aspergillus tamarii was cultivated in the presence of galactomannan, were purified by a procedure including chromatography on hydroxyapatite and DEAE-cellulose columns. Each of these enzymes showed a single protein band, corresponding to their respective activities, on polyacrylamide-gel electrophoresis. Both enzymes were shown to be glycoproteins containing N-acetylglucosamine, mannose and galactose, with molar proportions of 1:6:1.5 for alpha-D-galactosidase and 1:13:8 for beta-D-mannanase. Mr values as determined by polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate and by the electrophoretic method of Hedrick & Smith [(1968) Arch. Biochem. Biophys. 126, 155-164] were 56000 and 53000 respectively. The alpha-D-galactosidase differed markedly from the mycelial forms I and II studied in the preceding paper [Civas, Eberhard, Le Dizet & Petek (1984) Biochem. J. 219, 849-855] with regard to both its kinetic and structural properties.
Full text
PDF

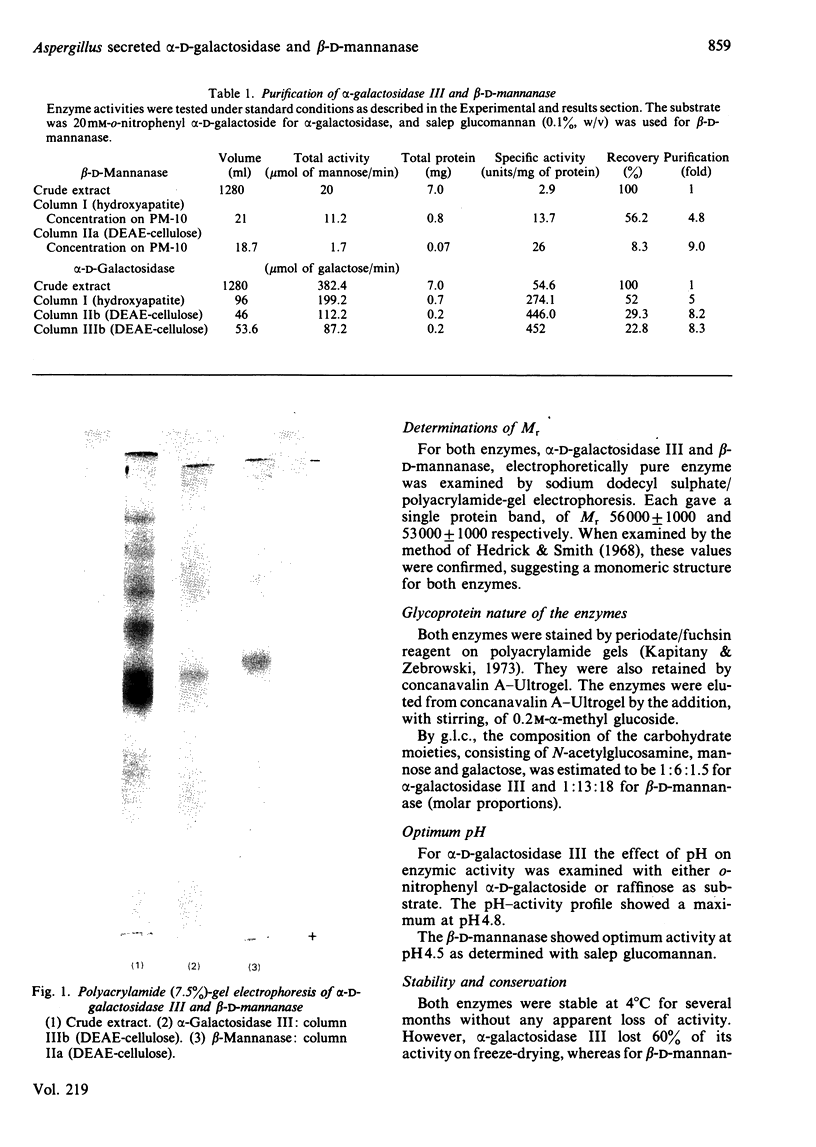
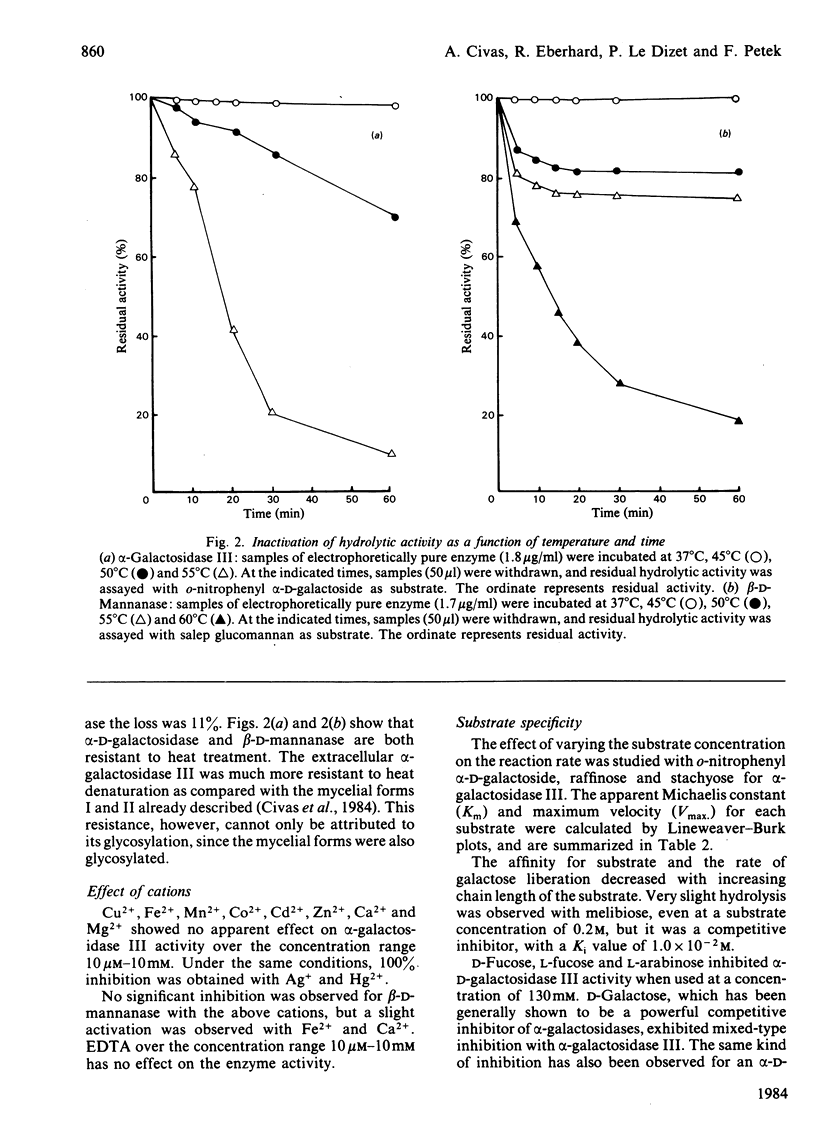

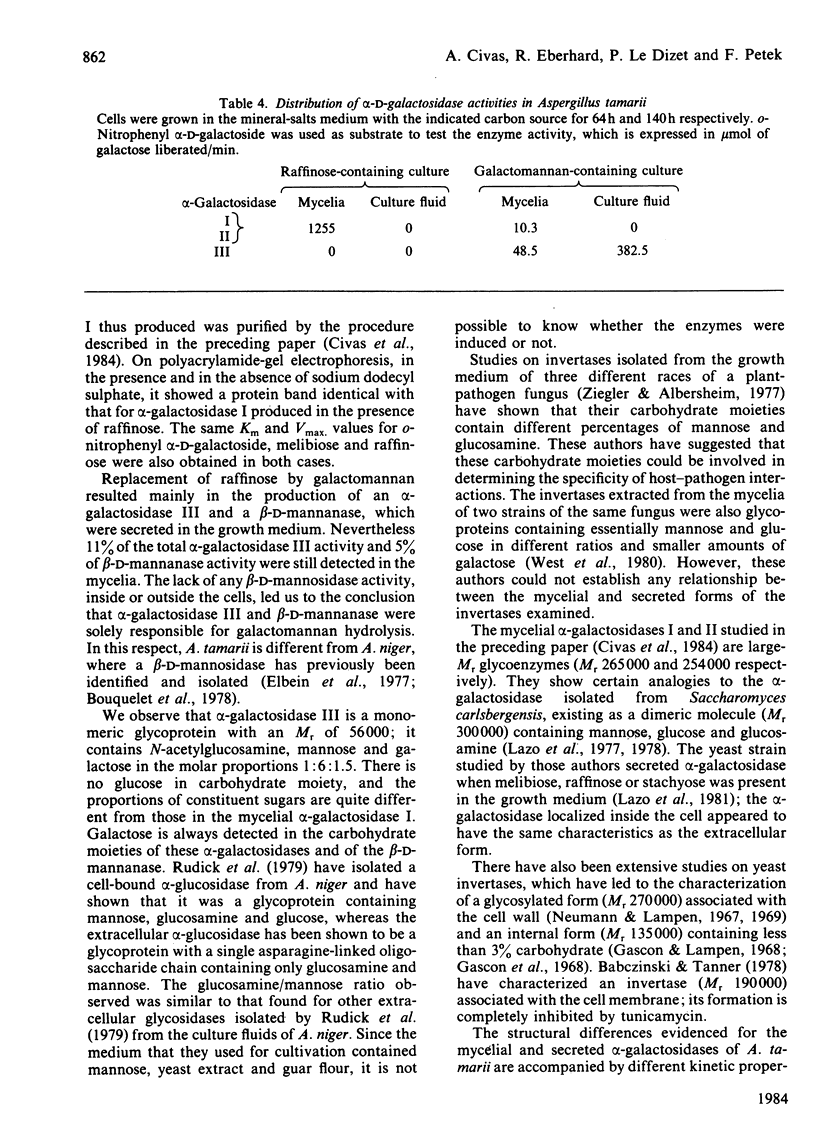

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babczinski P., Tanner W. A membrane-associated isozyme of invertase in yeast. Precursor of the external glycoprotein. Biochim Biophys Acta. 1978 Feb 1;538(3):426–434. doi: 10.1016/0304-4165(78)90404-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bouquelet S., Spik G., Montreuil J. Properties of a beta-D-mannosidase from Aspergillus niger. Biochim Biophys Acta. 1978 Feb 10;522(2):521–530. doi: 10.1016/0005-2744(78)90084-0. [DOI] [PubMed] [Google Scholar]
- Civas A., Eberhard R., Le Dizet P., Petek F. Glycosidases induced in Aspergillus tamarii. Mycelial alpha-D-galactosidases. Biochem J. 1984 May 1;219(3):849–855. doi: 10.1042/bj2190849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Adya S., Lee Y. C. Purification and properties of a beta-mannosidase from Aspergillus niger. J Biol Chem. 1977 Mar 25;252(6):2026–2031. [PubMed] [Google Scholar]
- Gascón S., Lampen J. O. Purification of the internal invertase of yeast. J Biol Chem. 1968 Apr 10;243(7):1567–1572. [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Lazo P. S., Flórez I. G., Ochoa A. G., Gascón S. Induction and catabolite repression of alpha-galactosidase from saccharomyces carlsbergensis. Cell Mol Biol Incl Cyto Enzymol. 1981;27(6):615–622. [PubMed] [Google Scholar]
- Lazo P. S., Ochoa A. G., Gascón S. alpha-Galactosidase (melibiase) from Saccharomyces carlsbergensis: structrual and kinetic properties. Arch Biochem Biophys. 1978 Nov;191(1):316–324. doi: 10.1016/0003-9861(78)90094-2. [DOI] [PubMed] [Google Scholar]
- Lazo P. S., Ochoa A. G., Gascón S. alpha-Galactosidase from Saccharomyces carlsbergensis. Cellular localization, and purification of the external enzyme. Eur J Biochem. 1977 Jul 15;77(2):375–382. doi: 10.1111/j.1432-1033.1977.tb11677.x. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. The glycoprotein structure of yeast invertase. Biochemistry. 1969 Sep;8(9):3552–3556. doi: 10.1021/bi00837a010. [DOI] [PubMed] [Google Scholar]
- Rudick M. J., Fitzgerald Z. E., Rudick V. L. Intra- and extracellular forms of alpha-glucosidase from Aspergillus niger. Arch Biochem Biophys. 1979 Apr 1;193(2):509–520. doi: 10.1016/0003-9861(79)90058-4. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Li S. C., Li Y. T. Alpha-galactosidase from Mortierella vinacea. Crystallization and properties. J Biol Chem. 1970 Feb 25;245(4):781–786. [PubMed] [Google Scholar]
- West C., Wade M., McMillan C., 3rd, Albersheim P. Purification and properties of invertases extractable from Phytophthora megasperma var. sojae mycelia. Arch Biochem Biophys. 1980 Apr 15;201(1):25–35. doi: 10.1016/0003-9861(80)90483-x. [DOI] [PubMed] [Google Scholar]
- Williams J., Villarroya H., Petek F. alpha-Galactosidases II, III and IV from seeds of Trifolium repens. Purification, physicochemical properties and mode of galactomannan hydrolysis in vitro. Biochem J. 1978 Dec 1;175(3):1069–1077. doi: 10.1042/bj1751069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E., Albersheim P. Host-Pathogen Interactions: XIII. Extracellular Invertases Secreted by Three Races of a Plant Pathogen Are Glycoproteins Which Possess Different Carbohydrate Structures. Plant Physiol. 1977 Jun;59(6):1104–1110. doi: 10.1104/pp.59.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]