Abstract
ABSTRACT
Objective
This study aims to explore the associated impairments of cerebral palsy (CP) and their correlates among children with CP in Vietnam.
Design
Descriptive cross-sectional study using hospital-based surveillance.
Setting
National Children’s Hospital, Hanoi, Vietnam between June and November 2017.
Participants
765 children with CP were recruited.
Outcome measures
We assessed clinical characteristics of CP, associated impairments (epilepsy, intellectual, visual, hearing, speech impairments) and their correlates. We performed descriptive analyses (median, IQR and proportion). χ2 test and Fisher’s exact test were used for categorical variables. Univariate logistic regression and multivariate logistic regression models were established and associated impairments were included as independent variables.
Results
The median age of children was 1.7 years (IQR=2.7). Quadriplegia was the predominant subtype (69.5%) and 46.5% were at Gross Motor Function Classification System level IV–V. Of children, 76.3% had ≥one associated impairment, most commonly speech or intellectual impairments (59.1% and 57.8%, respectively). Severity of motor impairment, type of CP, maternal and perinatal factors (eg, gestational age, perinatal asphyxia, timing of injury causing CP) were associated with greater risk of associated impairments.
Conclusion
Children with CP have a high burden of associated impairments. Findings from our study will inform the development and implementation of appropriate screening and interventions and reduce the long-term adverse effects of these impairments on individuals with CP and their socioeconomic impact.
Keywords: EPIDEMIOLOGY, Community child health, Developmental neurology & neurodisability
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We established active, hospital-based surveillance in one of the largest hospitals in Vietnam but hospital-based data are not representative of cerebral palsy (CP) in other settings.
Surveillance was modelled on the Pediatric Active Enhanced Disease Surveillance system operating in Australia.
Clinical assessment and data collection were completed by trained paediatricians using a modification of a widely used CP register form to generate comparable data.
Lack of comprehensive assessments and a young cohort likely led to under-reporting of associated impairments.
The challenges of confirming diagnoses of CP and intellectual impairment in young children may have influenced findings among study participants.
Introduction
Cerebral palsy (CP), the most common physical disability of childhood, is a group of permanent disorders of motor impairment that result from a non-progressive injury to the developing brain.1 CP is often related to a range of associated impairments, some of which result directly from the brain injury causing CP and some that are secondary to the motor impairments of CP.2 One of the most widely used definition of CP outlines that ‘the motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication and behaviour, by epilepsy and by secondary musculoskeletal problems’.1 Associated impairments of CP adversely impact participation,3 quality of life3 and survival2 of individuals with CP, often more than the motor impairment itself. This reaffirms the need for better understanding of comorbid conditions, their impact on the lives of individuals with CP and their caregivers and the management of CP.
A systematic review by Novak reports that rates of comorbidity and associated impairments are strongly linked to the severity of motor impairment of CP.4 Although there are reports of a decline in the prevalence and severity of CP from high-income countries, severe motor impairment is increasingly documented from emerging CP registers in low and middle-income countries.5,8 This indicates a potentially greater burden of associated impairments of CP in low resource settings and highlights the need for better identification of associated impairments and development and implementation of appropriate interventions to reduce their adverse effects and improve participation and quality of life of the children and their families.
In Vietnam, it is estimated that there are 60 000 children living with CP and that CP comprises 30%–40% of all childhood disability.9 However, these figures came from a report which did not provide detailed information about associated impairments in CP. Furthermore, to the best of our knowledge, there has been no research investigating the prevalence of impairments among Vietnamese children with CP. Therefore, in 2017, we established hospital-based surveillance to prospectively document the clinical characteristics of children with CP. In the present study, we explore the associated impairments and their various correlates among those children.
CP negatively affects children and their caregivers, parents, families and healthcare providers. Caregivers play a vital role in the rehabilitation of children with CP10 and often face a wide range of challenges due to the heavy physical burden of caregiving, guilt about their child’s condition and financial burden.11 Also, a study about children with neurodevelopmental disorders reported that half of their caregivers had stress at moderate and high levels.12 In Bangladesh, caregivers of adolescents with CP had a significantly high risk of depression and stress.13 Consequently, the quality and effectiveness of rehabilitation for children may be negatively affected. Providing information on factors associated with impairments in children with CP helps clinicians develop evidence-based rehabilitation interventions that focus on the most severely affected groups and reduce caregiver stress. In addition, understanding the risk factors that lead to associated impairments may inform their prevention in children with CP.
The aim of the study was to describe associated impairments (ie, epilepsy, intellectual, visual, hearing, speech impairments) among children with CP and their various correlates (eg, sex, type and topography of CP, Gross Motor Function Classification System (GMFCS) level, Manual Ability Classification System (MACS) level, etc).
Materials and methods
Study design
This is a descriptive cross-sectional study using hospital-based surveillance.
Procedure
We established hospital-based surveillance modelled on the Paediatric Active Enhanced Disease Surveillance system operating in Australia to identify children with CP.14 In this study, we conducted active, prospective case finding involving daily identification of patients as they presented to the National Children’s Hospital (NCH) Vietnam, as detailed in protocol for this study.9
The questionnaire used for data collection is a modified version of the Australian Cerebral Palsy Register (ACPR) record form which was used for the Bangladesh CP Register (BCPR) Study.6 15 Clinical information was collected by trained Vietnamese paediatricians at the NCH. Responses were recorded in Vietnamese and translated into English by two study investigators. Translated data were entered into a database.16
Study setting
NCH is an 1800 bed tertiary paediatric hospital in Hanoi that provides services for about 100 000 inpatients and 1 million outpatients each year. The rehabilitation department sees approximately 80–100 children per day. Baseline case identification by Vietnamese paediatricians took place between June and November 2017.
Participants
Participants were children aged less than 18 years, who attended the rehabilitation department at NCH and were newly diagnosed with CP, according to the definition used in Surveillance of Cerebral Palsy in Europe and the ACPR.5 7 15 Diagnosis of CP was confirmed by paediatricians following clinical assessment, history taking, review of existing hospital records and investigations using our study protocol. The definition, inclusion and exclusion criteria used for CP are described in the study protocol.9
Outcomes measures
CP characteristics
Detailed clinical assessment was conducted to describe the type and topography of CP. Clinical information including age at the time of diagnosis and presumed timing of the injury causing CP was collected. The GMFCS was used to classify and describe motor function.17 The MACS, which reports on use of hands and upper limbs in different activities, was used to assess children over 4 years of age.17 18
Associated impairments
The presence and severity of associated impairments were documented based on clinical history, detailed clinical assessment, report by the parents/primary caregivers according to the Washington Group conceptual framework19 and review of relevant medical reports.
Diagnosis of epilepsy was based on a clinical history of one or more unprovoked seizures in the previous 3 months beyond the neonatal period and review of medical records.
Intellectual impairment was defined as notable deficits in age-appropriate intellectual functioning, adaptive skills and developmental delays. Assessment of children to identify those with and/or likely to develop intellectual impairment was based on clinical history, and/or assessment of adaptive function and/or report by the parents/primary caregivers and/or review of available medical records including IQ by clinicians. In the absence of relevant medical records of formal assessment, which was the case for the majority of our study participants, a clinical assessment was made by experienced paediatricians at the NCH. Assessment of probable severity of intellectual impairment was based on each child’s age-appropriate intellectual and adaptive functioning, and daily skills in accordance with the DSM-5 criteria for diagnosis and classification of severity of intellectual disability20 and classified as follows: ‘children who were clinically assessed to be slower in all areas of conceptual development and social and daily living skills’ relative to age were classified as mild to moderate; ‘children with major delays in development, poor intellectual and adaptive functioning skills’ for age were classified as severe.
Due to certain known limitations of the DSM-5 in the assessment of severity of intellectual impairment among such a young cohort, the experienced clinicians used additional assessment tools to support their clinical assessment and decision-making about the severity. This included the additional use of the American Association on Intellectual Developmental Disabilities-AAIDD)21 to evaluate the extent of support required, thus certain adaptive skills of each child relative to their age to determine the extent of developmental delays to support the DSM-5 criteria, which confirms that ‘major developmental delays’ reflect severe or profound intellectual impairment. Collective use of these multiple tools, completion of detailed history taking and clinical assessment by experienced paediatricians and review of available medical records ensured a rigorous approach to confirmation and classification of intellectual impairment among these children. This multipronged approach additionally enabled classification consistent with the categories used by the Australian Cerebral Palsy Register and hence allow estimation of the IQ category: normal (IQ >70), mild impairment (IQ 50–69), moderate impairment (IQ 35–49), severe impairment (IQ<35).22 23
Visual impairment was determined by an ophthalmologist through history, clinical assessment of visual acuity and functional vision (including counting fingers, hand motion and light perception) and medical records and classified using WHO guidelines24 as: normal: no impairment, mild to moderate: some impairment, severe: Blindness.
Assessment of hearing impairment was based on history and examination, including the child’s response to name call, clap and vehicle horns; previous ear infections, symptoms of ear pain or purulent drainage; family history; prescription and use of over-the-counter ototoxic medication and previous hearing loss interventions. Otoscopic examination was performed to evaluate the pinna, external auditory canal and tympanic membrane for conditions that could contribute to hearing loss or may require further evaluation and treatment. Assessment was done by otolaryngology and audiometry and based on WHO guideline25 as follow: normal: no hearing loss, mild to moderate: some impairment, severe: deafness. Audiology was not performed.
Assessment of speech was based on the history taken from the primary caregiver, and speech and language assessment by a medical practitioner. Expressive and respective language, naming quality and quality of conversational speech were also observed. Speech impairment was classified as follows: normal: no impairment, mild to moderate: some impairment, severe: Non-verbal.
These are the common associated impairments among children with CP often resulting from the causal injury to the developing brain. We assessed and collected data on each associated impairment using evidence-based tools and definitions (ILEA,26 DSM-521 and WHO24 25), which are used extensively by clinicians. Chosen assessments and classification of the measures are widely used by CP registers internationally, including the Australian CP Register, Surveillance of CP in Europe and BCPR, which has ensured generation of robust comparable data.
Risk factors
Data were collected on the known risk factors for CP through history taking, review of hospital records and clinical assessment. Data were collected on maternal febrile illness during pregnancy, gestational age at birth, mode of delivery, birth weight of children, timing of injury causing CP, perinatal asphyxia, neonatal jaundice, maternal infection during pregnancy, consanguinity and multiple births.
Maternal febrile illness during pregnancy was defined as self-reported fever any time during pregnancy when the temperature was measured ≥37.8°C.
Data on timing of injury causing CP was collected in two categories: (1) prenatally or perinatally acquired CP and (2) postneonatally acquired CP. Those with no clear known prenatal, perinatal or postneonatal cause were reported as ‘unknown’.
Perinatal asphyxia was defined as failure of a newborn to cry at the time of birth, experiencing delayed onset of breathing (>1 min) or requiring assistance to initiate breathing. Non-physiological jaundice confirmed by physicians was reported.
Neuroimaging patterns
Detailed reports of MRI scans, completed by radiologists at the NCH and stored in the hospital records, were obtained. MRI findings from scans completed at any age were included in the study. The MRI findings were classified into five groups using the MRI Classification System: maldevelopment, predominant white matter injury, predominant grey matter injury, miscellaneous patterns and normal.27 These findings will be separately published.
Nutritional status
Anthropometric measurements (height and weight) were documented to assess the nutritional status of children using a WHO guideline.28 We reported these data separately in a previous publication.29 Three indices were used to describe the nutritional status of children with CP: weight for age (WA), height for age (HA) and weight for height (WH). The Z scores for these three indices (ie, WAZ, HAZ and WHZ) were calculated using WHO Anthro (V.3.2) and WHO AnthroPlus software. WAZ was calculated for children aged less than 121 months and WHZ was calculated for children aged less than 61 months. Nutritional status of children was categorised using the WHO cut-offs for the z scores (ie, −2 SD to +2 SD: normal; <−2 SD to −3 SD: moderately undernourished and <−3SD: severely undernourished).
Head circumference
Using a tape measure 1–2 cm wide and marked in 0.1 cm increments, maximum head circumference was measured. Measurements were repeated three times and the largest measurement to the nearest 0.1 cm was selected.30 Head circumference z-scores (calculated using WHO charts31) were categorised to indicate microcephaly (head circumference >2 SD below the mean or <3rd centile for age and sex), severe microcephaly (head circumference >3 SD below the mean for age and sex or <1st centile); macrocephaly (occipitofrontal circumference >2 SD above the mean for given age and sex or >97th centile) and normocephaly.30
All of these outcome measures have been widely used and investigated in similar research contexts.32,35 The chosen outcome measures allow us to assess and analyse the specific variables and parameters that are essential to answering the research questions.
Statistical analysis
We performed descriptive analyses (median, IQR and proportion). χ2 test and Fisher’s exact test were used for categorical variables. Univariate logistic regression analyses were conducted, variables reported in previous studies as associated factors with associated impairments were included as independent variables.35,45 Then multivariate logistic regression model was established based on impact/relation of each variable in univariate models. The dependent variables of both models were the absence of each associated impairment. All tests were considered significant at p<0.05. All analyses were performed using SPSS Statistics software V.24 (IBM Corporation, Chicago, Illinois).
Patient and public involvement
Patients or the public were not directly involved in the conception, design and planning of this study.
Result
A total of 765 children with CP participated in this study. The median age of participants was 1.7 years (IQR=2.7) and 64.2% were men. Quadriplegia was the predominant subtype (69.5%) followed by monoplegia/hemiplegia (20.5%). Nearly half the children had motor function at GMFCS level IV–V (46.5%) (table 1). About a third of children had malnutrition. The median gestational age at birth was 38 weeks (IQR=3). The majority had a vaginal delivery, were born at term and had a pre/perinatal injury causing CP (table 1)
Table 1. Characteristics of the study participants.
| Characteristics (N=765) | n | % |
| Sex | ||
| Male | 491 | 64.2 |
| Female | 274 | 35.8 |
| Age groups | ||
| 0–4 | 644 | 84.2 |
| 5–9 | 101 | 13.2 |
| 10–14 | 20 | 2.6 |
| Type and topography of CP | ||
| Spastic | ||
| Monoplegia/hemiplegia | 157 | 20.5 |
| Diplegia | 40 | 5.2 |
| Quadriplegia | 532 | 69.5 |
| Ataxic | 1 | 0.1 |
| Dyskinetic | 35 | 4.6 |
| GMFCS level (n=754) | ||
| I–III | 403 | 53.5 |
| VI–V | 351 | 46.5 |
| MACS (n=188)* | ||
| I–III | 170 | 90.4 |
| VI–V | 18 | 9.6 |
| Weight-for-age z score (n=737)† | ||
| Normal | 524 | 71.1 |
| Moderate underweight | 139 | 18.9 |
| Severe underweight | 74 | 10.0 |
| Height-for-age z score (n=716)‡ | ||
| Normal | 442 | 61.7 |
| Stunted | 111 | 15.5 |
| Moderate | 103 | 14.4 |
| Severe | 60 | 8.4 |
| Weight-for-height z score (n=579)§ | ||
| Normal | 397 | 68.6 |
| Wasted | 64 | 11.1 |
| Moderate | 80 | 13.8 |
| Severe | 38 | 6.6 |
| Head circumference (n=629)¶ | ||
| Normocephalic | 341 | 54.2 |
| Microcephalic | 267 | 42.5 |
| Macrocephalic | 21 | 3.3 |
| Neuroimaging patterns (n=264) | ||
| Maldevelopments | 14 | 5.3 |
| Predominant white matter injury | 118 | 44.7 |
| Predominant grey matter injury | 25 | 9.5 |
| Miscellaneous | 45 | 17.1 |
| Normal | 62 | 23.5 |
| Maternal febrile illness during pregnancy (n=762) | 102 | 13.4 |
| Mode of delivery (n=756) | ||
| Vaginal birth | 504 | 66.7 |
| Caesarean section | 238 | 31.5 |
| Instrumental delivery (eg, forceps or ventouse suction) | 14 | 1.8 |
| Gestational age at birth (n=762) | ||
| Term (≥37–41 weeks) | 541 | 71.0 |
| Preterm (≤36 weeks) | 202 | 26.5 |
| Post-term (42–43 weeks) | 19 | 2.5 |
| Low birth weight (<2500 gram) (n=761) | 200 | 26.3 |
| Timing of injury causing CP | ||
| Pre/perinatal | 416 | 54.4 |
| Postneonatal | 98 | 12.8 |
| Unknown | 251 | 32.8 |
| Perinatal asphyxia (n=761) | 276 | 36.3 |
| Neonatal jaundice | 81 | 10.5 |
| Maternal infection | 69 | 5.8 |
| Consanguinity (n=744) | 11 | 1.5 |
| Multiple births (n=733) | 42 | 5.7 |
MACS was assessed for children over 4 years old.
Weight-for-age z score was calculated for children aged ≤121 months.
Missing data (n=49).
Weight-for-height z score was calculated for children aged ≤61 months.
Head circumference was calculated for children aged ≤61 months.
CPcerebral palsyGMFCSGross Motor Function Classification SystemMACSManual Ability Classification System
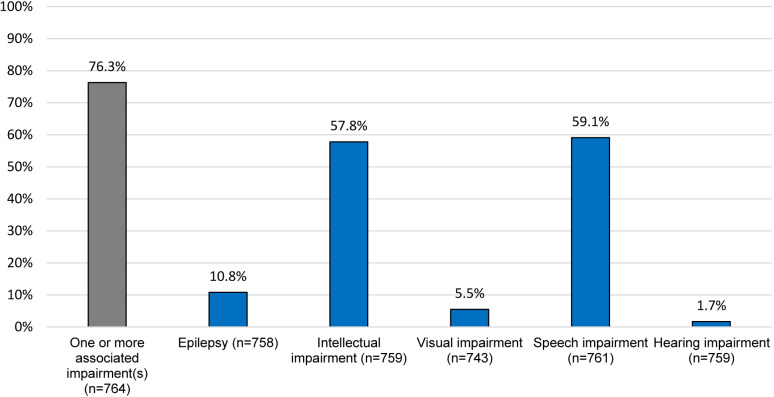
Three quarters (76.3%) of the children had at least one associated impairment with speech and intellectual impairments being the most common (59.1% and 57.8%, respectively) (figure 1).
Figure 1. Associated impairments among children with CP. CP, cerebral palsy.
Most children with intellectual or visual impairment had mild to moderate impairment. In contrast, over one-third of the individuals with hearing impairment had severe impairment (table 2).
Table 2. Distribution of associated impairments and their severity among children with CP.
| Impairments | Severe n (%) | Mild to moderate n (%) | No impairment n (%) |
| Intellectual | 13 (1.9) | 426 (55.7) | 230 (34.4) |
| Visual | 1 (0.1) | 40 (5.4) | 695 (94.4) |
| Hearing | 3 (0.4) | 10 (1.3) | 743 (98.3) |
| Speech | 258 (36.6) | 192 (27.2) | 255 (36.2) |
Children without information on impairment severity of impairment were excluded.
CPcerebral palsy
Over 50% children with either dyskinesia or quadriplegia had intellectual or speech impairments. Epilepsy, visual and hearing impairment were observed in under 15% for all CP subtypes. Ataxia group had one participant and this child had intellectual and visual impairments (online supplemental figure S1).
The proportion of children with selected associated impairments by GMFCS level is shown in online supplemental figure S2. Of the children with GMFCS levels II, III, IV and V, 50%–70% had intellectual and/or speech impairments. Epilepsy was more commonly observed with more severe motor functional impairment (from 5% in GMFCS level I to 15.9% in GMFCS level V, p=0.007).
In a univariate logistic model, children with quadriplegia, GMFCS level IV-V, intellectual impairment or speech impairment had higher risk of epilepsy compared with other children with CP (p<0.01). Children with miscellaneous findings (cerebellar atrophy, cerebral atrophy, delayed myelination, ventriculomegaly and haemorrhage not covered by white matter injury, brainstem lesions, calcifications) on brain MRI (OR=2.47 (95% CI 1.13 to 5.4) and postneonatal injury causing CP (OR=4.56, p<0.001) had a higher risk of epilepsy. Factors associated with intellectual impairment included quadriplegia, GMFCS levels IV–V, MACS level IV–V, the other four impairments (epilepsy, speech, visual and hearing impairments) and term birth (p<0.01) (table 3). Factors significantly associated with visual impairment were intellectual or speech impairments, MACS levels IV–V and perinatal asphyxia. Intellectual impairment was the only significant association with hearing impairment (OR=9.05, 95% CI 1.17 to 69.96). Associated factors of speech impairment were quadriplegia, intellectual and visual impairments, GMFCS/MACS level IV–V, miscellaneous findings on brain MRI and perinatal asphyxia (p<0.05) (table 3). The full version of table 3 (includes number, percentage) is in online supplemental appendix.
Table 3. Association between associated impairments and factors (univariate logistic regression model).
| Characteristics | Epilepsy (n=82)OR (95% CI) | Intellectual (n=439)OR (95% CI) | Visual (n=41)OR (95% CI) | Hearing (n=13)OR (95% CI) | Speech (n=450)OR (95% CI) |
| Sex | |||||
| Female | 1.15 (0.72 to 1.84) | 0.90 (0.67 to 1.22) | 0.8 (0.41 to 1.57) | 2.92 (0.95 to 9.02) | 0.9 (0.66 to 1.20) |
| Type and topography of CP | |||||
| Spastic | |||||
| Mono/hemi | 0.63 (0.33 to 1.19) | 0.32 (0.22 to 0.46)*** | 0.63 (0.26 to 1.52) | 0.69 (0.15 to 3.17) | 0.26 (0.18 to 0.37)*** |
| Diplegia | – | 0.20 (0.09 to 0.43)*** | 0.43 (0.06 to 3.18) | – | 0.13(0.06to0.31)*** |
| Quadriplegia | 2.31 (1.27 to 4.20)** | 3.54 (2.56 to 4.89)*** | 1.42 (0.68 to 2.95) | 2.48 (0.54 to 11.3) | 3.82 (2.76 to 5.28)*** |
| Ataxic | – | – | – | – | – |
| Dyskinetic | 0.49 (0.11 to 2.07) | 0.86 (0.44 to 1.70) | 1.15 (0.27 to 4.99) | – | 2.06 (0.95 to 4.47) |
| Associated impairments | |||||
| Epilepsy | 3.66 (2.05 to 6.53)*** | 1.79 (0.77 to 4.18) | 2.88 (0.76 to 10.9) | 2 (1.19 to 3.33) | |
| Intellectual | 3.66 (2.05 to 6.53)*** | 3.22 (1.47 to 7.07)** | 9.05 (1.17 to 69.96)* | 5.49 (4 to 7.53)*** | |
| Visual | 1.79 (0.76 to 4.18) | 3.21 (1.46 to 7.05)** | 1.6 (0.2 to 12.74) | 2.19 (1.06 to 4.56)* | |
| Hearing | 2.87 (0.76 to 10.84) | 9.02 (1.17 to 69.74)* | 1.6 (0.2 to 12.74) | – | |
| Speech | 1.99 (1.19 to 3.32)** | 5.46 (3.98 to 7.50)*** | 2.19 (1.06 to 4.56)* | – | |
| GMFCS level | |||||
| VI–V | 2.23 (1.38 to 3.61)** | 2.30 (1.71 to 3.10)*** | 1.17 (0.62 to 2.21) | 0.98 (0.32 to 2.95) | 1.93 (1.44 to 2.6)*** |
| MACS level | |||||
| VI–V | 1.80 (0.47 to 6.85) | 5.50 (1.54 to 19.70)** | 3.90 (0.95 to 16.2)* | – | 8.79 (1.96 to 39.4)** |
| Neuroimaging patterns | |||||
| Normal | 0.93 (0.38 to 2.27) | 0.68 (0.38 to 1.22) | 0.91 (0.18 to 4.5) | 0.80 (0.09 to 7.32) | 0.65 (0.36 to 1.16) |
| Miscellaneous | 0.94 (0.34 to 2.60) | 1.52 (0.75 to 3.07) | 0.62 (0.08 to 5.11) | 1.24 (0.14 to 11.4) | 2.47 (1.13 to 5.4)* |
| Predominant grey matter injury | 1.01 (0.28 to 3.60) | 1.60 (0. 64 to 3.97) | 2.83 (0.56 to 14.4) | 2.54 (0.27 to 23.7) | 1.2 (0.5 to 2.9) |
| Predominant white matter injury | 0.49 (0.11 to 2.17) | 0.38 (0.17 to 0.81)* | 0.93 (0.11 to 7.59) | – | 1.67 (0.72 to 3.86) |
| Maldevelopments | 3.26 (0.96 to 11.1)* | 0.78 (0.26 to 2.33) | – | – | 0.54 (0.18 to 1.58) |
| Maternal febrile illness during pregnancy | 0.88 (0.44 to 1.76) | 0.96 (0.63 to 1.46) | 0.49 (0.15 to 1.62) | 0.53 (0.07 to 4.12) | 0.66 (0.43 to 1.0) |
| Mode of delivery | |||||
| Vaginal birth | 0.90 (0.56 to 1.45) | 1.06 (0.79 to 1.45) | 0.83 (0.43 to 1.6) | 0.8 (0.26 to 2.47) | 1.03 (0.76 to 1.41) |
| Caesarean section | 1.15 (0.71 to 1.88) | 0.91 (0.67 to 1.24) | 1.17 (0.6 to 0.08) | 1.36 (0.44 to 4.21) | 0.9 (0.66 to 1.23) |
| Gestational age | |||||
| Term (≥37–41 weeks) | 1.13 (0.67 to 1.89) | 1.42 (1.04 to 1.95)* | 0.99 (0.49 to 1.97) | 2.26 (0.50 to 10.3) | 1.26 (0.92 to 1.73) |
| Preterm (≤36 weeks) | 0.89 (0.52 to 1.51) | 0.73 (0.53 to 1.01) | 1.16 (0.58 to 2.32) | 0.50 (0.11 to 2.28) | 0.83 (0.6 to 1.15) |
| Low birth weight (<2500 gram) | 0.65 (0.37 to 1.16) | 0.88 (0.64 to 1.22) | 1.83 (0.96 to 3.51) | 0.51 (0.11 to 2.31) | 0.95 (0.68 to 1.31) |
| Timing of injury causing CP | |||||
| Pre/perinatal | 0.66 (0.41 to 1.04) | 1.00 (0.75 to 1.34) | 1.21 (0.64 to 2.29) | 0.52 (0.17 to 1.60) | 1.12 (0.84 to 1.5) |
| Postneonatal | 4.56 (2.71 to 7.67)*** | 1.51 (0.97 to 2.36) | 2.02 (0.93 to 4.37) | 3.12 (0.94 to 10.3) | 1.03 (0.67 to 1.6) |
| Unknown | 0.50 (0.29 to 0.88)* | 0.82 (0.60 to 1.11) | 0.48 (0.22 to 1.05) | 0.91 (0.28 to 2.98) | 0.87 (0.64 to 1.19) |
| Perinatal asphyxia | 1.31 (0.82 to 2.10) | 1.09 (0.80 to 1.47) | 1.97 (1.05 to 3.71)* | 0.52 (0.17 to 1.60) | 1.71 (1.25 to 2.32)** |
| Neonatal jaundice | 0.53 (0.21 to 1.35) | 1.08 (0.67 to 1.73) | 0.43 (0,10 to 1.81) | 3.12 (0.94 to 10.3) | 1.25 (0.77 to 2.02) |
| Maternal infection | 1.31 (0.62 to 2.76) | 1.31 (0.79 to 2.20) | 0.83 (0.25 to 2.77) | 0.91 (0.28 to 2.98) | 1.53 (0.9 to 2.6) |
| Consanguinity | 1.93 (0.41 to 9.11) | 1.94 (0.51 to 7.34) | – | – | 0.81 (0.24 to 2.68) |
| Multiple births | 0.88 (0.31 to 2.53) | 0.50 (0.26 to 0.94)* | 0.50 (0.26 to 0.94)* | – | 0.9 (0.48 to 1.7) |
n (%) is presented for categorical variables.
Chiχ2 square test and Fisher´’s exact test were used for comparison between groups and for p- value (2two-sided). *p, **p, ***p.
*p<0.05, **p<0.01, ***p<0.001.
CPcerebral palsyGMFCSGross Motor Function Classification System
A univariate logistic model was used to investigate the association between anthropometric characteristics and associated impairments (online supplemental table S1). Severe underweight and stunting were significantly associated with intellectual impairment.
In multivariate logistic models, children with monoplegia/hemiplegia and diplegia were found to have lower risk of intellectual and speech impairments than children with quadriplegia (p<0.05). GMFCS levels IV–V and postneonatal timing of injury causing CP were associated with higher risk of epilepsy (OR=2.75, 95% CI 1.25 to 6.06 and OR=9.08, p<0.001). Children with postneonatal injury causing CP had a significantly higher risk of visual impairment (OR=3.84) than children with pre/perinatal injury causing CP. Perinatal asphyxia and maternal infection were associated with visual and speech impairments (p<0.05) (table 4).
Table 4. Association between type of associated impairments and clinical characteristics in children with CP (multivariate logistic regression model).
| Epilepsy (n=82)OR (95% CI) | Intellectual (n=439)OR (95% CI) | Visual (n=41)OR (95% CI) | Hearing (n=13)OR (95% CI) | Speech (n=450)OR (95% CI) | |
| Sex | |||||
| Male | 1 | 1 | 1 | 1 | 1 |
| Female | 0.74 (0.33 to 1.66) | 1.27 (0.78 to 2.07) | 0.95 (0.30 to 2.96) | 2.75 (0.53 to 14.3) | 1.03 (0.63 to 1.68) |
| Type and topography of CP | |||||
| Spastic | |||||
| Mono/hemi | 0.18 (0.02 to 1.58) | 0.99 (0.26 to 3.80) | 0.98 (0.30 to 3.21) | 0.45 (0.07 to 3.13) | 0.22 (0.05 to 0.92)* |
| Diplegia | – | 0.09 (0.01 to 0.66)* | 2.24 (0.20 to 25.6) | – | 0.09 (0.02 to 0.57)* |
| Quadriplegia | 0.41 (0.05 to 3.00) | 3.10 (0.84 to 11.5) | – | – | 0.94 (0.24 to 3.71) |
| Ataxic | – | – | – | – | – |
| Dyskinetic | – | – | – | – | – |
| GMFCS level | |||||
| I–III | 1 | 1 | 1 | 1 | 1 |
| VI–V | 2.75 (1.25 to 6.06)* | 1.43 (0.92 to 2.22) | 1.37 (0.49 to 3.78) | 0.29 (0.05 to 1.74) | 1.10 (0.70 to 1.73) |
| Weight-for-age z score (n=737) | |||||
| Normal | 1 | 1 | 1 | 1 | 1 |
| Moderate underweight | 0.80 (0.28 to 2.31) | 1.39 (0.73 to 2.64) | 1.11 (0.31 to 4.00) | 4.49 (0.50 to 40.3) | 1.12 (0.59 to 2.11) |
| Severe underweight | 2.27 (0.55 to 9.34) | 1.59 (0.60 to 4.24) | 0.21 (0.01 to 3.30) | – | 2.35 (0.84 to 6.56) |
| Height-for-age z score (n=716) | |||||
| Normal | 1 | 1 | 1 | 1 | 1 |
| Stunted | 0.91 (0.33 to 2.52) | 1.66 (0.91 to 3.06) | 2.23 (0.66 to 7.47) | 0.63 (0.05 to 8.10) | 1.16 (0.63 to 2.15) |
| Moderate | 0.54 (0.16 to 1.82) | 1.72 (0.80 to 3.71) | 1.73 (0.36 to 8.29) | 2.34 (0.20 to 28.0) | 0.83 (0.39 to 1.77) |
| Severe | 2.09 (0.62 to 7.00) | 1.27 (0.56 to 2.91) | 0.83 (0.09 to 7.98) | – | 0.93 (0.39 to 2.20) |
| Weight-for-height z score (n=579) | |||||
| Normal | 1 | 1 | 1 | 1 | 1 |
| Wasted | 0.89 (0.29 to 2.71) | 1.70 (0.81 to 3.56) | 2.57 (0.77 to 8.60) | 1.30 (0.08 to 22.1) | 1.64 (0.77 to 3.52) |
| Moderate | 0.58 (0.17 to 1.98) | 0.78 (0.35 to 1.76) | – | 0.61 (0.02 to 19.9) | 1.17 (0.51 to 2.70) |
| Severe | 1.69 (0.49 to 5.90) | 1.20 (0.48 to 3.02) | 2.17 (0.39 to 12.0) | – | 1.51 (0.61 to 3.73) |
| Head circumference | |||||
| Normocephalic | 5.81 (0.56 to 60.2) | 1.40 (0.42 to 4.46) | 0.64 (0.21 to 1.94) | 0.14 (0.01 to 2.00) | 1.68 (0.50 to 5.61) |
| Microcephalic | 2.82 (0.27 to 29.7) | 1.61 (0.47 to 5.50) | – | 0.12 (0.01 to 1.87) | 1.62 (0.47 to 5.54) |
| Macrocephalic | – | – | – | – | |
| Maternal febrile illness during pregnancy | 0.90 (0.35 to 2.30) | 1.35 (0.73 to 2.49) | – | 0.78 (0.06 to 9.44) | 0.52 (0.29 to 0.95) |
| Mode of delivery | |||||
| Vaginal birth | 1 | 1 | 1 | 1 | 1 |
| Caesarean section | 1.01 (0.51 to 6.29) | 1.03 (0.67 to 1.59) | 2.08 (0.80 to 5.40) | 2.50 (0.55 to 11.3) | 0.75 (0.48 to 1.16) |
| Instrumental delivery | 2.22 (0.21 to 23.04) | 1.21 (0.18 to 7.94) | – | – | – |
| Gestational age | |||||
| Term (≥37–41 weeks) | 1 | 1 | 1 | 1 | 1 |
| Preterm (≤36 weeks) | 2.28 (0.82 to 6.29) | 0.82 (0.43 to 1.57) | 0.30 (0.07 to 1.34) | 2.33 (0.18 to 30.9) | 0.78 (0.39 to 1.52) |
| Post-term (>41 weeks) | 1.22 (0.09 to 16.5) | 0.49 (0.09 to 2.62) | – | – | 0.08 (0.01 to 0.80)* |
| Low birth weight (<2500 gram) | 0.51 (0.17 to 1.51) | 0.55 (0.29 to 1.04) | 3.96 (0.98 to 16.0) | 0.13 (0.00 to 3.49) | 0.64 (0.33 to 1.25) |
| Timing of injury causing CP | |||||
| Pre/perinatal | 1 | 1 | 1 | 1 | 1 |
| Postneonatal | 9.08 (3.63 to 22.7)*** | 1.78 (0.85 to 3.69) | 3.84 (1.07 to 8.78)* | 2.96 (0.41 to 21.0) | 0.93 (0.46 to 1.89) |
| Unknown | 1.14 (0.45 to 2.87) | 0.79 (0.47 to 1.32) | 1.10 (0.31 to 3.97) | 0.70 (0.08 to 5.84) | 0.83 (0.49 to 1.41) |
| Perinatal asphyxia | 1.65 (0.80 to 3.40) | 1.11 (0.70 to 1.76) | 3.06 (1.07 to 8.78)* | 1.65 (0.32 to 8.56) | 1.77 (1.10 to 2.83)* |
| Neonatal jaundice | 0.29 (0.07 to 1.24) | 0.80 (0.40 to 1.61) | 1.46 (0.26 to 8.09) | 1.24 (0.12 to 13.2) | 0.84 (0.40 to 1.75) |
| Maternal infection | 0.89 (0.29 to 2.70) | 1.45 (0.68 to 3.12) | 0.18 (0.02 to 1.61) | 3.28 (0.52 to 20.7) | 2.28 (1.02 to 5.08)* |
| Consanguinity | 3.30 (0.33 to 43.5) | 2.87 (0.19 to 43.4) | – | – | 0.34 (0.05 to 2.39) |
| Multiple births | 1.05 (0.27 to 4.17) | 0.55 (0.24 to 1.27) | 1.06 (0.19 to 5.91) | – | 0.90 (0.39 to 2.09) |
*p<0.05, **p<0.01, ***p<0.001. MACS classification and neuroimaging patterns were not included because of a small number of available data. – indicates that calculation of OR and 95% CI is non-applicable, since both groups are omitted or empty).
Analysis only for children under 61 months.
CPcerebral palsyGMFCSGross Motor Function Classification System
Discussion
This is the first study reporting about significant factors related to associated impairments. We investigated 765 Vietnamese children with CP and found that children with CP have a considerably higher burden of associated impairments than the general child population in Vietnam.46 47 The majority (76.3%) of children had at least one impairment; intellectual and speech impairments being the most common. The type and motor severity of CP are significantly associated with associated impairments among children with CP. Specifically, children with quadriplegia had a significantly higher risk of epilepsy and intellectual and speech impairments, and children with severe motor functional impairment (ie, GMFCS IV–V) were more likely to have epilepsy, intellectual and speech impairments.
Epilepsy was also associated with brain neuroimaging patterns, timing of injury causing CP and other associated impairments, that is, intellectual and speech impairment (p<0.05). Perinatal asphyxia, the timing of injury causing CP, intellectual and speech impairments were associated with visual impairment (p<0.05). Gestational age, perinatal asphyxia and maternal infection were associated factors of speech impairment (p<0.05). Intellectual impairment was associated with gestational age, multiple births, types of CP, GMFCS levels, MACS levels, head circumference, and other associated impairments (p<0.05).
Characteristics of associated impairments
The proportion of all associated impairments among children with CP was higher than their peers in the general population.46 47 Although our study was hospital based, the proportion of children with CP and at least one associated impairment (76.3%) were comparable to findings from population-based studies of CP cohorts in other LMICs (79.6% in Bangladesh and 77.7% in Indonesia).6 48 Consistent with findings from CP cohorts in Europe and Ethiopia, intellectual and speech impairments were the most common associated impairments in our study.49 50 Both these impairments considerably limit communication and participation in education and society, thus negatively impacting life achievements and quality of life.51
Similar to an Indonesian and a large European study,48 52 over half of the children in our study had intellectual impairment, and almost one-third had severe or moderate impairment. This is lower than the reported rates from a hospital-based study of children with CP in Israel51 and could be in part be due to methodological differences in assessment and definition of intellectual impairment and its severity. Furthermore, the children in our study were younger than in the studies from Indonesia and Israel and older than in the European study, which may have limited testing and influenced the reported differences.48 51 52
The prevalence of speech, visual and hearing impairments in our cohort were lower than reported rates in CP cohorts in other countries such as Bangladesh, Ethiopia, Indonesia.6 48 50 Therein, the children with CP in the Ethiopian article were of similar age to the children in our study.50 Epilepsy was also reported in a considerably lower proportion of children in our study (10.8%) compared with hospital-based studies in Israel and Ethiopia50 51 and population-based studies conducted in Bangladesh,6 Indonesia,48 Europe.52 The lower proportion of some of the associated impairments, particularly epilepsy, in our study could be because the study participants were recruited from a tertiary paediatric hospital where they had access to treatment and interventions informed by thorough neurological assessments by trained paediatricians, and comprehensive investigations including MRI. Although most of our participants had mild to moderate impairment, such impairments are linked to reduced quality of life,53 which is lower than that of the general population.2 The higher burden of associated impairments in children with CP compared with their peers is explained by the excess risk of disturbances that are as cocausal with CP and complications of CP as well.39 This suggests a need for standardised screening of children with CP for the commonly associated impairments, complemented by education of health professionals and caregivers on their diagnosis and management.
GMFCS levels and associated impairments
In our study, the multivariate logistic regression model showed that children with GMFCS levels IV–V had a higher risk of epilepsy (OR: 2.75, 95% CI 1.25 to 6.06). Furthermore, the univariate model showed that GMFCS levels IV–V were associated with epilepsy, intellectual and speech impairments (p<0.001) (table 3), similar to findings from Turkey (OR: 2.56).36 If has been previously reported that children with CP and GMFCS levels IV–V have more severe impairment37 than those with GMFCS levels I–III. GMFCS IV–V is often associated with more profound injury to the brain, increasing the risk for associated impairments. Although findings from a study in Germany reported that children with severe CP were at greater risk of severe visual impairment,38 we did not find a significant association between GMFCS level and visual impairment. This could be due to the small number of children with visual impairment in our cohort. Another German study suggested that identification and intervention targeting various associated impairments should best be directed towards children with GMFCS levels IV to V.40 GMFCS can be assessed early in life with measurable consistency and reliability,54 55 thus providing clinicians a simple mechanism to identify children at risk for associated impairments.
Type of CP and associated impairments
Type of CP was associated with or predictive of associated impairments in our study. Children with quadriplegia had higher risk of epilepsy, intellectual and speech impairments (p<0.01) (table 3). Similar findings were reported by CP registers in Canada and Norway.39 40 In contrast, a German study reported no association between CP type and epilepsy.36
Association among associated impairments
Univariate logistic analysis showed that each associated impairment was associated with an increased likelihood of other associated impairments (p<0.01) (table 3). Children with epilepsy were 3.66 times more likely to have intellectual impairment and 1.99 times more likely to have speech impairment (p<0.01). Children with intellectual impairment were also more likely to have other impairments compared with those without any known intellectual impairment (p<0.001), with OR ranging from 3.21 for visual impairment to 9.02 for hearing impairment. This is unsurprising because CP results from widespread injury to the developing brain,2 which can lead to cocausal impairments.
Risk factors and associated impairments
Several perinatal factors are known to contribute to the causation of CP,36 but their relationships with the associated impairments of CP are poorly understood. We explored the relationship between a range of known risk factors and associated impairments of CP. Term-born children had a higher risk of intellectual impairment (OR=1.42, p<0.05). Although birth weight was not significantly related to associated impairments in our study, previous studies report that children with CP and low birth weight are at significantly higher risk of associated impairments.3541,43
Our study did not find any significant relationship between gestational age and epilepsy. Previous studies report conflicting findings; a Turkish study showed no relationship between prematurity and epilepsy,56 although studies from Israel and Germany reported that epilepsy was more frequent in term infants compared with premature infants.36 44 A study based on data from 17 European registers also showed an association between epilepsy and birth at term.45 It is well known that low gestational age and low birth weight are associated with intellectual impairment and epilepsy in large cohort studies on children without CP.57 58 However, in our cohort, we found that those born at term were at greater risk for intellectual impairment and epilepsy, and no significant association was found between low birth weight and associated impairments. These require further exploration to better understand the various factors potentially contributing to this disparity among the children with CP in our study. Our univariate model showed that only children with maldevelopment of the brain on neuroimaging were more likely to have epilepsy (OR=3.26, p<0.05).
Children with a postneonatal cause of CP had a nine times higher risk of epilepsy and about a four times higher risk of visual impairment compared with those with a pre/perinatal cause (p<0.001). Furthermore, children with a history of perinatal asphyxia had higher risk of visual and speech impairments (OR=3.06 and OR=1.77, p<0.05, respectively). Maternal infection during pregnancy increased the risk of visual impairment (OR=2.28, p<0.05). Microcephalic children were at greater risk for intellectual impairment (OR=1.42, 95% CI 1.03 to 1.96). Future studies should further investigate the causal mechanisms relating to these associations. Several studies explored associated factors of epilepsy in CP. A study conducted in Israel reported that the mode of delivery, head circumference, adjusted birth weight, gender and ethnic group, consanguineous marriage and prematurity were not risk factors for epilepsy in children with CP.44 Similarly, maternal febrile illness during pregnancy, mode of delivery, perinatal asphyxia, neonatal jaundice, infection and nutritional status were not associated with epilepsy.36 Studies which investigated risk factors for other associated impairments were scarce. Although speech impairment is often accompanied by functional limitations of the muscles involved in chewing and swallowing, and thus nutritional status, we did not find any association between nutritional status and speech impairment in our study.
Study imitations
Despite our considerable efforts, we acknowledge certain limitations. First, our study design (ie, descriptive cross-sectional study) limits exploration of causal relationships.
Second, the presence and severity of associated impairments were documented based on clinical history, detailed clinical assessment conducted by clinicians, report by the parents/primary caregivers and review of relevant medical records. In the absence of more comprehensive assessments in our young cohort, some associated impairments, particularly speech, visual and hearing impairment, may have been under-reported. In regions like Vietnam, where the use of best practice tools such as Prechtl’s General Movements Assessment (GMA), Hammersmith Infant Neurological Examination (HINE) and brain MRI is limited for early detection of CP, confirmation of diagnosis can be challenging particularly in young children. Within the National Children Hospital, alternative diagnostic methods employed by experienced paediatricians emphasise on the reliability of comprehensive history taking, clinical assessment and review of medical records. These methods, though effective and often the best alternative in several LMICs in absence of professionals trained in GMA and HINE, come with acknowledged limitations. These challenges have guided our team’s continued advocacy for improved access to these tools in LMIC including Vietnam. Our broad research programme has since ensured training of the first certified GM scorer in Vietnam (THHK), which is a key step towards the implementation of the use of the best practice tools in addition to clinical assessment by experienced clinicians for enhanced diagnostic accuracy for CP in Vietnam.
In our study, experienced paediatricians relied on the DSM-5 criteria and AAIDD guideline for confirmation and classification of severity of intellectual impairment.20 21 This had additional acknowledged limitations, which can be addressed in future studies through professional training and implementation of use of other tools for the assessment of cognitive function among infants and toddlers.59 It is imperative to ensure continued follow-up and referral of all children identified to have probable intellectual impairment through the methods employed in our study for further assessment using age-appropriate validated tests for adaptive function and IQ at the appropriate age.
Third, data on whether caesarean delivery was emergency or planned were not collected. Finally, information on risk factors relied on caregiver report, which could lead to recall bias.
Conclusion
We provided a comprehensive exploration of impairments associated with CP. We confirmed a substantial burden of these impairments among children with CP in a hospital-based cohort in Vietnam and identified a range of factors associated with them including type of CP, GMFCS levels, gestational age, timing of injury causing CP and perinatal asphyxia. Currently, there is no routine screening in place for CP and its associated impairments among children in Vietnam, which should be considered to ensure best outcomes. Findings from our study will inform development and implementation of appropriate interventions necessary to reduce the adverse effects of these impairments on individuals with CP, and thus improve the quality of life of children with CP in Vietnam.
supplementary material
Acknowledgements
We would like to sincerely thank all the families who made this research possible. Thanks to Vinh Son Tran, Xuan Hoa Nguyen and Hong Tuyet Nguyen for their support in data collection.
Footnotes
Funding: This study was supported by the Research Foundation of Cerebral Palsy Alliance (PG03317 and PG6115). EE is supported by an Australian Medical Research Futures Fund and National Health and Medical Research Council (NHMRC) Next Generation Fellowship (1135959). GK is supported by National Health and Medical Research Council (NHMRC) Investigator Grant (APP2009873). TK is supported by the Cerebral Palsy Alliance Research Foundation (ERG01421). The study sponsor played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-075820).
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient consent for publication: Consent obtained from parent(s)/guardians.
Ethics approval: Ethics approval was obtained from the University of Sydney Human Research Ethics Committee (HREC) (2016/456), Hanoi Medical University (HMU) (1722/QD-DHYHN) and NCH (812/QD-BVNTU) in Hanoi, Vietnam. Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Thi Hong Hanh Khuc, Email: honghanhkhuc@gmail.com.
Tasneem Karim, Email: tasneem.karim.tk@gmail.com.
Van Anh Thi Nguyen, Email: nguyenvananh@hmu.edu.vn.
Nguyen Thi Huong Giang, Email: hgiang70@gmail.com.
Trịnh Quang Dũng, Email: quangdzungnip@gmail.com.
Rachael Dossetor, Email: rachael.dossetor@gmail.com.
Chau Cao Minh, Email: chau.caominh@phenikaa-uni.edu.vn.
Nguyen Van Bang, Email: bsbangbmnhiyhn@gmail.com.
Nadia Badawi, Email: nadia.badawi@health.nsw.gov.au.
Gulam Khandaker, Email: gulam.khandaker@health.nsw.gov.au.
Elizabeth Elliott, Email: elizabethelliott1956@gmail.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 2.Colver A, Fairhurst C, Pharoah POD. Cerebral palsy. Lancet. 2014;383:1240–9. doi: 10.1016/S0140-6736(13)61835-8. [DOI] [PubMed] [Google Scholar]
- 3.Fluss J, Lidzba K. Cognitive and academic profiles in children with cerebral palsy: A narrative review. Ann Phys Rehabil Med. 2020;63:447–56. doi: 10.1016/j.rehab.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Novak I, Hines M, Goldsmith S, et al. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics. 2012;130:e1285–312. doi: 10.1542/peds.2012-0924. [DOI] [PubMed] [Google Scholar]
- 5.Sellier E, Platt MJ, Andersen GL, et al. Decreasing prevalence in cerebral palsy: a multi-site European population-based study, 1980 to 2003. Dev Med Child Neurol. 2016;58:85–92. doi: 10.1111/dmcn.12865. [DOI] [PubMed] [Google Scholar]
- 6.Khandaker G, Muhit M, Karim T, et al. Epidemiology of cerebral palsy in Bangladesh: a population-based surveillance study. Dev Med Child Neurol. 2019;61:601–9. doi: 10.1111/dmcn.14013. [DOI] [PubMed] [Google Scholar]
- 7.Australian Cerebral Palsy Register Report of the australian cerebral palsy register, birth years 1995–2012. 2018 https://cpregister.com/wp-content/uploads/2019/02/Report-of-the-Australian-Cerebral-Palsy-Register-Birth-Years-1995-2012.pdf Available.
- 8.McIntyre S, Goldsmith S, Webb A, et al. Global prevalence of cerebral palsy: A systematic analysis. Dev Med Child Neurol. 2022;64:1494–506. doi: 10.1111/dmcn.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandaker G, Van Bang N, Dũng TQ, et al. Protocol for hospital based-surveillance of cerebral palsy (CP) in Hanoi using the Paediatric Active Enhanced Disease Surveillance mechanism (PAEDS-Vietnam): a study towards developing hospital-based disease surveillance in Vietnam. BMJ Open. 2017;7:e017742. doi: 10.1136/bmjopen-2017-017742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palisano RJ, Di Rezze B, Stewart D, et al. Life course health development of individuals with neurodevelopmental conditions. Dev Med Child Neurol. 2017;59:470–6. doi: 10.1111/dmcn.13402. [DOI] [PubMed] [Google Scholar]
- 11.Vadivelan K, Sekar P, Sruthi SS, et al. Burden of caregivers of children with cerebral palsy: an intersectional analysis of gender, poverty, stigma, and public policy. BMC Public Health. 2020;20:645. doi: 10.1186/s12889-020-08808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purpura G, Tagliabue L, Petri S, et al. Caregivers’ Burden of School-Aged Children with Neurodevelopmental Disorders: Implications for Family-Centred Care. Brain Sci. 2021;11:875. doi: 10.3390/brainsci11070875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power R, Muhit M, Heanoy E, et al. Depression, anxiety and stress among caregivers of adolescents with cerebral palsy in rural Bangladesh. Disabil Rehabil. 2021;43:2123–30. doi: 10.1080/09638288.2019.1692378. [DOI] [PubMed] [Google Scholar]
- 14.Zurynski Y, McIntyre P, Booy R, et al. Paediatric active enhanced disease surveillance: a new surveillance system for Australia. J Paediatr Child Health. 2013;49:588–94. doi: 10.1111/jpc.12282. [DOI] [PubMed] [Google Scholar]
- 15.ACPR . Australia: 2018. [10-Aug-2022]. Report of the Australian cerebral palsy register, birth years 1995–2012.https://cpregister.com/wp-content/uploads/2019/02/Report-of-the-Australian-Cerebral-Palsy-Register-Birth-Years-1995-2012.pdf Available. accessed. [Google Scholar]
- 16.Karim T, Dossetor R, Huong Giang NT, et al. Data on cerebral palsy in Vietnam will inform clinical practice and policy in low and middle-income countries. Disabil Rehabil. 2022;44:3081–8. doi: 10.1080/09638288.2020.1854872. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PL, Palisano RJ, Bartlett DJ, et al. Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. 2008;50:249–53. doi: 10.1111/j.1469-8749.2008.02045.x. [DOI] [PubMed] [Google Scholar]
- 18.Eliasson A-C, Krumlinde-Sundholm L, Rösblad B, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–54. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 19.The Washington Group on Disability Statistics Conceptual framework. [10-Jul-2017]. https://www.washingtongroup-disability.com/about/conceptual-framework/ Available. Accessed.
- 20.Boat T. Mental Disorders and Disabilities Among Low-Income Children. Washington (DC): National Academies Press (US); 2015. Clinical characteristics of intellectual disabilities. [PubMed] [Google Scholar]
- 21.Schalock RL, Borthwick-Duffy SA, Bradley VJ, et al. Intellectual disability: definition, classification, and systems of supports: eric. 2010
- 22.Committee to Evaluate the Supplemental Security Income Disability Program for Children with Mental Disorders, Board on the Health of Select Populations, Board on Children Y, and Families . Boat TF, Wu JT, ed. Washington (DC): National Academies Press (US); 2015. Clinical characteristics of intellectual disabilities, vol 9.https://www.ncbi.nlm.nih.gov/books/NBK332877/ Available. [PubMed] [Google Scholar]
- 23.Cerebral Palsy Alliance Research Instutite Australian cerebral palsy register report. 2018
- 24.World Health Organization Blindness and vision impairment. [10-Jul-2017]. https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment Available. Accessed.
- 25.World Health Organization Hearing screening: considerations for implementation. [10-Jul-2022]. https://www.who.int/publications/i/item/9789240032767 Available. Accessed.
- 26.Fisher RS. The New Classification of Seizures by the International League Against Epilepsy 2017. Curr Neurol Neurosci Rep. 2017;17:1–6. doi: 10.1007/s11910-017-0758-6. [DOI] [PubMed] [Google Scholar]
- 27.Himmelmann K, Horber V, De La Cruz J, et al. MRI classification system (MRICS) for children with cerebral palsy: development, reliability, and recommendations. Dev Med Child Neurol. 2017;59:57–64. doi: 10.1111/dmcn.13166. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization . Geneva, Switzerland: 1997. [08-Aug-2022]. WHO global database on child growth and malnutrition.http://apps.who.int/iris/bitstream/10665/63750/1/WHO_NUT_97.4.pdf Available. accessed. [Google Scholar]
- 29.Karim T, Jahan I, Dossetor R, et al. Nutritional Status of Children with Cerebral Palsy-Findings from Prospective Hospital-Based Surveillance in Vietnam Indicate a Need for Action. Nutrients. 2019;11:2132. doi: 10.3390/nu11092132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sydney local health district SLHD guideline: neonatal anthropometry – measurement and reporting of newborn size and growth. 2021
- 31.World Health Organization Head circumference for age. [22-Aug-2022]. https://www.who.int/tools/child-growth-standards/standards/head-circumference-for-age Available. Accessed.
- 32.Andrews C, Kakooza-Mwesige A, Almeida R, et al. Impairments, functional limitations, and access to services and education for children with cerebral palsy in Uganda: a population-based study. Dev Med Child Neurol. 2020;62:454–62. doi: 10.1111/dmcn.14401. [DOI] [PubMed] [Google Scholar]
- 33.Aubert AM, Costa R, Johnson S, et al. Risk factors for cerebral palsy and movement difficulties in 5-year-old children born extremely preterm. Pediatr Res. 2023;94:771–80. doi: 10.1038/s41390-022-02437-6. [DOI] [PubMed] [Google Scholar]
- 34.Jahan I, Al Imam MH, Muhit M, et al. Epidemiology of cerebral palsy among children in the remote Gorkha district of Nepal: findings from the Nepal cerebral palsy register. Disabil Rehabil. 2023;45:2808–17. doi: 10.1080/09638288.2022.2118871. [DOI] [PubMed] [Google Scholar]
- 35.Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr Dis Treat. 2020;16:1505–18. doi: 10.2147/NDT.S235165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karatoprak E, Sözen G, Saltık S. Risk factors associated with epilepsy development in children with cerebral palsy. Childs Nerv Syst. 2019;35:1181–7. doi: 10.1007/s00381-019-04152-w. [DOI] [PubMed] [Google Scholar]
- 37.Cerebral Palsy Alliance Gross motor function classification system. 2018. [31-Jul-2022]. https://cerebralpalsy.org.au/our-research/about-cerebral-palsy/what-is-cerebral-palsy/severity-of-cerebral-palsy/gross-motor-function-classification-system/ Available. Accessed.
- 38.Rauchenzauner M, Schiller K, Honold M, et al. Visual Impairment and Functional Classification in Children with Cerebral Palsy. Neuropediatrics. 2021;52:383–9. doi: 10.1055/s-0040-1722679. [DOI] [PubMed] [Google Scholar]
- 39.Hollung SJ, Bakken IJ, Vik T, et al. Comorbidities in cerebral palsy: a patient registry study. Dev Med Child Neurol. 2020;62:97–103. doi: 10.1111/dmcn.14307. [DOI] [PubMed] [Google Scholar]
- 40.Shevell MI, Dagenais L, Hall N, et al. Comorbidities in cerebral palsy and their relationship to neurologic subtype and GMFCS level. Neurology (ECronicon) 2009;72:2090–6. doi: 10.1212/WNL.0b013e3181aa537b. [DOI] [PubMed] [Google Scholar]
- 41.Horber V, Fares A, Platt MJ, et al. Severity of Cerebral Palsy-The Impact of Associated Impairments. Neuropediatrics. 2020;51:120–8. doi: 10.1055/s-0040-1701669. [DOI] [PubMed] [Google Scholar]
- 42.Ream MA, Lehwald L. Neurologic Consequences of Preterm Birth. Curr Neurol Neurosci Rep. 2018;18:48. doi: 10.1007/s11910-018-0862-2. [DOI] [PubMed] [Google Scholar]
- 43.Ego A, Lidzba K, Brovedani P, et al. Visual-perceptual impairment in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2015;57 Suppl 2:46–51. doi: 10.1111/dmcn.12687. [DOI] [PubMed] [Google Scholar]
- 44.Zelnik N, Konopnicki M, Bennett-Back O, et al. Risk factors for epilepsy in children with cerebral palsy. Eur J Paediatr Neurol. 2010;14:67–72. doi: 10.1016/j.ejpn.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Sellier E, Uldall P, Calado E, et al. Epilepsy and cerebral palsy: characteristics and trends in children born in 1976-1998. Eur J Paediatr Neurol. 2012;16:48–55. doi: 10.1016/j.ejpn.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 46.General Statistics Office. Unicef Children with disabilities in viet nam. 2018. [8-Aug-2022]. https://www.unicef.org/vietnam/media/2776/file/children%20with%20disabilities%20survey%20findings%20vn.pdf Available. Accessed.
- 47.Tuan NA, Cuong LQ, Allebeck P, et al. The prevalence of epilepsy in a rural district of Vietnam: a population-based study from the EPIBAVI project. Epilepsia. 2008;49:1634–7. doi: 10.1111/j.1528-1167.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 48.Jahan I, Al Imam MH, Karim T, et al. Epidemiology of cerebral palsy in Sumba Island, Indonesia. Dev Med Child Neurol. 2020;62:1414–22. doi: 10.1111/dmcn.14616. [DOI] [PubMed] [Google Scholar]
- 49.Surveillance of Cerebral Palsy in Europe Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002;44:633–40. doi: 10.1111/j.1469-8749.2002.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsige S, Moges A, Mekasha A, et al. Cerebral palsy in children: subtypes, motor function and associated impairments in Addis Ababa, Ethiopia. BMC Pediatr. 2021;21:544. doi: 10.1186/s12887-021-03026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabis LV, Tsubary NM, Leon O, et al. Assessment of Abilities and Comorbidities in Children With Cerebral Palsy. J Child Neurol. 2015;30:1640–5. doi: 10.1177/0883073815576792. [DOI] [PubMed] [Google Scholar]
- 52.Germany L, Ehlinger V, Klapouszczak D, et al. Trends in prevalence and characteristics of post-neonatal cerebral palsy cases: a European registry-based study. Res Dev Disabil. 2013;34:1669–77. doi: 10.1016/j.ridd.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Mitry D, Williams C, Northstone K, et al. Perceptual visual dysfunction, physical impairment and quality of life in Bangladeshi children with cerebral palsy. Br J Ophthalmol. 2016;100:1245–50. doi: 10.1136/bjophthalmol-2015-307296. [DOI] [PubMed] [Google Scholar]
- 54.Shevell MI, Majnemer A, Poulin C, et al. Stability of motor impairment in children with cerebral palsy. Dev Med Child Neurol. 2008;50:211–5. doi: 10.1111/j.1469-8749.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288:1357–63. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- 56.Mert GG, Incecik F, Altunbasak S, et al. Factors affecting epilepsy development and epilepsy prognosis in cerebral palsy. Pediatr Neurol. 2011;45:89–94. doi: 10.1016/j.pediatrneurol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Pisani F, Spagnoli C. Outcome in preterm infants with seizures. Handb Clin Neurol. 2019;162:401–14. doi: 10.1016/B978-0-444-64029-1.00019-9. [DOI] [PubMed] [Google Scholar]
- 58.Schüssler SC, Schmidt M, Deiters L, et al. Long-term outcomes of very-low-birth-weight and low-birth-weight preterm newborns with neonatal seizures: A single-center perspective. Eur J Paediatr Neurol. 2022;36:137–42. doi: 10.1016/j.ejpn.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Weiss LG, Oakland T, Aylward GP. Bayley-III Clinical Use and Interpretation. Academic Press; 2010. [Google Scholar]