Abstract
Abstract
Objectives
This study represents a pioneering attempt to quantify the contribution of age, sex and socioeconomic status (SES) to the observed inequalities in lipid profile components.
Design
Cross-sectional study.
Setting
The data from the Ravansar Non-Communicable Disease (RaNCD) Cohort Study were used.
Participants
10 000 individuals aged 35–65 years.
Main outcome measures
Principal component analysis was used to determine the SES of individuals. Using the concentration index (C-index) and curves, the study assessed socioeconomic inequalities in dyslipidaemia in different age groups and genders. Decomposition analysis was used to determine the contribution of sex, age and SES to the observed inequality in the prevalence of dyslipidaemia components between the wealthiest and poorest groups.
Results
The prevalence of dyslipidaemia was 72.39% of the population and was significantly higher in women than in men (excluding hypertriglyceridaemia). Overall, no significant SES-based inequality in dyslipidaemia was observed (C-index=−0.045, p=0.116), but after adjustment for age and sex, individuals with high SES had increased odds of dyslipidaemia (OR=1.16, 95% CI: 1.03 to 1.31). Hypercholesterolaemia and hyper-low-density lipoprotein (LDL) were more common in individuals with lower SES (C-index=−0.117 and −0.105), while hypo-high-density lipoprotein (HDL) was more prevalent in individuals with higher SES (C-index=0.029), regardless of adjustment for age, sex and confounding factors. SES played a significant role in hypercholesterolaemia and hyper-LDL (322.11% and 400.14%), while sex dominated in hypertriglyceridaemia and hypo-HDL (814.05% and −615.26%) and contributed to the existing inequalities.
Conclusion
The results highlight the existing inequalities in lipid profiles due to SES, sex and age. Consideration of these factors in interventions and policy decisions is critical to reduce abnormalities and inform future interventions.
Keywords: Public Health, Health Equity, Epidemiologic Studies
Strengths and limitations of this study.
This study was the first to examine the quantified role of age, sex and socioeconomic status in lipid profile component inequality in the context of a population-based study.
The current sample is derived from the Ravansar Non-Communicable Disease Cohort Study which is part of the larger PERSIAN (Prospective Epidemiological Research Studies in IrAN) Cohort.
This study did not examine inequality in access to and adoption of therapeutic interventions, whereas the existing inequality in dyslipidaemia components may decrease or increase with treatment adoption.
As diet plays a central and influential role in the components of the lipid profile, the lack of this data prevented the investigation of the dietary behaviour of the participants in the different groups.
Introduction
Cardiovascular disease (CVD) is the leading cause of worldwide mortality, resulting in approximately 17.9 million deaths annually.1 In Iran, a developing country, CVD is responsible for half of mortality and contributes to 20–23% of the total disease burden.2 On the other hand, atherosclerosis is a major risk factor for CVD and dyslipidaemia, characterised by abnormal levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol, or a combination of these components has been shown contribute to the development of atherosclerosis.3 Evidence shows that a 1% reduction in mean population levels of TC leads to a decrease in CVD mortality of approximately 2.5%.4 Similarly, a 40 mg/dL reduction in LDL cholesterol is associated with a corresponding 22% decrease in CVD mortality and morbidity.5
The prevalence of dyslipidaemia varies in different regions; approximately 30–60% of the population is affected by dyslipidaemia, with hypercholesterolaemia ranging from 22.6% to 54% in Africa, Southeast Asia, Europe and the Americas.6,9 Notably, in the Iranian population, the prevalence rates were reported as 41.6% for hypercholesterolaemia, 46.0% for hypertriglyceridaemia, 35.5% for hyper-LDL and 43.9% for hypo-HDL.10
In addition, considering the multifaceted nature of dyslipidaemia, it is clear that this condition is influenced by numerous factors, including age, sex, socioeconomic status (SES), level of fat intake and obesity.11 12 SES is commonly defined as a combination of education, income level, occupational status and place of residence.13 14 Numerous studies have been conducted to investigate the socioeconomic indicators contributing to dyslipidaemia development in the Iranian population.1015,17 A national study by Soleimani et al on urban-dwelling and rural-dwelling adults in all 31 provinces of Iran showed lower HDL levels and higher rates of hypercholesterolaemia and hypertriglyceridaemia among urban male population with lower income, lower urbanisation and lower education levels. While in women, lower levels of urbanisation and education were associated with higher rates of hypercholesterolaemia and hypertriglyceridaemia and lower income levels were associated with hypertriglyceridaemia.15 In addition, a systematic review of 29 studies by Tabatabaei-Malazy et al indicated a sex-specific difference in the prevalence of dyslipidaemia such that hypercholesterolaemia, hyper-LDL and hypo-HDL were more prevalent in women and hypertriglyceridaemia was more reported in men.10
The differences in the prevalence of individual lipid profile components and dyslipidaemia as a whole may be due to the prevailing socioeconomic inequalities between people. Inequalities can be defined as systematic disparities in health that could be mitigated by appropriate interventions, as they are due to an unequal distribution of health risks and resources.18 19 According to a widely accepted definition, the perpetuation of avoidable and unnecessary health inequalities is considered unjustifiable.20 Age and sex are important determinants of the emergence of these inequalities. While younger people tend to have better access to educational and health resources and a wider range of employment opportunities,21 they often have fewer financial resources and are more affected by housing insecurity than their older peers within and across socioeconomic groups.22 These circumstances can have a tangible impact on the lifestyle choices they make, which translates into unequal health status. In terms of sex, women in different socioeconomic groups face different educational and occupational conditions, as well as differences in work-life balance, which in turn has a significant impact on their overall health.23
Assessing the extent of inequalities within a society is crucial for setting targets to promote change. Although previous studies conducted in Iran and other countries have examined the association between socioeconomic factors and components of lipid profile, these studies have not comprehensively assessed the extent of inequalities in the components of lipid profile or identified the factors responsible for these inequalities. Therefore, the present study aims to improve our understanding of socioeconomic inequalities in dyslipidaemia and lipid profile components by analysing the effects of sex and age on these inequalities, as well as the contribution of age, sex and SES in the existing inequalities among participants in the Ravansar cohort study.
Methods
Study population and measurements
For this cross-sectional study, data were used from the Ravansar Non-Communicable Disease (RaNCD) Cohort Study, which is part of the larger PERSIAN (Prospective Epidemiological Research Studies in IrAN) Cohort. The RaNCD cohort is a population-based prospective study that includes at least 10 000 individuals aged 35–65 years. More details on this cohort can be found elsewhere.24 Trained interviewers used a pretested demographic and clinical information form of the Persian cohort questionnaire to collect the data on age, sex, smoking status, alcohol consumption, physical activity, anthropometric characteristics and comorbidities (CVD, diabetes and hypertension). The comprehensive data collection and measurement details have been described elsewhere.25,29 Briefly, the participants were categorised into never-smokers and passive smokers and current and ex-smokers. Physical activity was measured based on physical activity over 24 hours and a 22-item questionnaire (metabolic equivalents=METs). Then METs were classified as low (24–36.5 MET/hours per day), moderate (36.6–44.4 MET/hours per day) and vigorous (≥44.5 MET/hours per day).30 Weight and height measurements were conducted using the InBody 770 BioSpace device (Korea) and the BSM 370 (Biospace, Seoul, Korea), respectively, with 0.5 kg and 0.1 cm precision. The body mass index (BMI) was calculated using the formula weight (kg)/height2 (m). In addition, the waist-to-height ratio (WHtR) was defined as the waist circumference (cm) divided by the height (cm).29 Seven missing data on SES, 71 missing data on lipid profile and 136 pregnant women were excluded from the analysis and data from 9832 individuals at baseline were included in the study. All participants provided written informed consent, and the Kermanshah University of Medical Sciences Review Board approved the study.
Definition and assessment
Dyslipidaemia and its components
Dyslipidaemia was defined as hypercholesterolaemia, and/or hypertriglyceridaemia, and/or hyper-LDL, and/or hypo-HDL based on the National Cholesterol Education Program Adult Treatment Panel III classification of lipid profile.31 Hypercholesterolaemia was defined as TC≥200 mg/dL, hypertriglyceridaemia as TG≥150 mg/dL, hyper-LDL as LDL≥130 mg/dL and hypo-HDL as HDL<40 mg/dL in men and <50 mg/dL in women.
Socioeconomic status
Principal component analysis (PCA) was used to determine the SES of individuals. The PCA analysis considered the possession of various assets (such as freezers, washing machines, dishwashers, microwaves, vacuum cleaners, television at the household, personal computer access to the internet, motorcycle and car (based on its price), having a mobile phone, computer, laptop, access to the internet and car (based on its price) for personal use), the status of the house (owned, rented or leased, relative’s house, etc), area per capita (house area per family number), rooms per capita (number of bedrooms per family number), number of books read in the last year (excluding school books, those required for a job and religious scriptures), international trips in a lifetime (never, pilgrimage only, both pilgrimage and non-pilgrimage trips), residency and education level. The asset index derived from the PCA served as a simple and efficient method for collecting data on SES. Polychoric PCA was used for this study because it includes both quantitative and qualitative variables that are highly correlated. Subsequently, all SES-related variables were transformed into a cardinal variable representing SES. Then, SES was classified into five ranks, ranging from the poorest to the richest (five groups: the poorest, poor, middle, rich and the richest).
Statistical analysis
Descriptive statistics was performed using the mean (standard deviation=SD) and number (percentage). The different variables in both sexes and according to age groups were compared using t-test and χ2 test. The concentration index (C-index) and a concentration curve were used to assess socioeconomic inequality in dyslipidaemia by sex and age. The concentration index was calculated using the normalised Wagstaff et al formula with the ‘conindex’ command.32 The concentration curve was obtained by plotting the cumulative percentage of dyslipidaemia and its components on the y-axis against the cumulative percentage of the ratio of the poorest to the richest socioeconomic groups plotted on the x-axis. When the curve is above the diagonal line, the C-index takes negative values, indicating a concentration of dyslipidaemia in low-SES groups. On the other hand, when the curve is below the line of equality, positive values of the C-index indicate the concentration of the outcome in high SES groups.33 34 In addition to the C-index, logistic regression was performed to examine the association between SES and the prevalence of dyslipidaemia and its components. Odds Ratio (OR) and 95% confidence intervals (CI) were reported. Decomposition analysis was used to determine the contributions of sex, age and SES to the differences in the prevalence of dyslipidaemia components between the poorest and richest groups based on the study by Mosquera et al35 and a probit regression model was used to estimate the marginal effects of socioeconomic determinants on the health variable. Weighted means of the dyslipidaemia components and concentration indices for each determinant were calculated. The elasticity of the dyslipidaemia components considering the determinants was also calculated. The unique contribution of each determinant was quantified to understand its role in explaining SES inequalities in dyslipidaemia components. Data analysis was performed using Stata V.14 software, and a significance level of p<0.05 was used.
Patient and public involvemen
Results
The mean age of the 9832 participants was 47.36±8.27 years. 51.96% (5109) of participants were women (sex ratio: 1.08 women/men). 59.74% of participants resided in urban areas. More than 90% of individuals were married. The frequency of current and ex-smokers is found to be higher in men than in women, with a prevalence of 36.96% in men. Alcohol consumption was lower in women and individuals ≥50 years, with a total frequency of 4.88%. Individuals with low physical activity were more common among men and individuals ≥50 years, with a total frequency of 30.34%. In addition, mean anthropometric indices were higher among women. About 25.74% of participants reported at least one comorbidity, and the frequency was higher among women and those ≥50 years (table 1).
Table 1. Frequency and distribution of different variables by sex and age group.
| Variables | Total (9832) N (%) | Male (4723)N (%) | Female (5109)N (%) | P value* | <50 years (6069)N (%) | ≥50 years (3763)N (%) | P value* |
| Age (year)† | 47.36 (8.27) | 47.01 (8.08) | 47.68 (8.43) | 0.001 | – | – | – |
| Sex (male) | 4723 (48.04) | – | – | – | 3002 (49.46) | 1721 (45.73) | <0.001 |
| Education (year)† | 5.41 (4.82) | 7.47 (4.95) | 3.50 (3.81) | <0.001 | 6.94 (4.65) | 2.94 (4.01) | <0.001 |
| Marital status | |||||||
| Single | 417 (4.24) | 94 (1.99) | 323 (6.32) | <0.001 | 398 (6.56) | 19 (0.50) | <0.001 |
| Married | 8871 (90.23) | 4590 (97.18) | 4281 (83.79) | 5483 (90.34) | 3388 (90.03) | ||
| Other | 414 (5.53) | 39 (0.83) | 505 (9.88) | 188 (3.10) | 356 (9.46) | ||
| Residency | |||||||
| Urban | 5874 (59.74) | 2921 (61.85) | 2953 (57.30) | <0.001 | 3781 (62.30) | 2093 (55.62) | <0.001 |
| Rural | 3958 (40.26) | 1802 (38.15) | 2156 (42.20) | 2288 (37.70) | 1670 (44.38) | ||
| Socioeconomic status | |||||||
| The poorest | 1944 (19.77) | 464 (9.82) | 1480 (28.97) | <0.001 | 905 (14.91) | 1039 (27.61) | <0.001 |
| Poor | 1958 (19.91) | 723 (15.31) | 1235 (24.17) | 1095 (18.04) | 863 (22.93) | ||
| Middle | 1969 (20.03) | 931 (19.71) | 1038 (20.32) | 1242 (20.46) | 727 (19.32) | ||
| Rich | 1976 (20.10) | 1117 (23.65) | 859 (16.81) | 1331 (21.93) | 645 (17.14) | ||
| The richest | 1985 (20.19) | 1488 (31.51) | 497 (9.73) | 1496 (24.65) | 489 (12.99) | ||
| Smoking status | |||||||
| Non-smoker and passive smoker | 7767 (79.43) | 2961 (63.04) | 4806 (94.57) | <0.001 | 5029 (83.26) | 2738 (73.23) | <0.001 |
| Current and ex-smokers | 2012 (20.57) | 1736 (36.96) | 276 (5.43) | 1011 (16.74) | 1001 (26.77) | ||
| Alcohol consumption | |||||||
| Yes | 480 (4.88) | 478 (10.12) | 2 (0.04) | <0.001 | 354 (5.83) | 126 (3.35) | <0.001 |
| No | 9352 (95.12) | 4245 (89.88) | 5107 (99.96) | 5715 (94.17) | 3637 (96.65) | ||
| Physical activity (MET hour/day) | |||||||
| Low | 2983 (30.34) | 1646 (34.85) | 1337 (26.17) | <0.001 | 1759 (28.98) | 1224 (32.53) | 0.001 |
| Moderate | 4656 (47.36) | 1445 (30.59) | 3211 (62.85) | 2951 (48.62) | 1705 (45.31) | ||
| High | 2193 (22.30) | 1632 (34.55) | 561 (10.98) | 1359 (22.39) | 834 (22.16) | ||
| Anthropometry† | |||||||
| Body mass index, kg/m2 | 27.45 (4.75) | 26.29 (4.20) | 28.51 (4.98) | <0.001 | 27.57 (4.75) | 27.24 (4.74) | 0.001 |
| Waist circumference, cm | 97.28 (10.51) | 96.20 (9.69) | 98.27 (11.13) | <0.001 | 96.56 (10.44) | 98.44 (10.53) | <0.001 |
| Waist-to-height ratio | 0.59 (0.07) | 0.56 (0.05) | 0.63 (0.07) | <0.001 | 0.59 (0.07) | 0.61 (0.07) | <0.001 |
| Comorbidities (yes) | |||||||
| Cardiovascular disease | 1676 (17.05) | 583 (12.34) | 1093 (21.39) | <0.001 | 494 (8.14) | 1182 (31.41) | <0.001 |
| Diabetes | 854 (8.69) | 385 (8.15) | 469 (9.18) | 0.071 | 335 (5.52) | 519 (13.79) | <0.001 |
| Hypertension | 1550 (15.76) | 689 (14.59) | 861 (16.85) | 0.002 | 475 (7.83) | 1075 (28.57) | <0.001 |
| Dyslipidaemia and its components (yes) | |||||||
| Dyslipidaemia | 7117 (72.39) | 3239 (68.58) | 3878 (75.91) | <0.001 | 4276 (70.46) | 2841 (75.50) | <0.001 |
| Hypercholesterolaemia | 3105 (31.58) | 1336 (28.29) | 1769 (34.63) | <0.001 | 1588 (26.17) | 1517 (40.31) | <0.001 |
| Hypertriglyceridaemia | 3103 (31.56) | 1710 (36.21) | 1393 (27.27) | <0.001 | 1838 (30.29) | 1265 (33.62) | 0.001 |
| Hyper-LDL | 2506 (25.49) | 1113 (23.57) | 1393 (27.27) | <0.001 | 1268 (20.89) | 1238 (32.90) | <0.001 |
| Hypo-HDL | 4835 (49.18) | 2025 (42.88) | 2810 (55.00) | <0.001 | 3065 (50.50) | 1770 (47.04) | 0.001 |
HypercholesterolemiaHypercholesterolaemia was defined as TC≥200; Hypertriglyceridemiahypertriglyceridaemia was defined as TG≥150 mg/dL; Hhyper-LDL was defined as LDL≥130 mg/dL; Hhypo-HDL was defined as HDL<40 mg/dL in men and <50 mg/dL in women.
Based on χ2 test. Bold values are significant.
Mean (SD) and p value based on t-test.
HDLhigh-density lipoprotein LDLlow-density lipoprotein METmetabolic equivalentTCtotal cholesterol TGtriglycerides
Prevalence of dyslipidaemia by sex and age group
The prevalence of dyslipidaemia was 72.39%, and the prevalence of hypercholesterolaemia, hypertriglyceridaemia, hyper-LDL and hypo-HDL were 31.58%, 31.56%, 25.49% and 49.18%, respectively. The prevalence of dyslipidaemia and all its components was significantly higher in women than in men (except hypertriglyceridaemia). The prevalence of dyslipidaemia was 70.46% in persons <50 years and 75.50% in persons ≥50 years (table 1). Online supplemental figure 1 shows the stratification of TC, TG, LDL and HDL by SES and age groups for men and women. TC levels increased with age, and women with higher SES tended to have lower TC levels compared with men. In women, TG levels increased with age. In addition, TG levels were generally higher in men and individuals with high SES, particularly in individuals <50 years. LDL levels in women showed higher values in lower SES for two age groups. Men generally had lower LDL levels than women, especially at lower SES, and also individuals ≥50 years. Across all age groups, men had lower HDL levels than women and HDL levels decreased with increasing SES (online supplemental figure 1).
Prevalence of dyslipidaemia and its components in men and women and age groups by socioeconomic status
Dyslipidaemia was found to be most prevalent in women and individuals ≥50 years of age with low SES. Conversely, the highest prevalence of dyslipidaemia in men and individuals aged <50 years was found in those with high SES. Both men and women of low SES had a higher prevalence of hypercholesterolaemia and hyper-LDL, while the prevalence of hypertriglyceridaemia were higher in men of high SES and in every age group, but higher in women of low SES. Hypo-HDL prevalence increased with increasing socioeconomic level, but decreased in the richest individuals (table 2).
Table 2. Prevalence of dyslipidaemia and its components in men and women and age groups by socioeconomic status.
| Variables | SES group | Total (%) | Male (%) | Female (%) | <50 years (%) | ≥50 years (%) |
| Dyslipidaemia | The poorest | 73.04 | 64.22 | 75.81 | 68.50 | 76.99 |
| Poor | 72.98 | 65.00 | 77.65 | 70.50 | 76.12 | |
| Middle | 72.52 | 67.45 | 77.07 | 71.01 | 75.10 | |
| Rich | 72.72 | 71.35 | 74.50 | 71.67 | 74.88 | |
| The richest | 70.68 | 70.29 | 71.83 | 70.05 | 72.59 | |
| P for trend | 0.116 | <0.001 | 0.077 | 0.430 | 0.058 | |
| Hypercholesterolaemia | The poorest | 39.91 | 32.54 | 42.22 | 30.82 | 47.83 |
| Poor | 33.40 | 29.87 | 35.46 | 25.93 | 42.87 | |
| Middle | 29.35 | 26.20 | 32.17 | 24.07 | 38.37 | |
| Rich | 29.45 | 29.63 | 29.22 | 27.12 | 34.26 | |
| The richest | 25.94 | 26.47 | 24.34 | 24.39 | 30.67 | |
| P for trend | <0.001 | 0.029 | <0.001 | 0.010 | <0.001 | |
| Hypertriglyceridaemia | The poorest | 28.65 | 31.03 | 27.90 | 24.53 | 32.22 |
| Poor | 31.00 | 34.71 | 28.82 | 28.40 | 34.29 | |
| Middle | 31.38 | 35.33 | 27.84 | 30.67 | 32.59 | |
| Rich | 33.14 | 37.51 | 27.47 | 32.15 | 35.19 | |
| The richest | 33.55 | 38.10 | 19.91 | 33.15 | 34.76 | |
| P for trend | <0.001 | 0.003 | 0.009 | <0.001 | 0.270 | |
| Hyper-LDL | The poorest | 31.58 | 28.44 | 32.56 | 23.64 | 38.49 |
| Poor | 27.01 | 23.37 | 29.14 | 21.00 | 34.64 | |
| Middle | 24.12 | 23.41 | 24.75 | 19.00 | 32.87 | |
| Rich | 24.64 | 25.24 | 23.86 | 22.61 | 28.83 | |
| The richest | 20.20 | 20.96 | 17.90 | 19.18 | 23.31 | |
| P for trend | <0.001 | 0.008 | <0.001 | 0.071 | <0.001 | |
| Hypo-HDL | The poorest | 44.13 | 32.97 | 47.63 | 47.07 | 41.57 |
| Poor | 50.35 | 36.09 | 58.70 | 51.78 | 48.55 | |
| Middle | 51.85 | 44.79 | 58.18 | 52.97 | 49.93 | |
| Rich | 50.96 | 45.47 | 58.09 | 50.63 | 51.62 | |
| The richest | 48.51 | 46.10 | 55.73 | 49.46 | 45.60 | |
| P for trend | 0.009 | <0.001 | <0.001 | 0.727 | 0.006 |
Bold values are significant.
HDLhigh-density lipoproteinLDLlow-density lipoproteinSES, socioeconomic status
Socioeconomic inequality in dyslipidaemia and its components by sex and age based on SES
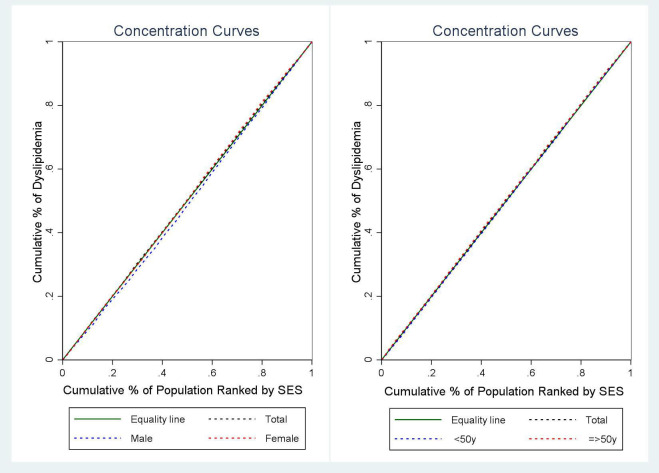
Regarding SES, the concentration index for the prevalence of dyslipidaemia was 0.058 in men (95% CI: 0.024, 0.093; p=0.001), –0.027 in women (95% CI: −0.063, 0.008; p=0.136) and −0.020 in the entire participant population (95% CI: −0.045, 0.004; p=0.116). The CI indicates statistical significance only for men. Furthermore, considering age groups, the concentration index for the prevalence of dyslipidaemia was 0.011 in individuals <50 years old (95% CI: −0.020, 0.042; p=0.488) and −0.039 in those aged 50 years or older (95% CI: −0.081, 0.002; p=0.063). The results indicate that there is no significant inequality in dyslipidaemia by age group (figure 1).
Figure 1. Concentration curve of dyslipidaemia based on SES by sex and age group. SES, socioeconomic status.
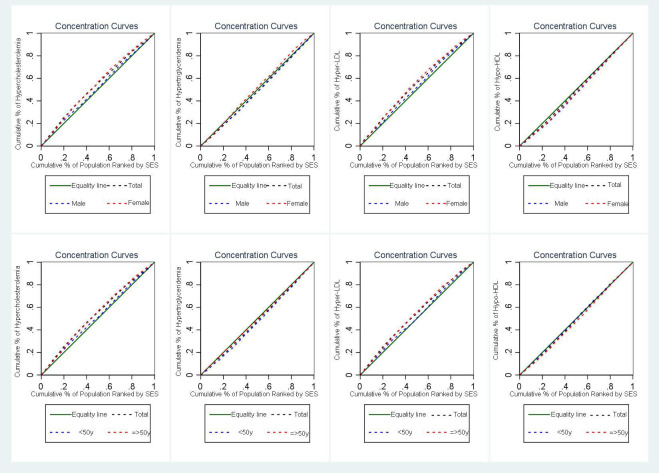
The concentration index for the prevalence of hypercholesterolaemia, hypertriglyceridaemia, hyper-LDL and hypo-HDL was −0.117 (95% CI: −0.141 to –0.093; p<0.001), 0.044 (95% CI: 0.020, 0.068; p<0.001), –0.105 (95% CI: −0.131 to –0.080; p<0.001), 0.029 (95% CI: 0.007, 0.051; p=0.010), respectively. In addition, the concentration index for hypercholesterolaemia and hyper-LDL was significantly negative according to sex, indicating that the prevalence of these conditions is higher in individuals with lower SES in both men and women. The C-index for hypo-HDL was significantly positive by sex, suggesting that the prevalence of hypo-HDL is higher in individuals with higher SES in both men and women. The C-index for hypertriglyceridaemia was significantly positive in men and negative in women, indicating that the prevalence of hypertriglyceridaemia is higher in individuals with higher SES in men and individuals with lower SES in women.
When age group was considered, the C-index was negative for hypercholesterolaemia and hyper-LDL, but statistically significant for hypercholesterolaemia in both age groups and for hyper-LDL only in individuals ≥50 years. The C-index was significantly positive for hypertriglyceridaemia only in individuals <50 years and for hypo-HDL only in individuals 50 years or older (figure 2).
Figure 2. Distribution of mean of total cholesterol, triglycerides, low-density lipoprotein (LDL) and high density lipoprotein (HDL) by socioeconomic status in men and women for age group. SES, socioeconomic status.
Association of dyslipidaemia and its components with SES
The univariable logistic regression analysis showed no significant association between dyslipidaemia and SES level. However, after adjustment for sex and age, the odds of dyslipidaemia was higher in individuals with high SES than in individuals with low SES (OR=1.16; 95% CI: 1.03 to 1.31). In addition, both the univariable and multivariable models showed that the odds of hypercholesterolaemia and hyper-LDL were significantly lower in those with higher SES than in those with lower SES (between 20% and 38% for hypercholesterolaemia and between 19% and 34% for hyper-LDL). Conversely, the OR for hypo-HDL was significantly higher in individuals with lower SES than in individuals with higher SES (ranging from 13% to 39%). Furthermore, in the multivariable model adjusted for sex and age, the odds of hypertriglyceridaemia were higher in individuals with higher SES than in individuals with lower SES, while this association was found to be inversely significant after adjustment for other variables, indicating a 16% lower prevalence of hypertriglyceridaemia in individuals with high SES (online supplemental table 1).
Decomposition of dyslipidaemia components
The concentration index for the predictor variables (CIk) indicates a disproportionate concentration of women among the poor for all dyslipidaemia components, as shown by the negative values. In addition, individuals aged <50 years were mainly concentrated among the rich, as shown by the positive CIk values. The positive marginal effect for women for hypercholesterolaemia and for women and persons <50 years for hypo-HDL implies that the determinant has a positive association with the outcome and a higher probability of hypercholesterolaemia and hypo-HDL. The results of the decomposition analysis showed that SES was the largest contributor to the observed inequalities in hypercholesterolaemia (322.11%), hyper-LDL (400.14%) and hypo-HDL (473.07%), whereas in hypertriglyceridaemia, sex was the largest contributor to the observed inequalities (814.05%). In addition, sex acts as an equalising factor in hypo-HDL (−615.26%). This means that, on average, women have a more even SES distribution than men, leading to a reduction in overall SES inequality (table 3).
Table 3. Decomposition results for inequality in dyslipidaemia components.
| Dyslipidaemia components | Marginal effect* | Elasticity | CIk | Contribution | % | Summed % |
| Hypercholesterolaemia | ||||||
| Age <50 | −0.490 | −0.959 | 0.094 | −0.090 | 10.76 | 10.76 |
| Female | 0.018 | 0.030 | −0.196 | −0.006 | 7.170 | 7.170 |
| Poor | −0.250 | −0.158 | −0.399 | 0.063 | −75.26 | 322.11 |
| Middle | −0.407 | −0.257 | 0.000 | 0.000 | 0.030 | |
| Rich | −0.387 | −0.245 | 0.400 | −0.098 | 116.36 | |
| The richest | −0.468 | −0.296 | 0.800 | −0.237 | 280.98 | |
| Hypertriglyceridaemia | ||||||
| Age <50 | 0.000 | 0.000 | 0.094 | 0.000 | −0.060 | −0.060 |
| Female | −0.795 | −1.309 | −0.196 | 0.256 | 814.05 | 814.05 |
| Poor | −0.043 | −0.027 | −0.399 | 0.011 | 34.860 | −457.15 |
| Middle | −0.108 | −0.068 | 0.000 | 0.000 | −0.020 | |
| Rich | −0.129 | −0.082 | 0.400 | −0.032 | −104.10 | |
| The richest | −0.241 | −0.152 | 0.800 | −0.122 | −387.89 | |
| Hyper-LDL | ||||||
| Age <50 | −0.511 | −1.238 | 0.094 | −0.117 | 146.07 | 146.07 |
| Female | −0.044 | −0.092 | −0.196 | 0.017 | −22.081 | −22.081 |
| Poor | −0.175 | −0.138 | −0.399 | 0.055 | −68.785 | 400.14 |
| Middle | −0.322 | −0.253 | 0.000 | 0.000 | 0.032 | |
| Rich | −0.273 | −0.214 | 0.400 | −0.085 | 106.92 | |
| The richest | −0.462 | −0.363 | 0.800 | −0.290 | 361.98 | |
| Hypo-HDL | ||||||
| Age <50 | 0.194 | 0.244 | 0.094 | 0.023 | 132.45 | 132.45 |
| Female | 0.518 | 0.547 | −0.196 | −0.107 | −615.26 | −615.26 |
| Poor | 0.231 | 0.094 | −0.399 | −0.0377 | −215.98 | 473.07 |
| Middle | 0.313 | 0.127 | 0.000 | 0.000 | 0.074 | |
| Rich | 0.269 | 0.109 | 0.400 | 0.043 | 251.47 | |
| The richest | 0.234 | 0.095 | 0.800 | 0.076 | 437.51 | |
Marginal effects from the probit model; Aadjusted for BMIbody mass index, WHtRwaist-to-height ratio, physical activity, smoking status, alcohol consumption, and comorbidities.
CIkconcentration index for the predictor variablesHDLhigh-density lipoproteinLDLlow-density lipoprotein
Discussion
Our results showed distinct patterns that are both sex-specific and age-specific. This indicates that the effects observed in our study vary between different sexes and across age groups. While hypertriglyceridaemia and hypo-HDL had a higher prevalence in men aged ≥50 years and women aged <50 years, respectively, dyslipidaemia and other components of the lipid profile were significantly more prevalent in women aged ≥50 years than in their peers. Socioeconomic inequality was reflected in a higher prevalence of hypercholesterolaemia and hyper-LDL in lower-SES individuals and a higher prevalence of hypo-HDL in higher-SES individuals, whether or not age, sex and other confounders were taken into account. Dyslipidaemia was associated with higher SES only in the age-adjusted and sex-adjusted analysis. Sex was found to be the most important factor contributing to inequalities in hypertriglyceridaemia and hypo-HDL, exceeding the influences of SES and age. In contrast, SES was more strongly associated with hypercholesterolaemia and hyper-LDL. Of note, age had the least influence on all lipid profile components.
The prevalence of dyslipidaemia and lipid abnormalities in plasma may vary by region, population and over time due to changes in lifestyle and health practices. Our results show that approximately 72.39% of the population studied had at least one abnormality in lipid profile components, with a significantly higher prevalence in women than in men. This sex disparity extends to all lipid profile components, with the exception of TG. In addition, women had a higher incidence of comorbidities, particularly CVD, which may be due to a higher rate of the aforementioned abnormalities. The exact mechanisms contributing to these sex differences are not yet fully understood, but are likely influenced by hormonal, genetic and lifestyle factors.36 Our findings are consistent with existing research suggesting a correlation between unfavourable anthropometric indices such as BMI, waist circumference and WHtR in women, justifying the observed higher rates of plasma lipid abnormalities. Considering participant characteristics in our study, it is noticeable that women participants had lower educational attainment, lower SES and lower engagement in physical activity compared with their men counterparts. Research shows that lower levels of education and lower social status can negatively impact health literacy, access to healthcare and the ability to make informed decisions related to well-being, such as dietary choices and health-related behaviours.37 In addition, the observed gender disparities in access to physical activity equipment and resources in Iran may contribute to these findings.38 These findings highlight the need for targeted interventions to mitigate the effects of dyslipidaemia and associated cardiovascular risks, particularly in vulnerable populations.
Consistent with the studies conducted on the prevalence of dyslipidaemia and plasma lipid abnormalities in different age groups, the current study showed that except for a significant prevalence of hypo-HDL in individuals younger than 50 years with the older age group, dyslipidaemia and other lipid profile components are more prevalent in the older age group.39,41 The literature suggests that ageing plays a crucial role in the increase of lipid profile components, which is due to a decrease in metabolic rate and changes in body fat distribution.42 43 In addition, in women, hormonal fluctuations, especially in the postmenopausal phase, which is characterised by a decrease in oestrogen levels, contribute to an increase in LDL and a decrease in HDL.44 Furthermore, ageing provides a prolonged period for the cumulative effects of unhealthy lifestyle habits, including unhealthy dietary habits, physical inactivity and smoking.45 These factors are known to influence the components of the lipid profile. The elderly population, characterised by a higher prevalence of disease, higher medication use and particular age-related characteristics, requires increased attention in the study of factors associated with lipid abnormalities in plasma.
In this study, the socioeconomic distribution showed that 51.16% of men and 26.54% of women belonged to the rich groups. Socioeconomic differences were always evident in the prevalence of hypercholesterolaemia, hyper-LDL and hypo-HDL, regardless of the influence of confounding factors; thus, hypercholesterolaemia and hyper-LDL were more common in lower SES individuals, whereas hypo-HDL was more common in higher SES individuals. Despite the absence of socioeconomic inequality in dyslipidaemia in women, which may be due to the significant and high prevalence of hypo-HDL (55%); socioeconomic inequalities in lipid profile components such as hypercholesterolaemia, hypertriglyceridaemia and hyper-LDL were mainly found in poor participants. Among men, the prevalence of hypercholesterolaemia and hyper-LDL was significantly higher in participants with lower SES. However, there was a shift in the overall prevalence of dyslipidaemia towards higher SES individuals, as the prevalence of hypertriglyceridaemia and hypo-HDL was higher in both the overall male population and in the rich group. One possible reason for the observed patterns could be the difference in access to health resources and lifestyle factors, which is associated with different SES in men. Specifically, higher SES in men may facilitate access to unhealthy behaviours, which include a diet of unhealthy and processed foods and high alcohol consumption and tobacco use.46 In contrast, lower SES in men may be associated with a work environment that emphasises physical activity, while sedentary work habits are prevalent among higher SES individuals.15 With regard to women’s health, a high socioeconomic level may manifest itself in greater participation in health management practices. This can include the inclusion of organic food in the diet and active participation in health-related events.47 However, it is necessary to acknowledge the complexity of these relationships and to exercise caution when generalising health behaviours between different sexes and strata with different SES, as the determinants of health status are multifaceted (including individual choices, socioeconomic influences and access to resources) and collectively shape the complex landscape of health outcomes.
The study showed that SES makes the largest contribution to existing inequalities, while age makes the smallest contribution. Moreover, the effects of sex and SES cancel each other out for most inequalities in the dyslipidaemia components (except hypercholesterolaemia). These results indicate that the implementation of preventive and therapeutic measures to improve the components of the lipid profile requires separate interventions. For example, for hypercholesterolaemia and hyper-LDL, planning and implementing interventions for low-SES groups is more effective than sex-specific interventions, whereas for hypo-HDL and hypertriglyceridaemia, planning for women and men, respectively, is preferable to planning based on groups with different SES.
Strength and limitations
Although previous studies conducted in Iran and other countries have mainly investigated the relationship between socioeconomic factors and lipid profile components, this study was the first to examine the quantified role of age, sex and SES in lipid profile component inequality. However, this study also had limitations. This study did not examine inequality in access to and adoption of therapeutic interventions, whereas the existing inequality in dyslipidaemia components may decrease or increase with treatment adoption. In addition, it should be noted that information on participants’ nutritional status was not available. As diet plays a central and influential role in the components of the lipid profile, the lack of this data prevented the investigation of the dietary behaviour of the participants in the different groups. Another limitation of the study is the reliance on self-reported data for some questions and variables. The generalisability of the results is limited to communities with similar cultural and socioeconomic characteristics.
In conclusion, our results emphasise the importance of existing inequalities in lipid profile components among participants, with SES, sex and age contributing significantly. Consequently, strategic planning of interventions and preventive measures based on the contribution of these determinants is necessary in a population where 7 out of 10 individuals have abnormalities in at least one component. Policy decisions based on this method and monitoring the results of implemented measures can provide information on the change in the status of inequalities. This approach facilitates a comprehensive understanding of the impact of policy and enables future planning of practical measures.
supplementary material
Acknowledgements
We would like to thank all the participants in this study and the people who supported us in carrying out this research project.
Footnotes
Funding: This study was supported by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Grant No 43007063. The funding agency did not play any role in the planning, conduct and reporting or in the decision to submit the paper for publication.
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-085035 ).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: All procedures performed in the study were approved by the Research Ethics Committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences (IR.SBMU.ENDOCRINE.REC.1402.089). Also, informed consent was obtained from all participants. All methods were carried out following relevant guidelines and regulations.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Neda Izadi, Email: neda.izady@yahoo.com.
Reza Yari-Boroujeni, Email: rezayari.sbmu@yahoo.com.
Moslem Soofi, Email: moslemsoof@gmail.com.
Mahdieh Niknam, Email: ma_niknam@sbmu.ac.ir.
Parisa Amiri, Email: amiri@endocrine.ac.ir.
Farid Najafi, Email: farid_n32@yahoo.com.
Data availability statement
Data are available upon reasonable request.
References
- 1.World Health Organisation Cardiovascular Diseases (CVDs) 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Available.
- 2.Sarrafzadegan N, Mohammmadifard N. Cardiovascular Disease in Iran in the Last 40 Years: Prevalence, Mortality, Morbidity, Challenges and Strategies for Cardiovascular Prevention. Arch Iran Med. 2019;22:204–10. [PubMed] [Google Scholar]
- 3.Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, et al. Pathophysiology of Atherosclerosis. Int J Mol Sci. 2022;23:3346. doi: 10.3390/ijms23063346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabatabaei-Malazy O, Qorbani M, Samavat T, et al. Prevalence of dyslipidemia in iran: a systematic review and meta-analysis study. Int J Prev Med. 2014;5:373–93. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcez MR, Pereira JL, Fontanelli M de M, et al. Prevalence of dyslipidemia according to the nutritional status in a representative sample of São Paulo. Arq Bras Cardiol. 2014;103:476–84. doi: 10.5935/abc.20140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opoku S, Gan Y, Fu W, et al. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: findings from the China National Stroke Screening and prevention project (CNSSPP) BMC Public Health. 2019;19:1500. doi: 10.1186/s12889-019-7827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC, Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–56. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 9.Mendis S. Global status report on noncommunicable diseases 2014: World health organization. 2014. [DOI] [PubMed]
- 10.Tabatabaei-Malazy O, Qorbani M, Samavat T, et al. Prevalence of dyslipidemia in iran: a systematic review and meta-analysis study. Int J Prev Med. 2014;5:373–93. [PMC free article] [PubMed] [Google Scholar]
- 11.Guptha S, Gupta R, Deedwania P, et al. Cholesterol lipoproteins and prevalence of dyslipidemias in urban Asian Indians: a cross sectional study. Ind Heart J. 2014;66:280–8. doi: 10.1016/j.ihj.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguilar-Salinas CA, Gómez-Pérez FJ, Rull J, et al. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2010;52 Suppl 1:S44–53. doi: 10.1590/s0036-36342010000700008. [DOI] [PubMed] [Google Scholar]
- 13.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Ouyang F, He J, et al. Associations of Socioeconomic Status and Healthy Lifestyle With Incidence of Dyslipidemia: A Prospective Chinese Governmental Employee Cohort Study. Front Public Health. 2022;10:878126. doi: 10.3389/fpubh.2022.878126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soleimani H, Ghasemi E, Saeedi Moghaddam S, et al. Assessing the effect of socioeconomic factors on prevalence of dyslipidemia among iranian adult population; district level analysis from 2016 STEPS national study using small area estimation. J Diabetes Metab Disord. 2022;21:647–55. doi: 10.1007/s40200-022-01027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikparvar M, Khaladeh M, Yousefi H, et al. Dyslipidemia and its associated factors in southern Iranian women, Bandare-Kong Cohort study, a cross-sectional survey. Sci Rep. 2021;11:9125. doi: 10.1038/s41598-021-88680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabrizi JS, Nikniaz L, Sadeghi-Bazargani H, et al. Prevalence of Dyslipidemia in Urban and Rural Areas of the Northwest of Iran: The Sociodemographic, Dietary and Psychological Determinants. Iran J Public Health . 2019;48:925–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Marmot M, Allen J, Bell R, et al. WHO European review of social determinants of health and the health divide. Lancet. 2012;380:1011–29. doi: 10.1016/S0140-6736(12)61228-8. [DOI] [PubMed] [Google Scholar]
- 19.Kawachi I, Subramanian SV, Almeida-Filho N. A glossary for health inequalities. J Epidemiol Community Health. 2002;56:647–52. doi: 10.1136/jech.56.9.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehead M. The concepts and principles of equity and health. Int J Health Serv. 1992;22:429–45. doi: 10.2190/986L-LHQ6-2VTE-YRRN. [DOI] [PubMed] [Google Scholar]
- 21.Hahn RA, Truman BI. Education Improves Public Health and Promotes Health Equity. Int J Health Serv. 2015;45:657–78. doi: 10.1177/0020731415585986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall GL, Ingraham B, Major J, et al. Modeling the impact of financial hardship and age on self-rated health and depressive symptoms pre/post the great recession. SSM Popul Health. 2022;18:101102. doi: 10.1016/j.ssmph.2022.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neil A, Russell JD, Thompson K, et al. The impact of socioeconomic position (SEP) on women’s health over the lifetime. Maturitas. 2020;140:1–7. doi: 10.1016/j.maturitas.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasdar Y, Najafi F, Moradinazar M, et al. Cohort Profile: Ravansar Non-Communicable Disease cohort study: the first cohort study in a Kurdish population. Int J Epidemiol. 2019;48:682–683f. doi: 10.1093/ije/dyy296. [DOI] [PubMed] [Google Scholar]
- 25.Hamzeh B, Farnia V, Moradinazar M, et al. Pattern of cigarette smoking: intensity, cessation, and age of beginning: evidence from a cohort study in West of Iran. Subst Abuse Treat Prev Policy. 2020;15:83. doi: 10.1186/s13011-020-00324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezaei M, Fakhri N, Pasdar Y, et al. Modeling the risk factors for dyslipidemia and blood lipid indices: Ravansar cohort study. Lipids Health Dis. 2020;19:176. doi: 10.1186/s12944-020-01354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedjat S, Hosseinpoor AR, Forouzanfar MH, et al. Decomposing socioeconomic inequality in self-rated health in Tehran. J Epidemiol Community Health. 2012;66:495–500. doi: 10.1136/jech.2010.108977. [DOI] [PubMed] [Google Scholar]
- 28.Safari-Faramani R, Rajati F, Tavakol K, et al. Prevalence, Awareness, Treatment, Control, and the Associated Factors of Diabetes in an Iranian Kurdish Population. J Diabetes Res. 2019;2019:5869206. doi: 10.1155/2019/5869206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darbandi M, Najafi F, Pasdar Y, et al. Structural equation model analysis for the evaluation of factors associated with overweight and obesity in menopausal women in RaNCD cohort study. Menopause. 2020;27:208–15. doi: 10.1097/GME.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 30.Moghaddam MB, Aghdam FB, Jafarabadi MA, et al. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18:1073–80. [Google Scholar]
- 31.NCEP Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 32.Wagstaff A, O’Donnell O, Van E, et al. Analyzing health equity using household survey data: a guide to techniques and their implementation. World Bank Publications; 2007. [Google Scholar]
- 33.Koolman X, van Doorslaer E. On the interpretation of a concentration index of inequality. Health Econ. 2004;13:649–56. doi: 10.1002/hec.884. [DOI] [PubMed] [Google Scholar]
- 34.Regidor E. Measures of health inequalities: part 2. J Epidemiol Community Health. 2004;58:900–3. doi: 10.1136/jech.2004.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosquera PA, San Sebastian M, Waenerlund A-K, et al. Income-related inequalities in cardiovascular disease from mid-life to old age in a Northern Swedish cohort: A decomposition analysis. Soc Sci Med. 2016;149:135–44. doi: 10.1016/j.socscimed.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–93. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995-2015. Arch Public Health. 2020;78:20. doi: 10.1186/s13690-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohebi F, Mohajer B, Yoosefi M, et al. Physical activity profile of the Iranian population: STEPS survey, 2016. BMC Public Health. 2019;19:1266. doi: 10.1186/s12889-019-7592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katulanda P, Dissanayake HA, De Silva SDN, et al. Prevalence, patterns, and associations of dyslipidemia among Sri Lankan adults-Sri Lanka Diabetes and Cardiovascular Study in 2005-2006. J Clin Lipidol. 2018;12:447–54. doi: 10.1016/j.jacl.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Yu S, Mao Z, et al. Dyslipidemia prevalence, awareness, treatment, control, and risk factors in Chinese rural population: the Henan rural cohort study. Lipids Health Dis. 2018;17:119. doi: 10.1186/s12944-018-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MH, Kim HC, Ahn SV, et al. Prevalence of Dyslipidemia among Korean Adults: Korea National Health and Nutrition Survey 1998-2005. Diabetes Metab J. 2012;36:43–55. doi: 10.4093/dmj.2012.36.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zampino M, AlGhatrif M, Kuo P-L, et al. Longitudinal Changes in Resting Metabolic Rates with Aging Are Accelerated by Diseases. Nutrients. 2020;12:3061. doi: 10.3390/nu12103061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–5. doi: 10.1016/j.nut.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko SH, Kim HS. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients. 2020;12:202. doi: 10.3390/nu12010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cockerham WC, D Wolfe J, Bauldry S. Health Lifestyles in Late Middle Age. Res Aging. 2020;42:34–46. doi: 10.1177/0164027519884760. [DOI] [PubMed] [Google Scholar]
- 46.Espírito Santo LR, Faria TO, Silva CSO, et al. Socioeconomic status and education level are associated with dyslipidemia in adults not taking lipid-lowering medication: a population-based study. Int Health. 2022;14:346–53. doi: 10.1093/inthealth/ihz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheraghian B, Asadi-Lari M, Mansournia MA, et al. Prevalence and associated factors of self-reported hypertension among Tehran adults in 2011. 28:105. n.d. [PMC free article] [PubMed] [Google Scholar]