Abstract
Background
Reducing the prevalence of hospital-acquired complications (HACs) is paramount for both patient safety and hospital financial performance because of its impact on patient’s recovery and health service delivery by diverting resources away from other core patient care activities. While numerous reports are available in the literature for projects that successfully reduce specific HAC, questions remain about the sustainability of this isolated approach and there may be benefits for more wholistic programmes that aim to align prevention strategies across a hospital. This study describes such a programme that uses evidence and theories in the literature to achieve and sustain a reduction in HACs in an Australian local health service between 2019 and 2022.
Methods
An organisation-wide HACs Reduction Programme underpinned by a 3-pillar strategic framework (complete documentation, accurate coding, clinical effectiveness) and a 5-year roadmap to clinical excellence was developed. Priorities were identified through Pareto analysis and aligned at organisational, service and specialty levels. The Institute for Healthcare Improvement (IHI) 90-day cycle was modified to implement contextualised evidence-based interventions supported by the application of the Awareness, Desire, Knowledge, Ability and Reinforcement change management model. Under this wholistic umbrella, specific projects were data-driven, evidence-based and outcome-oriented to promote clinical engagement and a continuous improvement culture.
Results
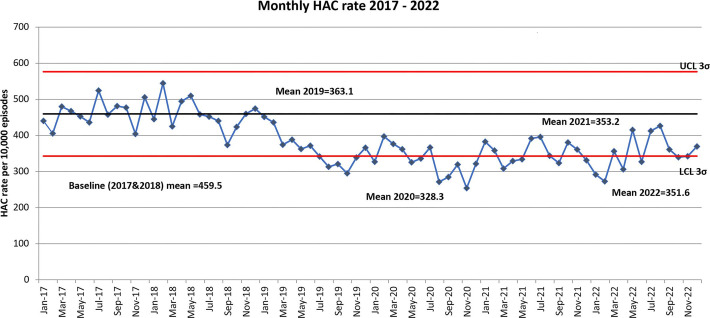
Overall mean HAC rate per 10 000 episodes of care decreased from a baseline of 459.5 across 2017 and 2018 to 363.1 in 2019 and remained lower through to the end of 2022 indicating sustained improvement in performance.
Conclusion
A wholistic approach to reduce HACs increased the likelihood of multidisciplinary integration for contextualised strategies and interventions. Improvement work, particularly in relation to patient outcomes, is a dynamic process that needs to be intentionally cultivated, targeted and coordinated. The modified IHI 90-day cycle proved to be an effective tool for implementation that contributed to sustained change.
Keywords: Patient safety; Quality improvement; Outcome assessment, Health care; Healthcare quality improvement; Implementation science
WHAT IS ALREADY KNOWN ON THIS TOPIC
While a lot of energy and cost are invested in safety and quality by hospitals, studies suggest that individual activities do not necessarily result in improvement or sustained improvement due to suboptimal communication between teams and a focus on assurance and compliance rather than factors that promote change.
WHAT THIS STUDY ADDS
Demonstrates an applied wholistic approach to align prevention strategies in a hospital and contextualise evidence in the literature to improve patient outcomes including the application of a modified version of the IHI 90-day cycle for implementation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
If safety and quality governance in hospitals are to be outcome-oriented, targeted contextualised interventions are essential in balance with other compliance and assurance measures.
Problem
A hospital-acquired complication (HAC) refers to a complication for which clinical mitigation strategies may reduce (but not necessarily eliminate) the prevalence of that complication occurring.1 In 2016, the Australian Commission on Safety and Quality in Healthcare (ACSQHC) released a list of 16 high-impact HACs table 1, with details in (supplementary document) based on preventability, patient impact (severity), health service impact and clinical priority.1 In July 2018, the Independent Hospital and Aged Care Pricing Authority (IHACPA) introduced a funding adjustment model for HACs as a financial signal to hospitals for actions required to reduce systemic risks related to the delivery of care.2 Subsequently, national and jurisdictional benchmarking reports became available for health services. This study outlines the work by an Australian local health service that had the highest HAC rate in the state and ranked in the bottom 25% quartile in a national benchmarking report. Alarmed by the impact of this on patient safety and the financial implications for the organisation, the health service executives drove an organisation-wide programme to promote awareness of HACs in late 2018 and endorsed the implementation of a hospital-wide HACs Reduction Programme that was developed in 2019 to improve performance. The aim of the programme was to reduce hospital HAC rate by 25% in 12 months and systematically address preventable HACs in a sustainable manner.
Table 1. Hospital-acquired complications list V.3.1 Australian Commission on Safety and Quality in Health Care and interventions applied in this study.
| HAC group | Interventions | Modified IHI 90-day cycle |
| Pressure injury |
|
|
| Falls resulting in fracture or intracranial injury |
|
|
| Healthcare-associated infection |
|
|
| Surgical complications requiring an unplanned return to theatre |
|
|
| *Unplanned intensive care unit admission |
|
|
| Respiratory complications |
|
|
| Venous thromboembolism |
|
|
| Renal failure |
|
|
| Gastrointestinal bleeding |
|
|
| Medication complications |
|
|
| Delirium |
|
|
| Incontinence |
|
|
| Endocrine complications |
|
|
| Cardiac complications |
|
|
| *Third and fourth degree of perineal laceration during delivery |
|
|
| *Neonatal birth trauma |
|
|
Currently not considered for the IHACPAIndependent Hospital and Aged Care Pricing Authority risk adjustment model therefore no funding adjustment.
AFatrial fibrillationHAChospital-acquired complicationIHIInstitute for Healthcare Improvement
Background
Cost to the Australian public sector for admissions associated with HACs was estimated to be A$4.1 billion or 8.9% of total hospital expenditure in the financial year (FY) 2017–20183 due to an almost fourfold increase in the mean length of stay per episode and increase in hospital cost.4 Identification of patients who develop HAC can be retrospectively determined from their hospital separation data by the diagnosis code and a condition onset flag assigned by clinical coders which indicates if the condition arose during the episode of care or not.2 5 However, concerns exist about using administrative coded data for performance measures due to potential ambiguity and incompleteness in clinical documentation and errors in coding.6,8 The gold standard of manual chart review is therefore necessary to identify ‘true’ HACs and understand their clinical implications9 as the most prevalent HACs are healthcare-associated infections (HAIs) which accounted for 55% of total HACs in Australian hospitals in 2021–2022 FY followed by delirium (17%), cardiac complications (16%) and respiratory complications (15%).10
As the underlying causes of HACs consist of a variety of hospital and patient-related factors,9 11 the ability to prevent them varies between the different conditions.1 For example, a retrospective study in an Australian tertiary hospital by Canning et al12 suggests that less than 50% of medication-related HACs were preventable while for HAI up to 70% are potentially preventable when evidence-based measures are effectively applied.13 14 Similarly, variations in the approach used by hospitals to address safety and quality issues can also have an impact on the outcomes with studies showing that a high volume of individual activities in hospitals did not always result in improvement due to suboptimal communication between teams,15 being overly focused on assurance and compliance16 and results from programmes such as hospital accreditation were inconsistent.17 18 Cost pressure,3 4 system complexity19 and competing priorities are just a few of the challenges that hospitals are facing, calling for data-driven, evidence-based and outcome-oriented wholistic improvement programmes to align priorities, enable clearer communication, improve the targeting of multiple interventions that may be aimed at the same patient cohort and to break down entrenched silos. While IHACPA has initiated reforms that integrate safety and quality (sentinel events, HACs, avoidable hospital readmissions) into the pricing and funding of public hospital services in Australia20 which provide strong incentives for hospitals to strive for best practices that result in better patient outcomes, changes at an organisational level, compared with those limited within a ward/unit, involve greater complexity to mobilise larger groups of stakeholders of multiple business units and across the organisational hierarchy.21
Despite the growing realisation of the need for a more wholistic and integrated programme to reduce the risk of patient harm during their hospital stay, there are very few studies that report on hospital-wide improvement programmes where HACs or adverse events are the primary measures,22 especially in the Australian context. Similarly, while some models and frameworks to reduce HACs23 have been proposed by a few hospitals, the impact of the implementation of these approaches is still pending. This study seeks to address this gap in knowledge by describing a wholistic approach that contextualises evidence and theories in the literature to achieve and sustain a reduction in HACs in an Australian local health service that includes a main tertiary hospital with 783 beds and a secondary hospital with 241 beds. The Standards for QUality Improvement Reporting Excellence reporting guidelines are used for this paper.24
Measurement
The primary outcome measure for this study was the HAC rate per 10 000 episodes of care. In this study, the mean HAC rate across 2017 and 2018 was set as a baseline to have enough data points for statistical process control (SPC) development.25 Two other supporting measures were developed through clinical case reviews to assist with the interpretation of HAC performance and goal setting. One was per cent of HACs that were considered as ‘false’ either due to ambiguity in clinical documentation or errors in clinical coding, another was per cent of HACs that were preventable or possibly preventable based on the judgement of clinicians who conducted the reviews.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this study.
Outcome data analysis
Data source
This study used HAC data between 2017 and 2022. Monthly HAC rates were extracted from the hospital’s internal HAC dashboard using coded episode information. De-identified episodes level information for all admissions in scope over the 6 years were provided in an Excel spreadsheet by the digital data intelligence unit who developed the HAC dashboard per V.3.1 HACs list specifications by the ACSQHC.5 Variables in the Excel include HAC group (table 1) applied, year, age, admission type, admission transfer status, diagnosis-related group (DRG) type, major diagnosis category 10 (MDC), Charlson Score and the dependent variable ‘Is HAC’ where 1 equal ‘yes’ and 0 equals ‘no’.
Data analysis and performance comparison
Monthly HAC rates were populated on SPC charts in Excel. Episodes-level data were analysed using IBM SPSS Statistics V.24. HAC rate was modelled as a function of year using Poisson regression, adjusted for the risk factors age, admission type, DRG type, MDC, admission transfer status and Charlson Score in accordance with the IHACPA risk adjustment model.2 Intensive care unit (ICU) status was excluded from the analysis due to data limitation to ascertain if an ICU stay occurred prior to or after an HAC diagnosis.2 P value less than 0.05 was considered as evidence for an effect. All estimated rates were presented with 95% CIs.
Design
To overcome the common issue of clinical engagement being impacted by concerns of coding accuracy, this study established a multidisciplinary HACs data integrity working group whose membership included the HAC programme manager, clinical coding manager, physicians, finance manager and data analysts. An HAC project officer with a clinical background and a few willing clinicians conducted audits and case reviews to ascertain if the condition was truly developed during the admission and if it was preventable, likely preventable or not preventable. Case reviews were not well-structured initially as clinicians had their own preferences on the level of details and how to feedback their findings. However, the educational and reflective benefits of such exercise became evident after a few months when a shift in languages used at meetings was noted whereby gaps in clinical care processes gained more attention than data issues. Medical and nursing clinical champions were nominated to review HACs in their respective specialties. HAC was increasingly endorsed as an agenda item on governance and clinical review committees.
Contemporaneously, a daily refreshed HAC dashboard was developed and became available 3 months post-establishment of the multidisciplinary HACs data integrity working group. In late 2020, an HAC clinical case review platform with structured questions and data triangulation to minimise manual data entry was developed on the Research Electronic Data Capture (REDCap) system to simplify and streamline the processes involved. The review questions were designed to gain insights on (1) per cent of HACs that were present or likely present on admission, (2) per cent of HAC diagnoses that were clinically accurate, (3) level of preventability and (4) possible risk mitigation strategies that could have been put in place for prevention.
Strategy
A 3-pillar strategic framework consists of Complete Documentation, Accurate Coding and Clinical Effectiveness was developed to be wholistic in addressing issues in the HAC data and gaps in clinical care practices complemented by a 5-year roadmap to clinical excellence.
The 5-year roadmap to clinical excellence
The roadmap consists of four phases.
Phase I focused on problem identification and culture preparation. Data issues were acknowledged and progressively addressed in the first 6 months. Education sessions and workshops were delivered to promote awareness and understanding of HACs along with processes for performance monitoring and reporting. Medical and nursing HAC clinical champions from each specialty were nominated whose main responsibility was to undertake case reviews and be the conduit of improvement strategies and changes in clinical care practice required.
Phase II witnessed the establishment of multidisciplinary improvement groups (MIGs) to research and implement interventions for priority HACs. The structure of the groups was an adaptation from the clinical communities described in the Johns Hopkins Performance Accountability Model26,28 and the Michigan ICU Project by Pronovost et al.29 30 Each group consisted of representatives from medical, nursing, allied health and pharmacy from priority specialties, a clinical subject matter expert, an executive sponsor, an expert in improvement and implementation who is the HAC programme manager and an HAC project officer. The MIGs were tasked with the development of contextualised interventions for piloting and implementation based on findings from literature review and clinical audits, experiences from other hospitals and clinical expert opinions. For example, the decision to implement oral care for hospital-acquired pneumonia (HAP) prevention was made following a literature review and clinical consultation. Published oral care protocol advocates three episodes of oral care per day per adult inpatient. Six high-risk wards were selected for piloting post Pareto analysis. On staff feedback and evaluation of outcome data, the oral care protocol was modified to be a minimum of two episodes per day focusing on morning and evening compliance. An average of 50% reduction in HAP was observed on piloting wards post-implementation. A hospital policy on oral care was developed and rolled out subsequently.
Phase III built on projects initiated in Phase II to embed effective interventions into everyday practice and to support teams for sustained outcomes and further improvement work. The HACs team led the design, communication and implementation of interventions organisation-wide, aligned priorities across services, specialties and teams and fostered collaboration among professions and teams. Forums were developed to foster a change-ready culture for evidence-based care. For example, an HACs prevention best practice facilitators’ group met regularly to share empirical findings in the literature, emerging trends in local data, lessons learnt from local projects and models applied by other institutions, etc. Resources were developed to support staff and patient education such as the Remain, Remove, Re-site decision-making tool for peripheral intravenous catheter management, Your Intravenous Cannula and Oral Care—Keeping Your Teeth Clean and Healthy posters for patients and consumers, HAI Prevention Bundle—BASICs Framework, ward round checklists and Think HYPOglycaemia poster.
Phase IV is a state of true patient safety whereby preventable patient harm is minimised. This remains an ongoing effort to mature in the hospital. Training on HACs prevention is built into staff orientation programmes that are reinforced by clinical champions and educators in local clinical areas to cultivate sustainable improvement effort.
Tools and frameworks applied
This study drew on several theories and frameworks and contextualised strategies reported in the literature to suit the needs of the organisation. The Institute of Healthcare Improvement (IHI) quality improvement tools31 including Driver Diagram, Pareto Charts and SPC were extensively used for planning and performance monitoring. For example, the prioritisation of HACs to be targeted was identified annually using Pareto analysis to focus on high volume and high-value complications as described by others.15
Due to the 3-monthly training rotation of the clinical workforce in the hospital and the importance of habit formation to embed evidence-based clinical care practice,32 33 we modified the IHI 90-day cycle to be solely for implementing targeted interventions over 12 weeks (84 days) instead of the three distinct phases for scan, focus and summarise.34 35 Each 12-week cycle was supported with tailored weekly feedback process using objective data.
Faced with challenges in clinical workforce shortage and recruitment, especially during and after the COVID-19 pandemic, we were unable to offer clinical staff protected time for improvement work as described by Pronovost et al29 30 but instead established MIGs and applied the agile values by Jay Arthur36 to help equip and empower others for change while collaborated for problem-solving over compliance monitoring and being responsive to change over following a plan. Change management applied the Awareness, Desire, Knowledge, Ability and Reinforcement model and the diffusion of innovation theory,37 38 both emphasised on awareness, desire (interest) and reinforcement (trial and adoption). We instigated intensive promotion of the HAC conditions and how the IHACPA model worked on various forums early in the programme and developed a large network of clinical champions to influence changes in local clinical areas and hospital-wide.
Interventions
Interventions were designed to be general that were used across the organisation for all HACs and targeted for high-priority complications. General interventions included clinical case reviews, education and training, monitoring and reporting. Targeted interventions were developed through literature review, clinical audits and clinical expert opinions for evidence-based care, then contextualised for implementation. These were complimented by other initiatives to influence organisational and local culture in clinical areas through data-driven dialogues, tailored feedback processes on performance, co-design of improvement strategies, activities to celebrate progress and success and forums to facilitate information and knowledge sharing.
Results
Of the 552 995 hospital discharges between 2017 and 2022, 14 434 (2.6%) had at least one HAC. Clinical review of 4061 HAC events on REDCap indicated that overall, 16.3% were present or likely present on admission, 74.1% developed during hospital stay, 5.3% were unable to be confirmed as developing while in hospital and 4.3% had no documentation of event found in notes therefore potential coding error. Regarding preventability, 5.2% of HACs were preventable, 44.6% possibly preventable and 50.1% non-preventable. Priority HACs identified through Pareto analysis and received targeted interventions included HAI, cardiac complications, surgical complications requiring unplanned return to theatre, medication complications, endocrine complications, venous thromboembolism (VTE) and delirium.
HACs performance on SPC chart
Figure 1 shows that overall mean HAC rate per 10 000 episodes of care decreased from 459.5 in 2017 and 2018 to be 363.1 in 2019, 328.3 in 2020, 353.2 in 2021 and 351.6 in 2022. Monthly HAC rate has been below the baseline of 459.5 since January 2019 indicating sustained improvement in performance.
Figure 1. Monthly HAC rate 2017–2022. HAC, hospital-acquired complication; LCL, lower control limit; UCL, upper control limit.
Adjusting for risk factors on HAC rate per episode
Our modelling of the HAC rate as a function of the year using the Poisson regression shows that there was an overall effect of the year (Χ2(4)=220.6, p<0.001). Compared to the baseline period of 2017–2018, the annual HAC rates in 2019 through 2022 were all significantly lower (p<0.001) with the average annual reduction in HAC events being 22.9%, 25.4%, 22.0% and 18.3% respectively (table 2).
Table 2. HAC rate modelling as a function of year with and without adjustment for risk factors.
| Complication | Year | Rate | Unadjusted 95% CI | P value | Rate | Adjusted 95% CI | P value |
| All HACs | Baseline (n=5419) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=2171) | 0.77 | 0.73 to 0.81 | <0.001 | 0.79 | 0.75 to 0.83 | <0.001 | |
| 2020 (n=2133) | 0.75 | 0.71 to 0.79 | <0.001 | 0.74 | 0.71 to 0.82 | <0.001 | |
| 2021 (n=2372) | 0.78 | 0.74 to 0.82 | <0.001 | 0.76 | 0.73 to 0.80 | <0.001 | |
| 2022 (n=2339) | 0.82 | 0.78 to 0.86 | <0.001 | 0.79 | 0.75 to 0.83 | <0.001 | |
| Pressure injury | Baseline (n=165) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=69) | 0.81 | 0.61 to 1.07 | 0.130 | 0.87 | 0.65 to 1.15 | 0.314 | |
| 2020 (n=46) | 0.53 | 0.38 to 0.73 | <0.001 | 0.52 | 0.38 to 0.73 | <0.001 | |
| 2021 (n=62) | 0.67 | 0.50 to 0.90 | 0.007 | 0.65 | 0.49 to 0.88 | 0.004 | |
| 2022 (n=86) | 0.99 | 0.76 to 1.28 | 0.916 | 0.90 | 0.69 to 1.17 | 0.440 | |
| Falls resulting in fracture or intracranial injury | Baseline (n=94) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=55) | 1.13 | 0.81 to 1.57 | 0.484 | 1.11 | 0.80 to 1.55 | 0.526 | |
| 2020 (n=47) | 0.95 | 0.67 to 1.35 | 0.746 | 0.90 | 0.63 to 1.29 | 0.568 | |
| 2021 (n=46) | 0.87 | 0.61 to 1.24 | 0.446 | 1.81 | 0.57 to 1.16 | 0.256 | |
| 2022 (n=46) | 0.93 | 0.65 to 1.32 | 0.669 | 0.86 | 0.60 to 1.22 | 0.388 | |
| (*T) Healthcare-associated infection | Baseline (n=2136) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=853) | 0.78 | 0.71 to 0.83 | <0.001 | 0.80 | 0.74 to 0.87 | <0.001 | |
| 2020 (n=791) | 0.70 | 0.65 to 0.76 | <0.001 | 0.69 | 0.64 to 0.75 | <0.001 | |
| 2021 (n=1053) | 0.88 | 0.82 to 0.95 | <0.001 | 0.84 | 0.78 to 0.90 | <0.001 | |
| 2022 (n=1083) | 0.96 | 0.89 to 1.03 | 0.265 | 0.91 | 0.84 to 0.98 | 0.011 | |
| (*T) Surgical complications requiring unplanned return to theatre | Baseline (n=109) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=43) | 0.76 | 0.53 to 1.08 | 0.126 | 0.81 | 0.57 to 1.16 | 0.253 | |
| 2020 (n=55) | 0.96 | 0.69 to 1.32 | 0.788 | 0.99 | 0.72 to 1.37 | 0.950 | |
| 2021 (n=72) | 1.18 | 0.87 to 1.59 | 0.284 | 1.19 | 0.88 to 1.60 | 0.263 | |
| 2022 (n=67) | 1.16 | 0.86 to 1.58 | 0.331 | 1.19 | 0.88 to 1.62 | 0.256 | |
| (*T) Respiratory complications | Baseline (n=653) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=262) | 0.77 | 0.67 to 0.89 | <0.001 | 0.81 | 0.70 to 0.94 | 0.005 | |
| 2020 (n=280) | 0.81 | 0.71 to 0.94 | 0.004 | 0.84 | 0.73 to 0.97 | 0.015 | |
| 2021 (n=357) | 0.97 | 0.86 to 1.11 | 0.689 | 0.99 | 0.87 to 1.12 | 0.819 | |
| 2022 (n=330) | 0.96 | 0.84 to 1.09 | 0.507 | 0.94 | 0.82 to 1.08 | 0.372 | |
| (*T) Venous thromboembolism | Baseline (n=190) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=55) | 0.56 | 0.41 to 0.75 | <0.001 | 0.59 | 0.43 to 0.78 | <0.001 | |
| 2020 (n=58) | 0.58 | 0.43 to 0.78 | <0.001 | 0.58 | 0.44 to 0.79 | <0.001 | |
| 2021 (n=64) | 0.60 | 0.45 to 0.80 | <0.001 | 0.59 | 0.45 to 0.79 | <0.001 | |
| 2022 (n=60) | 0.60 | 0.45 to 0.80 | <0.001 | 0.58 | 0.43 to 0.78 | <0.001 | |
| Renal failure | Baseline (n=73) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=33) | 0.87 | 0.58 to 1.31 | 0.507 | 0.95 | 0.63 to 1.44 | 0.817 | |
| 2020 (n=35) | 0.91 | 0.61 to 1.36 | 0.642 | 0.97 | 0.65 to 1.46 | 0.898 | |
| 2021 (n=21) | 0.51 | 0.32 to 0.83 | 0.007 | 0.51 | 0.31 to 0.83 | 0.007 | |
| 2022 (n=23) | 0.60 | 0.37 to 0.95 | 0.030 | 0.59 | 0.37 to 0.95 | 0.029 | |
| Gastrointestinal bleeding | Baseline (n=223) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=102) | 0.88 | 0.70 to 1.11 | 0.287 | 0.91 | 0.72 to 1.15 | 0.410 | |
| 2020 (n=100) | 0.85 | 0.67 to 1.08 | 0.177 | 0.83 | 0.66 to 1.06 | 0.132 | |
| 2021 (n=110) | 0.88 | 0.70 to 1.10 | 0.267 | 0.84 | 0.67 to 1.06 | 0.135 | |
| 2022 (n=106) | 0.90 | 0.71 to 1.13 | 0.368 | 0.85 | 0.67 to 1.07 | 0.167 | |
| (*T) Medication complications | Baseline (n=305) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=146) | 0.92 | 0.76 to 1.12 | 0.416 | 0.92 | 0.76 to 1.13 | 0.431 | |
| 2020 (n=116) | 0.72 | 0.58 to 0.89 | 0.003 | 0.74 | 0.60 to 0.92 | 0.006 | |
| 2021 (n=102) | 0.60 | 0.48 to 0.75 | <0.001 | 0.61 | 0.49 to 0.76 | <0.001 | |
| 2022 (n=95) | 0.59 | 0.47 to 0.74 | <0.001 | 0.60 | 0.47 to 0.75 | <0.001 | |
| (*T) Delirium | Baseline (n=933) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=387) | 0.79 | 0.71 to 0.90 | <0.001 | 0.81 | 0.72 to 0.91 | <0.001 | |
| 2020 (n=388) | 0.79 | 0.70 to 0.89 | <0.001 | 0.80 | 0.71 to 0.90 | <0.001 | |
| 2021 (n=389) | 0.74 | 0.66 to 0.84 | <0.001 | 0.74 | 0.65 to 0.83 | <0.001 | |
| 2022 (n=371) | 0.75 | 0.67 to 0.85 | <0.001 | 0.75 | 0.66 to 0.84 | <0.001 | |
| (*T) Incontinence | Baseline (n=89) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=22) | 0.48 | 0.30 to 0.76 | 0.002 | 0.49 | 0.30 to 0.79 | 0.003 | |
| 2020 (n=23) | 0.49 | 0.31 to 0.78 | 0.002 | 0.48 | 0.30 to 0.76 | 0.002 | |
| 2021 (n=33) | 0.66 | 0.44 to 0.99 | 0.042 | 0.63 | 0.42 to 0.93 | 0.022 | |
| 2022 (n=21) | 0.45 | 0.28 to 0.72 | 0.001 | 0.41 | 0.25 to 0.66 | <0.001 | |
| (*T) Endocrine complications | Baseline (n=739) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=295) | 0.77 | 0.67 to 0.88 | <0.001 | 0.80 | 0.70 to 0.92 | <0.001 | |
| 2020 (n=292) | 0.75 | 0.65 to 0.86 | <0.001 | 0.76 | 0.66 to 0.87 | <0.001 | |
| 2021 (n=366) | 0.88 | 0.78 to 1.00 | 0.050 | 0.89 | 0.78 to 1.00 | 0.069 | |
| 2022 (n=332) | 0.85 | 0.75 to 0.97 | 0.014 | 0.83 | 0.73 to 0.94 | 0.005 | |
| (T) Cardiac complications | Baseline (n=1090) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=354) | 0.63 | 0.56 to 0.71 | <0.001 | 0.65 | 0.57 to 0.73 | <0.001 | |
| 2020 (n=352) | 0.61 | 0.54 to 0.69 | <0.001 | 0.64 | 0.56 to 0.72 | <0.001 | |
| 2021 (n=294) | 0.48 | 0.42 to 0.55 | <0.001 | 0.49 | 0.43 to 0.56 | <0.001 | |
| 2022 (n=259) | 0.45 | 0.39 to 0.52 | <0.001 | 0.46 | 0.41 to 0.53 | <0.001 | |
| Third and fourth degree perineal laceration during delivery | Baseline (n=141) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=53) | 0.72 | 0.53 to 0.99 | 0.045 | 0.72 | 0.52 to 0.99 | 0.040 | |
| 2020 (n=53) | 0.71 | 0.52 to 0.98 | 0.035 | 0.79 | 0.58 to 1.09 | 0.147 | |
| 2021 (n=77) | 0.97 | 0.74 to 1.28 | 0.846 | 0.92 | 0.69 to 1.21 | 0.535 | |
| 2022 (n=75) | 1.00 | 0.76 to 1.33 | 0.965 | 0.80 | 0.60 to 1.06 | 0.114 | |
| Neonatal birth trauma | Baseline (n=51) | 1 | Reference | – | 1 | Reference | – |
| 2019 (n=21) | 0.79 | 0.48 to 1.32 | 0.370 | 0.82 | 0.49 to 1.37 | 0.447 | |
| 2020 (n=21) | 0.78 | 0.47 to 1.30 | 0.339 | 0.79 | 0.47 to 1.31 | 0.360 | |
| 2021 (n=20) | 0.70 | 0.42 to 1.17 | 0.174 | 0.63 | 0.37 to 1.08 | 0.092 | |
| 2022 (n=16) | 0.59 | 0.34 to 1.04 | 0.069 | 0.54 | 0.31 to 0.95 | 0.034 |
T indicates that targeted intervention/s were implemented for this HAC.
HAChospital-acquired complicationLCLlower control limitUCLupper control limit
All six risk factors (age, admission type, DRG type, MDC, admission transfer status and Charlson Score) influenced the HAC rate individually and in combination (p<0.001). After adjusting for the risk factors, the effect of the year on the HAC rate (Χ2(4)=232.3, p<0.001) remained significant so was the reduction in HAC rate (p<0.001) (see table 2 for details).
Changes in individual HAC groups
Significant sustained reductions were observed in eight HAC groups (HAI, VTE, renal failure, medication complications, delirium, incontinence, endocrine complications and cardiac complications), all receiving targeted contextualised interventions (table 1). Periodic reductions in pressure injury (2020 and 2021), respiratory complications (2019 and 2020), third-degree and fourth-degree perineal laceration during delivery (2019), neonatal birth trauma (2022) were observed but not sustained. No significant change was observed for falls resulting in fracture or intracranial injury, surgical complications requiring an unplanned return to theatre and gastrointestinal bleeding.
Lessons and limitations
The wholistic approach we undertook to reduce HACs was novel by contextualising strategies and interventions with multidisciplinary integration which resulted in a significant and sustained reduction in the hospital’s total HAC rate. Despite this success, the study also identified a few lessons and limitations.
First, targeted contextualised interventions and the availability of objective data to provide feedback were essential for achieving sustained HAC reduction. General and cultural interventions were necessary but not sufficient on their own. For example, a postoperative surgical site infection minimisation project was developed with comprehensive interventions derived from a literature review, clinical audits and expert opinions. However, due to resource constraints, no audit was undertaken during implementation therefore lack of objective data to feedback to clinical teams. As a result, no lasting change in performance was observed for surgical complications (see table 2). Similarly, no change in performance was observed for falls resulting in fracture or intracranial injury over the years whereby no targeted contextualised interventions were available despite general and cultural interventions from the HACs Reduction Programme and other safety and quality monitoring processes such as clinical incident management and policy compliance monitoring in the hospital. This finding resonates with recommendations from previous studies regarding limitations of programmes with a focus on compliance monitoring in achieving improvement in patient-related outcome measures16 17 highlighting that if safety and quality governance in hospitals are to be outcome-oriented, there need to be a balance between targeted contextualised interventions and compliance measures, subsequently implications on how safety and quality activities are budgeted and planned. The importance of having objective data for feedback to embed evidence-based practice which often involves behaviour change is supported by social science on habit formation whereby reinforcement plays a pivotal role.39
Second, responding to change over following a plan and being able to engage the human side of change were key success factors for clinical engagement. We faced unexpected challenges to implement the 3-pillar strategy fully due to contextual factors and the sudden onset of COVID-19 which almost stalled the programme. A decision was made early to focus on the strategic pillar of Clinical Effectiveness that resonated with the professional values of staff and be constant in testing and data over opinions and habits36 to guide decision-making and co-design of interventions. This approach helped develop trust in relationships with clinicians and key stakeholders and promoted a data-driven, evidence-based and outcome-oriented improvement culture. Clinical engagement (especially medical engagement) and clinical leadership modelling were critical to implement changes and sustain successful outcomes40 so was executive support.41
Third, deliberate actions were needed to ensure the work was not consumed by perfecting data at the expense of intervention. Clinicians often viewed HACs from the perspective of preventability and clinical implications while coders had to make judgement based on documentation and record of the onset of the condition. The lack of a clear definition for certain HACs in the national model further complicated the data issues. To help address this challenge, we openly acknowledged the different perspectives on the data and developed mechanisms early in the programme that allowed transparency in reporting and facilitated case reviews to add objectiveness in understanding the data and the clinical significance of HACs. We worked in partnership with coders and clinicians to verify queries raised and bridged mutual understanding through the process.
Fourth, HAC performance was sensitive to changes in contextual factors. In 2021, significant changes occurred in one of the services in the hospital and an exponential increase in HACs especially HAIs was noted in its patient cohort. We met with the service leadership team and clinical staff to understand the changes, adjusted priorities and developed tailored strategies for early identification and mitigation of clinical risks that mattered to patient outcomes most.42 Performance within the service improved within 3 months and has been sustained through the establishment of a multidisciplinary HACs prevention collaboration group.
Despite the positive outcome, our results are limited by the lack of baseline measurement for the two supporting measures, namely per cent of HACs that are considered as ‘false’ and per cent of HACs that are preventable to ascertain the potential impact of changes in the two measures on the overall HAC performance. However, data collected since October 2020 shows no significant changes in the measures and neither specific intervention on increasing coding accuracy nor improving clinical documentation until February 2022. Therefore, we are confident that improvement in HAC performance was largely attributed to the strategies outlined above. Financial implications of the HACs Reduction Programme and its impact on an organisation culture of multidisciplinary collaboration and using data to drive improvement will be reported in another study to shed light on decisions for similar investments in hospitals. Simulation-based, mastery learning intervention with deliberate practice will be explored in the future for staff training in prevention. Generalisation of the work to other hospitals is possible but will depend on contextual factors such as leadership support and level of clinical engagement.
Conclusion
A wholistic approach in reducing HACs increases the likelihood of multidisciplinary integration for contextualised strategies and interventions. Improvement work, particularly in relation to patient outcomes, is a dynamic process that needs to be intentionally planned, targeted and coordinated. The modified IHI 90-day cycle is an effective tool for implementation that creates lasting changes when complemented with objective data from audits and tailored feedback processes.
supplementary material
Acknowledgements
The HACs Reduction Programme is an organisation wide improvement strategy that involved many. We are indebted to the executive leadership vision for this programme and all who have contributed to the development and implementation of the programme at its different phases over the years.
Footnotes
Funding: This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. However, supervision support has been received through the Australian Government Research Training Program Scholarship.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: This study received ethics approval from the South Metropolitan Health Services Human Research Ethics Committee in Western Australia, reference number RGS0000003304; and approval from the University of Notre Dame Australia Human Research Ethics Committee, reference number 019068F.
Contributor Information
Qun Catherine Li, Email: catherine.li@health.wa.gov.au.
Jim Codde, Email: jim.codde@nd.edu.au.
Jonathan Karnon, Email: jon.karnon@flinders.edu.au.
Dana Hince, Email: dana.hince1@nd.edu.au.
Data availability statement
Data are available upon reasonable request.
References
- 1.Australian Commission on Safety and Quality in Health Care Hospital–acquired complications information kit. fact sheets to support safety and quality in australian health services. 2018. [24-May-2024]. https://www.safetyandquality.gov.au/sites/default/files/migrated/SAQ7730_HAC_InfomationKit_V2.pdf Available. Accessed.
- 2.Independent Hospital Pricing Authority Pricing and funding for safety and quality - risk adjusted model for hospital acquired complications. 2020. [24-May-2024]. https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.ihacpa.gov.au%2Fsites%2Fdefault%2Ffiles%2F2022-02%2FPricing%2520and%2520funding%2520for%2520safety%2520and%2520quality%2520-%2520HACs.docx&wdOrigin=BROWSELINK Available. Accessed.
- 3.Australian Commission on Safety and Quality in Health Care . ACSQHC Canberra (AUST); 2019. [4-Jun-2024]. The state of patient safety and quality in australian hospitals.https://www.safetyandquality.gov.au/sites/default/files/2019-07/the-state-of-patient-safety-and-quality-in-australian-hospitals-2019.pdf Available. Accessed. [Google Scholar]
- 4.Trentino KM, Swain SG, Burrows SA, et al. Measuring the incidence of hospital-acquired complications and their effect on length of stay using CHADx. Med J Aust. 2013;199:543–7. doi: 10.5694/mja12.11640. [DOI] [PubMed] [Google Scholar]
- 5.Australian Commission on Safety and Quality in Health Care Hospital-acquired complications (hacs) list - specifications - version 3.1 (12th edn) 2019. [31-Aug-2023]. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/hospital-acquired-complications-hacs-list-specifications-version-31-12th-edn Available. Accessed.
- 6.Bartley D, Panchasarp R, Bowen S, et al. How accurately is hospital acquired pneumonia documented for the correct assignment of a hospital acquired complication (HAC)? Inf Dis Health. 2021;26:67–71. doi: 10.1016/j.idh.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Herson M, Curtis SJ, Land G, et al. Performance of a hospital-acquired complication algorithm using administrative data for detection of central line-associated bloodstream infections: experience at an Australian healthcare facility. J Hosp Infect. 2021;112:116–8. doi: 10.1016/j.jhin.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Duke GJ, Bishara M, Hirth S, et al. Performance of hospital administrative data for detection of sepsis in Australia: The sepsis coding and documentation (SECOND) study. Hlth Inf Manag. 2024;53:61–7. doi: 10.1177/18333583221107713. [DOI] [PubMed] [Google Scholar]
- 9.Duke GJ, Loughnan D, De Frietas M, et al. Clinical evaluation of the national hospital-acquired complication programme. Intern Med J. 2022;52:1910–6. doi: 10.1111/imj.15468. [DOI] [PubMed] [Google Scholar]
- 10.Australian Institute of Health and Welfare . Canberra: Australian Institute of Health and Welfare; 2021. [4-Jun-2024]. Admitted patient safety and quality.https://www.aihw.gov.au/reports-data/myhospitals/intersection/quality/apc/hospital-acquiredcomplications Available. Accessed. [Google Scholar]
- 11.Duke GJ, Moran JL, Bersten AD, et al. Hospital-acquired complications: the relative importance of hospital- and patient-related factors. Med J Aust. 2022;216:242–7. doi: 10.5694/mja2.51375. [DOI] [PubMed] [Google Scholar]
- 12.Canning M, Lee CH, Bolitho R, et al. Evaluation of the nature, severity, likelihood and preventability of medication-related hospital-acquired complications. Aust Health Rev. 2020;44:935–40. doi: 10.1071/AH19215. [DOI] [PubMed] [Google Scholar]
- 13.Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32:101–14. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 14.Bearman G, Doll M, Cooper K, et al. Hospital Infection Prevention: How Much Can We Prevent and How Hard Should We Try? Curr Infect Dis Rep. 2019;21:2. doi: 10.1007/s11908-019-0660-2. [DOI] [PubMed] [Google Scholar]
- 15.Swensen SJ, Dilling JA, Milliner DS, et al. Quality: the Mayo Clinic approach. Am J Med Qual. 2009;24:428–40. doi: 10.1177/1062860609339521. [DOI] [PubMed] [Google Scholar]
- 16.Li QC, Karnon J, Codde J. Outcomes of completed quality activities in an Australian tertiary hospital, 2015-2019. Int J Qual Health Care. 2023;35:mzad074. doi: 10.1093/intqhc/mzad074. [DOI] [PubMed] [Google Scholar]
- 17.Hussein M, Pavlova M, Ghalwash M, et al. The impact of hospital accreditation on the quality of healthcare: a systematic literature review. BMC Health Serv Res. 2021;21:1057. doi: 10.1186/s12913-021-07097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flodgren G, Gonçalves-Bradley DC, Pomey M-P. External inspection of compliance with standards for improved healthcare outcomes. Cochrane Database Syst Rev. 2016;12:CD008992. doi: 10.1002/14651858.CD008992.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braithwaite J. Changing how we think about healthcare improvement. BMJ. 2018;361:k2014. doi: 10.1136/bmj.k2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Independent Hospital Pricing Authority Safety and quality. 2023. [4-Jun-2024]. https://www.ihacpa.gov.au/health-care/pricing/safety-and-quality Available. Accessed.
- 21.Cowie J, Nicoll A, Dimova ED, et al. The barriers and facilitators influencing the sustainability of hospital-based interventions: a systematic review. BMC Health Serv Res. 2020;20:588. doi: 10.1186/s12913-020-05434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sim MA, Ti LK, Mujumdar S, et al. Sustaining the Gains: A 7-Year Follow-Through of a Hospital-Wide Patient Safety Improvement Project on Hospital-Wide Adverse Event Outcomes and Patient Safety Culture. J Patient Saf. 2022;18:e189–95. doi: 10.1097/PTS.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 23.Jessup RL, Tacey M, Glynn M, et al. Evaluation of the effectiveness of a comprehensive care plan to reduce hospital acquired complications in an Australian hospital: protocol for a mixed-method preimplementation and postimplementation study. BMJ Open. 2020;10:e034121. doi: 10.1136/bmjopen-2019-034121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines From a Detailed Consensus Process. J Contin Educ Nurs. 2015;46:501–7. doi: 10.3928/00220124-20151020-02. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Healthcare Improvement Control charts. 2023. [4-Jun-2024]. https://www.ihi.org/resources/tools Available. Accessed.
- 26.Pronovost PJ, Armstrong CM, Demski R, et al. Creating a high-reliability health care system: improving performance on core processes of care at Johns Hopkins Medicine. Acad Med. 2015;90:165–72. doi: 10.1097/ACM.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 27.Pronovost PJ, Demski R, Callender T, et al. Demonstrating High Reliability on Accountability Measures at The Johns Hopkins Hospital. Jt Comm J Qual Patient Saf. 2013;39:531–AP5. doi: 10.1016/S1553-7250(13)39069-2. [DOI] [PubMed] [Google Scholar]
- 28.Pronovost PJ, Holzmueller CG, Callender T, et al. Sustaining Reliability on Accountability Measures at The Johns Hopkins Hospital. Jt Comm J Qual Patient Saf. 2016;42:51–60. doi: 10.1016/s1553-7250(16)42006-4. [DOI] [PubMed] [Google Scholar]
- 29.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 30.Pronovost PJ, Watson SR, Goeschel CA, et al. Sustaining Reductions in Central Line–Associated Bloodstream Infections in Michigan Intensive Care Units. Am J Med Qual. 2016;31:197–202. doi: 10.1177/1062860614568647. [DOI] [PubMed] [Google Scholar]
- 31.Institute for Healthcare Improvement . Institute for Healthcare Improvement; 2017. [3-Apr-2024]. Quality improvement essentials toolkit.https://www.ihi.org/resources/tools/quality-improvement-essentials-toolkit#downloads Available. Accessed. [Google Scholar]
- 32.Rothman AJ, Sheeran P, Wood W. Reflective and automatic processes in the initiation and maintenance of dietary change. Ann Behav Med. 2009;38 Suppl 1:S4–17. doi: 10.1007/s12160-009-9118-3. [DOI] [PubMed] [Google Scholar]
- 33.Lally P, van Jaarsveld CHM, Potts HWW, et al. How are habits formed: Modelling habit formation in the real world. Euro J Social Psych. 2010;40:998–1009. doi: 10.1002/ejsp.674. [DOI] [Google Scholar]
- 34.Martin LA, Mate K. Boston, Massachusetts: Institute for Healthcare Improvement; 2018. IHI innovation system. ihi white paper.https://www.ihi.org/sites/default/files/2023-10/IHIInnovationSystemWhitePaper.pdf Available. [Google Scholar]
- 35.Park ST. California Carnegie Foundation for the Advancement of Teaching; 2013. [29-Sep-2023]. 90-day cycle handbook.https://www.carnegiefoundation.org/wp-content/uploads/2014/09/90DC_Handbook_external_10_8.pdf Available. Accessed. [Google Scholar]
- 36.Arthur J. Agile Process Innovation; Hacking Lean Six Sigma for Maximum Results. Denver, Colorado: the United States of America Lifestar Publishing; 2019. [Google Scholar]
- 37.Creasey T. Applying the adkar model when change management is new. [29-Sep-2023]. https://www.prosci.com/blog/applying-the-adkar-model-when-change-management-is-new Available. Accessed.
- 38.Rogers EM, Singhal A, Quinlan MM. An integrated approach to communication theory and research. Routledge; 2014. Diffusion of innovations; pp. 432–48. [Google Scholar]
- 39.Jager W. Libor Amicorum for Charles Vlek, Groningen: University of Groningen; 2003. Breaking bad habits: a dynamical perspective on habit formation and change.https://d1wqtxts1xzle7.cloudfront.net/32308125/Jager_habits_chapter_2003-libre.pdf?1391568773=&response-content-disposition=inline%3B+filename%3DBreaking_bad_habits_a_dynamical_perspect.pdf&Expires=1717559569&Signature=LyMPFV-ljbqbFkfcEiluDoBeNTuC3a0DM4EbaZdPYzEeNS85iW~SpAUgsw1mYLBtGS5b7dXarkkswijZ9KbTO6w-V6rFio5nO0CDTswJkRKFmiE3a~nuViuxcJKXwBj48zW0dU4oOD-n5uxkvnkoiQkBKjO7LWOayytrkbbUeE20HmAzwCH3H0jxDXF3lAKKc6qPUnjXDJvacFdpbkhxfJrX2owc9dI4cm~GSgP7VJ7fL0y7l5plrOMpX4zZAwqD7Joxb7RVOC2sDYcG3d3y508HK~cmByg0VNdLBnu67Zc20fcestBQ-D6xIgB0lDNTiO3-XHYvKhRaF9aECXIHuA__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Available. [Google Scholar]
- 40.Daly J, Jackson D, Mannix J, et al. The importance of clinical leadership in the hospital setting. JHL. 2014;6:75. doi: 10.2147/JHL.S46161. [DOI] [Google Scholar]
- 41.Birken SA, Lee S-Y, Weiner BJ, et al. From strategy to action: how top managers’ support increases middle managers’ commitment to innovation implementation in health care organizations. Health Care Manage Rev. 2015;40:159–68. doi: 10.1097/HMR.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivers NM, Sales A, Colquhoun H, et al. No more 'business as usual' with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci. 2014;9:14. doi: 10.1186/1748-5908-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Women’s Healthcare Australasia . Australia Women’s Healthcare Australia; 2019. [2-Mar-2024]. Women’s healthcare australasia. the how to guide: wha cec perineal protection bundle.https://women.wcha.asn.au/wp-content/uploads/sites/3/2022/04/wha_national_collaborative_how_to_guide_21.1.20.pdf Available. Accessed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.