Abstract
Background
To date, evidence on late-onset immune-related adverse events (irAEs) with immune checkpoint inhibitors (ICIs) is limited to a small number of clinical cases. This study aimed to identify drug- and patient-related characteristics potentially associated with the reporting of late-onset irAEs with ICIs in VigiBase, the WHO global database of individual case safety reports (ICSRs).
Methods
Observational study comparing deduplicated ICSRs with ICIs reporting late-onset irAEs (occurred >90 days after ICI discontinuation) versus ICSRs with ICIs not reporting late-onset irAEs, collected in VigiBase from 2011 to December 31, 2022. Logistic regression was used to model the relationship between drug-related and patient-related characteristics of ICSRs and the reporting of late-onset irAEs. Significance was determined for variables with the lower bound of the 95% CI of the reporting OR (ROR) higher than 1 and a p value <0.05.
Results
The study population consisted of 6006 ICSRs with ICI-related irAEs (4574, 76.2%, originated from Europe; 3900, 64.9%, involved males; median patient age was 67 years, IQR 59–74 years). Of these, 344 (5.7%) ICSRs reported a total of 388 late-onset irAEs, among which the most frequent were thyroiditis (n=45), pneumonitis (n=37), interstitial lung disease (n=25), hepatitis (n=23) and vitiligo (n=19). Median time to onset since ICI discontinuation was 167 days (IQR 115–294 days), with negligible proportion (3.2%) of co-reported antineoplastic agents during the discontinuation period. Logistic regression models showed disproportionate reporting of late-onset irAEs with ICI combination therapy (ROR 2.33, 95% CI 1.19 to 4.57), reporting of multiple irAEs (ROR 3.96, 95% CI 2.85 to 5.52), reporting of cutaneous irAEs (ROR 1.83, 95% CI 1.24 to 2.71), and melanoma (ROR 1.47, 95% CI 1.04 to 2.06).
Conclusions
This global pharmacovigilance study provides the largest case series of late-onset irAEs with ICIs to date and identifies characteristics of ICSRs associated with disproportionate reporting. Dedicated prospective observational studies focused on long-term sequelae, quality of life and survival of patients developing late-onset irAEs with ICIs should be planned to confirm whether these reporting characteristics are predictors of actual occurrence. Furthermore, translational research should be encouraged to clarify the molecular mechanisms underlying late-onset irAE development.
Keywords: Immune Checkpoint Inhibitor, Immune related adverse event - irAE
WHAT IS ALREADY KNOWN ON THIS TOPIC
Late-onset immune-related adverse events (irAEs) in patients with cancer treated with immune checkpoint inhibitors (ICIs) can have a negative impact on their quality of life if not promptly recognized and appropriately treated.
Current evidence on late-onset irAEs with ICIs is limited to a small number of clinical cases.
WHAT THIS STUDY ADDS
By exploiting VigiBase, the global pharmacovigilance database, this study gathered a relatively large number of individual case safety reports reporting late-onset irAEs with ICIs and identified ICI combination regimen, reporting of multiple irAEs, reporting of cutaneous irAEs and melanoma as characteristics associated with disproportionate reporting of late-onset irAEs.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Future research should assess whether these characteristics associated with the reporting of late-onset irAEs may also predict their actual occurrence.
Translational studies are needed to elucidate the underlying molecular mechanisms.
Introduction
Since marketing authorization of the first-in-class ipilimumab in 2011 by the Food and Drug Administration (FDA), immunotherapy with immune checkpoint inhibitors (ICIs) has become a mainstay for the treatment of an expanding number of cancers in the adjuvant/neoadjuvant curative setting.1 2
By activating the immune system against tumor cells, ICIs are accompanied by a peculiar safety profile characterized by immune-related adverse events (irAEs) potentially affecting any organs3 and displaying sex differences.4 5
Although most irAEs occur within the first 3 months of ICI treatment,6 late-onset irAEs (ie, those manifesting more than 3 months after discontinuation of immunotherapy—cfr. Society for Immunotherapy of Cancer (SITC)7 8) may also occur,9 reaching an overall 5% incidence.10 11 Biological plausibility for late-onset irAEs is provided by the fact that, 1 year after drug discontinuation, ICIs are still detectable in the organism,12 and programmed cell death 1 (PD-1) occupancy by nivolumab is still 40% after 8 months from the last dose.13 Nonetheless, evidence on late-onset irAEs with ICIs is still limited to a small number of clinical cases detailing a heterogeneous spectrum of manifestations at the level of the culprit organs and a variable time elapsed since ICI discontinuation.1014,18 These series of cases did not assess drug-related and/or patient-related characteristics associated with the occurrence of late-onset irAEs. In this regard, the only observation made was that of Owen et al10 on a cohort of 118 patients with melanoma, who developed irAEs more than 12 months after commencement of an anti-PD-1 therapy. In this cohort, early irAEs occurred within the first 12 months of ICI treatment affected 58% of patients.10
This gap of knowledge about drug-related and patient-related characteristics contributing to the development of late-onset irAE leaves both clinicians and patients unable to promptly recognize and adequately treat them, with a potential negative impact on patients’ quality of life.
Considering the relative rarity of late-onset irAEs, post-marketing surveillance through individual case safety reports (ICSRs) of suspected adverse drug reactions (ADRs) represents a privileged setting to characterize their spectrum and relevant clinical features,19 20 together with their onset timing from drug discontinuation and possible drug-related and patient-related characteristics associated with their reporting. Therefore, by exploiting VigiBase, the WHO global database of adverse event reports for medicines and vaccines maintained by the Uppsala Monitoring Centre, we aimed to identify potential drug-related and patient-related characteristics associated with disproportionate reporting of late-onset irAEs with ICIs.
Methods
Study design and data source
This observational study compares deduplicated ICSRs with ICIs reporting late-onset irAEs versus ICSRs with ICIs not reporting late-onset irAEs, collected in VigiBase from 2011 to December 21, 2022 (accessed through VigiLyze on February 5, 2023). National pharmacovigilance centers of the WHO Program for International Drug Monitoring (WHO-PIDM) member countries are responsible for the electronic transfer of ICSRs to VigiBase. In VigiBase, events are coded according to MedDRA (the international Medical Dictionary for Regulatory Activities terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), V.26.0). This terminology is hierarchically structured, from the very specific level of “lowest level terms” that reflect how information is communicated by reporters, to the very general level of “system organ classes”, whereby events are grouped by etiology, manifestation site or purpose. Above “lowest level terms” are “preferred terms”, which refer to single medical concepts (eg, a symptom, sign, diagnosis, therapeutic indication, or investigation). To facilitate retrieval and analysis, MedDRA also includes validated, standard sets of terms, called Standardized MedDRA Queries (SMQs), concerning safety topics of interest. Drugs are standardized using WHODrug Global, the international reference drug dictionary for medicinal product information.21 This study was reported according to the READUS-PV Guidelines, The REporting of A Disproportionality analysis for drUg Safety signal detection using ICSRs in PharmacoVigilance.22 23
Selection criteria of ICSRs
ICSRs recording as suspect or interacting drugs at least one ICI among the following active ingredients, ipilimumab, pembrolizumab, nivolumab, cemiplimab, atezolizumab, avelumab, durvalumab and dostarlimab, were retrieved. ICSRs that co-reported additional suspect and/or interacting drugs and ICSRs with partial or missing information on the date of onset of ADRs and/or the dates of start and end of ICI treatment were excluded from the study population. Only ICSRs recording one or more irAEs were selected to constitute the study population. Among reported events, irAEs were identified on the basis of the SMQ “immune-related/autoimmune disorders” and through a critical appraisal of all reported events containing or ending with “-itis” based on a consensus among authors, comprising clinical pharmacologists (online supplemental table 1). ICSRs with late-onset irAEs (cases) were defined as those in which one or more irAEs occurred more than 3 months after ICI discontinuation7 and were compared with ICSRs not reporting late-onset irAEs (non-cases). A flowchart summarizing the selection of ICSRs is shown in online supplemental figure 1. With regard to the end date of ICI treatment, depending on how ICI administration schedule was reported, if multiple start/end dates were recorded (eg, if single administrations of a therapy cycle were recorded or if multiple cycles of ICI treatments were administered), the last dose (indicating ICI discontinuation) was defined as the one with the most recent end date.
Variables
The following characteristics of ICSRs were described: country of origin, reporting year, reporter type, and seriousness (defined based on the recording of one of the following outcomes: death, life-threatening, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability or incapacity, congenital anomaly, or otherwise medically significant condition); patient age and sex; regimen, duration and indication of ICI treatment; number of reported irAEs (single or multiple), time to onset (in days) since ICI discontinuation and organs affected by ICI toxicity. The reporting of irAEs occurred during the first 3 months after ICI initiation (when most of irAEs occur6) was also evaluated and defined as “early”. ICI regimen was categorized as anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) monotherapy (ipilimumab), anti-PD-1 monotherapy (pembrolizumab, nivolumab, cemiplimab, or dostarlimab), anti-PD-L1 (programmed cell death ligand 1) monotherapy (atezolizumab, avelumab, or durvalumab), and anti-PD-1/anti-CTLA-4 combination (with two ICIs with identical start dates). Based on the recent SITC consensus definition for multisystem irAEs,7 we assessed the reporting of multiple irAEs as the presence, within an ICSR, of irAEs that occurred concomitantly or at different times during and/or after ICI treatment, and that affected more than one organ or occurred in different tissues of the same organ. Organ toxicity was defined based on the “system organ class” MedDRA terms (ie, blood, cardiac, endocrine, eye, gastrointestinal, hepatic, immune, musculoskeletal, neurologic, renal, reproductive, respiratory, skin, vascular). Lastly, co-reporting of antineoplastic agents (of the anatomical therapeutic chemical—ATC—group L01) as concomitant drugs (ie, not suspected to be the cause of the reported adverse events according to reporters), administered after ICI discontinuation but before the date of onset of reported irAE(s), was evaluated.
Statistical analysis
Descriptive analyses were conducted using absolute and percentage frequencies for categorical variables and median with IQR for continuous variables. Demographic and clinical characteristics of cases and non-cases were compared using two-sided Mann-Whitney test for continuous variables and two-sided χ2 test for categorical variables. Significance was determined as a p value <0.05.
Logistic regression was used to model the relationship between drug-related and patient-related characteristics of ICSRs with ICIs and the reporting of late-onset irAEs. Variables were selected for inclusion using a forward stepwise regression approach, with retention of those variables with a p value <0.05. A multivariable logistic regression model was then created, including interaction variables between sex and the organ toxicities for which an association with the reporting of late-onset irAEs was previously found.
ICSRs with missing data for any of the variables included in the multivariable regression model were excluded from the analysis. Significance was determined for variables with the lower bound of the 95% CI of the reporting OR (ROR) higher than 1 and a p value <0.05. Analyses were conducted using Statistical Analysis System Software (V.9.4; SAS Institute, Cary, NC).
According to the Human Research Act (810.30, of 30 September 2011 - status as of 1 September 2023, Art. 2), from the Federal Assembly of the Swiss Confederation, ethical approval and written informed consents were not required.
Results
Characteristics of the study population
The study population consisted of 6006 ICSRs with ICI-related irAEs. As summarized in table 1, the majority of ICSRs originated from Europe (4574, 76.2%), with physicians being the most numerous reporters (4107, 68.4%). More ICSRs concerned males (3900, 64.9%), and the median age was 67 years (IQR 59–74 years). The most common ICI regimens were anti-PD-1 monotherapies, in particular with nivolumab (2648 ICSRs, 44.1%) and pembrolizumab (1512, 25.2%). Lung cancer (2367, 39.4%, of which 2130, 90.0%, explicitly reported as metastatic) and melanoma (1944, 32.4%, of which 1886, 97.0%, explicitly reported as metastatic) were the most frequent indications. The majority of ICSRs were categorized as serious (5111, 85.1%) and primarily related with new or prolonged hospitalization (2829, 47.1%).
Table 1. Demographics and clinical characteristics of the individual case safety reports included in the study population.
| Characteristic | n (%), N=6006 |
| Country | |
| Europe* | 4574 (76.2) |
| Asia | 1141 (19.0) |
| Australia | 218 (3.6) |
| South America | 58 (1.0) |
| North America | 13 (0.2) |
| Africa | 2 (0.0) |
| Year | |
| 2011 | 13 (0.2) |
| 2012 | 20 (0.3) |
| 2013 | 22 (0.4) |
| 2014 | 67 (1.1) |
| 2015 | 134 (2.2) |
| 2016 | 461 (7.7) |
| 2017 | 782 (13.0) |
| 2018 | 1067 (17.8) |
| 2019 | 956 (15.9) |
| 2020 | 833 (13.9) |
| 2021 | 863 (14.4) |
| 2022 | 788 (13.1) |
| Reporter | |
| Physician | 4107 (68.4) |
| Pharmacist | 941 (15.7) |
| Other health professional | 811 (13.5) |
| Patient/consumer | 60 (1.0) |
| Lawyer | 1 (0.0) |
| Missing | 86 (1.4) |
| Sex | |
| Male | 3900 (64.9) |
| Female | 2051 (34.2) |
| Missing | 55 (0.9) |
| Age | |
| Reported | 4981 (82.9) |
| Median (IQR), years | 67 (59–74) |
| Missing | 1025 (17.1) |
| Regimen | |
| Anti-CTLA-4 monotherapy | |
| Ipilimumab | 478 (8.0) |
| Anti-PD-1/PD-L1 monotherapy | |
| Nivolumab | 2648 (44.1) |
| Pembrolizumab | 1512 (25.2) |
| Cemiplimab | 82 (1.4) |
| Dostarlimab | 3 (0.1) |
| Atezolizumab | 265 (4.4) |
| Durvalumab | 358 (6.0) |
| Avelumab | 71 (1.2) |
| Anti-PD-1 and anti-CTLA-4 combination | |
| Ipilimumab and nivolumab | 586 (9.8) |
| Ipilimumab and pembrolizumab | 3 (0.1) |
| Indication (in ≥1% of ICSRs) | |
| Lung cancer | 2367 (39.4) |
| Melanoma | 1944 (32.4) |
| Renal cancer | 415 (6.9) |
| Urothelial carcinoma | 130 (2.2) |
| Bronchial cancer | 127 (2.1) |
| Head and neck cancer | 93 (1.6) |
| Mesothelioma | 62 (1.0) |
| Missing | 451 (7.5) |
| Seriousness | |
| Yes | 5111 (85.1) |
| Caused/prolonged hospitalization | 2829 |
| Other medically important condition | 1329 |
| Death | 519 |
| Life threatening | 356 |
| Disabling/incapacitating | 78 |
| No | 830 (13.8) |
| Missing | 65 (1.1) |
Out of 4574 ICSRs from Europe, 3618 (79.1%) originated from France (n=1817), Italy (n=837), Germany (n=678) and Spain (n=286).
CTLA-4, cytotoxic T-lymphocyte antigen-4; ICSRs, individual case safety reportsPD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1
Demographic and clinical characteristics of ICSRs with ICIs reporting late-onset irAEs versus ICSRs with ICIs not reporting late-onset irAEs
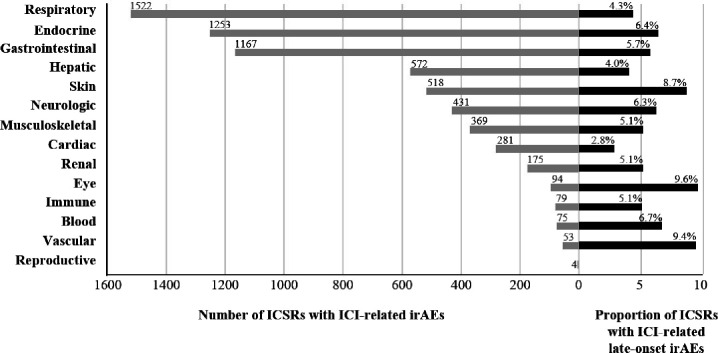
Of 6006 retained ICSRs with ICIs, 344 (5.7%) reported late-onset irAEs. The highest proportion of ICSRs with late-onset irAEs out of the total number of ICSRs by organ toxicity concerned the eyes (9.6%), vascular system (9.4%) and skin (8.7%) (figure 1). Among the 344 ICSRs, there were 388 late-onset irAEs. The most frequently reported late-onset irAEs were thyroiditis (n=45), pneumonitis (n=37), interstitial lung disease (n=25), hepatitis (n=23), and vitiligo (n=19). Online supplemental table 1 shows the spectrum of clinical presentations of late-onset irAEs by organ toxicity.
Figure 1. Number of individual case safety reports and percentage of those with late-onset immune-related adverse events according to organ toxicity. ICI, immune checkpoint inhibitor; ICSRs, individual case safety reports; irAEs, immune-related adverse events.
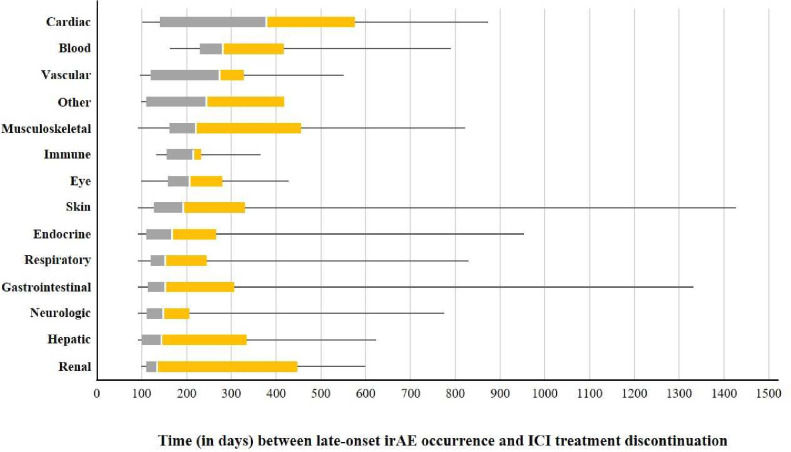
As compared with ICSRs not reporting late-onset irAEs, ICSRs reporting late-onset irAEs were associated with ICI combination regimen (16.9% vs 9.4%, p<0.0001), melanoma as indication (45.4% vs 31.6%, p<0.0001), shorter ICI treatment duration (median 42 days vs 63 days, p<0.0001), and more frequently reported multiple irAEs (25.3%–87 ICSRs vs 12.1%, p<0.0001) (table 2). Among cases, 11 ICSRs (3.2%) co-reported antineoplastic agents as concomitant drugs during the discontinuation period, before the date(s) of onset of reported irAE(s) (table 2). Out of 344 cases of late-onset irAEs, 38 (11.1%) reported early irAEs (affecting the same organ subsequently affected by late-onset irAEs in 15 ICSRs, 4.4%). For the 388 late-onset irAEs, median time to onset since ICI discontinuation was 167 days (IQR 115–294 days), and the most delayed onset was observed for cardiac toxicity (378 days, IQR 140–576 days) (figure 2).
Table 2. Demographics and clinical characteristics of individual case safety reports with ICIs reporting late-onset immune-related adverse event(s) versus individual case safety reports with ICIs not reporting late-onset immune-related adverse event(s).
| Characteristics | ICSRs with ICIs reporting late-onset irAEs | ICSRs with ICIs not reporting late-onset irAEs | P value | Adjusted p value* |
| N=344 | N=5662 | |||
| Sex | ||||
| Male | 209 (60.8) | 3691 (65.2) | 0.104 | 1.044 |
| Female | 131 (38.1) | 1920 (33.9) | ||
| Missing | 4 (1.2) | 51 (0.9) | ||
| Age, years | ||||
| Reported | 221 (64.2) | 4760 (84.1) | ||
| Median (IQR) | 68 (58–74) | 67 (59–74) | 0.861 | 1 |
| Missing | 123 (35.8) | 902 (15.9) | ||
| Regimen | ||||
| Anti-CTLA-4 monotherapy | 0.054 | 0.543 | ||
| Ipilimumab | 18 (5.2) | 460 (8.1) | ||
| Anti-PD-1/PD-L1 monotherapy | 0.031 | 0.306 | ||
| Nivolumab | 128 (37.2) | 2520 (44.5) | ||
| Pembrolizumab | 84 (24.4) | 1428 (25.2) | ||
| Cemiplimab | 10 (2.9) | 72 (1.3) | ||
| Dostarlimab | 1 (0.3) | 2 (0.0) | ||
| Atezolizumab | 10 (2.9) | 255 (4.5) | ||
| Durvalumab | 34 (9.9) | 324 (5.7) | ||
| Avelumab | 1 (0.3) | 70 (1.2) | ||
| Anti-PD-1 and anti-CTLA-4 combination | <0.0001 | <0.0001 | ||
| Ipilimumab and nivolumab | 58 (16.9) | 528 (9.3) | ||
| Ipilimumab and pembrolizumab | – | 3 (0.1) | ||
| Indication (in ≥1% of ICSRs) | ||||
| Melanoma | 156 (45.4) | 1788 (31.6) | <0.0001 | <0.0001 |
| Lung cancer | 105 (30.5) | 2262 (40.0) | <0.0001 | 0.0008 |
| Renal cancer | 22 (6.4) | 393 (6.9) | ||
| Bronchial cancer | 6 (1.7) | 121 (2.1) | ||
| Skin cancer | 6 (1.7) | – | ||
| Urothelial carcinoma | 5 (1.5) | 125 (2.2) | ||
| Head and neck cancer | – | 91 (1.6) | ||
| Mesothelioma | – | 59 (1.0) | ||
| Missing | 17 (4.9) | 237 (7.1) | ||
| Duration | ||||
| Median (IQR), days | 42 (0–125) | 63(21–165) | <0.0001 | <0.0001 |
| <90 | 233 (67.7) | 3499 (61.8) | ||
| 90–179 | 43 (12.5) | 857 (15.1) | ||
| 180–364 | 45 (13.1) | 815 (14.4) | ||
| ≥365 | 23 (6.7) | 491 (8.7) | ||
| Multiple irAEs | 87 (25.3) | 687 (12.1) | <0.0001 | <0.0001 |
| Seriousness | ||||
| Yes | 291 (84.6) | 4820 (85.1) | 0.918 | 9.179 |
| Caused/prolonged hospitalization | 189 | 2640 | ||
| Other medically important condition | 74 | 1255 | ||
| Death | 19 | 500 | ||
| Life threatening | 8 | 348 | ||
| Disabling/incapacitating | 1 | 77 | ||
| No | 48 (14.0) | 782 (13.8) | ||
| Missing | 5 (1.4) | 60 (1.1) | ||
| No. of ICSRs co-reporting antineoplastic agents† as concomitant drugs, administered after ICI discontinuation but before the date(s) of onset of reported irAE(s) | 11 (3.2) | 26 (0.5) | <0.0001 | <0.0001 |
Data are n (%).
Holm-Bonferroni correction for multiple tests.
ATC (anatomical therapeutic chemical) L01.
CTLA-4, cytotoxic T-lymphocyte antigen-4; ICI, immune checkpoint inhibitor; ICSRs, individual case safety reports; irAE, immune-related adverse event; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1
Figure 2. Boxplot displaying the time (in days) between late-onset immune-related adverse event occurrence and immune checkpoint inhibitor treatment discontinuation according to organ toxicity. Within each box, vertical white lines denote median values; boxes extend from the 25th to the 75th percentiles of each group’s distribution of values; horizontal extending lines denote the minimum and maximum values observed. “Other” included the following seven events reported in five individual case safety reports and not belonging to any of the predefined organ toxicities: immune-mediated adverse reaction (n=2), serositis, autoinflammatory disease, smooth muscle antibody positive, anti-neutrophil cytoplasmic antibody positive, anti-actin antibody positive (all n=1). ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events.
Drug-related and patient-related characteristics of ICSRs with ICIs associated with the reporting of late-onset irAEs
Forward stepwise regressions showed that monotherapy with a PD-1/PD-L1 agent, ICI combination therapy, duration of ICI treatment, reporting of multiple irAEs, endocrine and skin toxicities, lung cancer and melanoma, and reporting of early irAEs were associated with the reporting of late-onset irAEs (table 3). From the multivariable logistic regression model, disproportionate reporting of late-onset irAEs was observed in association with ICI combination therapy (ROR, 2.33, 95% CI 1.19 to 4.57), reporting of multiple irAEs (ROR 3.96, 95% CI 2.85 to 5.52), reporting of cutaneous irAEs (ROR 1.83, 95% CI 1.24 to 2.71), and melanoma (ROR 1.47, 95% CI 1.04 to 2.06) (table 3). Remarkably, no interaction was found between endocrine toxicity and patient’s sex (p=0.38) nor between skin toxicity and patient’s sex (p=0.35) in relation to the reporting of late-onset irAEs with ICIs.
Table 3. Logistic regression modeling the relationship between drug-related and patient-related characteristics and the reporting of late-onset immune-related adverse event(s) with immune checkpoint inhibitors in VigiBase.
| Characteristics (independent variables) | Reporting of late-onset irAE(s) with ICIs (dependent variable) | |||
| Forward stepwise modelROR (95% CI) | P value* | Multivariable modelROR (95% CI) | P value | |
| Sex (female vs male)† | 1.00 (0.66 to 1.04) | 0.105 | 0.94 (0.72 to 1.22) | 0.644 |
| Age (continuous) | 1.00 (0.99 to 1.02) | 0.434 | – | – |
| CTLA4 monotherapy (yes vs no) | 1.60 (0.99 to 2.60) | 0.057 | – | – |
| PD-1/PD-L1 monotherapy (yes vs no) | 1.34 (1.03 to 1.74) | 0.031 | 1.40 (0.74 to 2.62) | 0.300 |
| Combination therapy (yes vs no) | 0.51 (0.38 to 0.69) | <0.0001 | 2.33 (1.19 to 4.57) | 0.014 |
| Duration of ICI treatment (continuous) | 1.001 (1.000 to 1.002) | 0.023 | 0.992 (0.990 to 0.993) | <0.0001 |
| Multiple irAEs (yes vs no) | 0.41 (0.32 to 0.53) | <0.0001 | 3.96 (2.85 to 5.52) | <0.0001 |
| Blood toxicity (yes vs no) | 0.85 (0.34 to 2.12) | 0.725 | – | – |
| Cardiac toxicity (yes vs no) | 1.88 (0.96 to 3.68) | 0.066 | – | – |
| Endocrine toxicity (yes vs no) | 0.70 (0.54 to 0.89) | 0.004 | 1.26 (0.92 to 1.71) | 0.145 |
| Eye toxicity (yes vs no) | 0.57 (0.28 to 1.14) | 0.110 | – | – |
| Gastrointestinal toxicity (yes vs no) | 0.83 (0.64 to 1.07) | 0.154 | – | – |
| Hepatic toxicity (yes vs no) | 1.11 (0.75 to 1.63) | 0.602 | – | – |
| Immune toxicity (yes vs no) | 1.14 (0.42 to 3.14) | 0.798 | – | – |
| Musculoskeletal toxicity (yes vs no) | 1.06 (0.67 to 1.69) | 0.793 | – | – |
| Neurologic toxicity (yes vs no) | 0.87 (0.58 to 1.29) | 0.476 | – | – |
| Renal toxicity (yes vs no) | 1.00 (0.53 to 1.92) | 0.994 | – | – |
| Respiratory toxicity (yes vs no) | 1.25 (0.96 to 1.63) | 0.093 | – | – |
| Skin toxicity (yes vs no) | 0.57 (0.42 to 0.79) | 0.0007 | 1.83 (1.24 to 2.71) | 0.002 |
| Vascular toxicity (yes vs no) | 0.47 (0.20 to 1.11) | 0.085 | – | – |
| Lung cancer (yes vs no) | 1.61 (1.27 to 2.05) | <0.0001 | 0.85 (0.60 to 1.21) | 0.360 |
| Melanoma (yes vs no) | 0.57 (0.46 to 0.71) | <0.0001 | 1.47 (1.04 to 2.06) | 0.027 |
| Reporting of early irAEs (yes vs no) | 15.14 (10.76 to 21.30) | <0.0001 | 0.02 (0.01 to 0.03) | <0.0001 |
Variables with a p value <0.05 from the forward stepwise regression approach were included in the multivariable logistic regression analysis.
Patient’s sex was included as covariate in the multivariable regression model to assess its interaction with specific ICI organ toxicities.
CTLA4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; PD-1/PD-L1, programmed cell death protein 1/programmed cell death ligand 1ROR, reporting OR
Discussion
By analyzing ICSRs collected in the global pharmacovigilance database, VigiBase, this study expanded the limited series of clinical cases so far described in the scientific literature on late-onset irAEs with ICIs.1014,18 Moreover, it identified ICI combination therapy, reporting of multiple irAEs, reporting of cutaneous irAEs and melanoma as characteristics of ICSRs associated with disproportionate reporting of late-onset irAEs in the post-marketing setting.
The 344 ICSRs of late-onset toxicity with ICIs represent approximately 6% of the total number of ICSRs with ICIs included in the study population, an estimate in line with data derived from both clinical trial and observational settings.10 11 The largest number of late-onset irAEs was endocrine, also previously described among the most common chronic long-lasting irAEs,9 with important implications for toxicity management: clinicians should remain vigilant and initiate timely proper strategies to counteract potentially irreversible damage. When considering the proportion of ICSRs reporting late-onset irAEs out of the total number of ICSRs from the study population by organ toxicity, we observed that the eyes, vascular system and skin toxicity (mainly vitiligo, acknowledged among the consensus-based diagnosis of dermatological irAEs,24 followed by pemphigoid, dermatitis and psoriasis), showed the highest proportions, partly confirming previous clinical cases of ICI-related late-onset uveitis, vitiligo and pemphigoid.14 15
Although most of ICSRs from the study population described a single irAE, multiple irAEs were more frequent among ICSRs reporting late-onset irAEs, with early irAEs (usually affecting different organs) reported in about 10% of cases.
For late-onset irAEs, the observed median time to onset of about 6 months supports evidence from clinical reports,14 with differences across organ toxicities. The most delayed time to onset was observed for the few cases of cardiac late-onset irAEs (namely, five events of myocarditis and three of pericarditis), in line with previous observations,25,27 even if myocarditis generally occurs soon after the start of ICI therapy.28,30
Across the entire study population, ICI monotherapy was the most frequently reported regimen, reflecting earlier worldwide approval and consequently higher frequency of spontaneous reporting of related adverse events compared with combination therapy.31 32 Nevertheless, late-onset irAEs were more likely to be reported with ICI treatments of relatively short duration, in line with previous findings from scattered clinical cases,14 but contrary to them, were more commonly reported with ICI combination therapy.1014,16 It is possible that both anti-PD-1 and anti-CTLA-4 antibodies may occupy immune checkpoint receptors due to their long-term persistence in the body,12 13 thus synergistically increasing T cell activity and the possibility of developing not only early irAEs (sometimes even fulminant30) but also late-onset irAEs. Future translational studies should focus on better understanding the molecular mechanisms underlying the occurrence of late-onset irAEs. Lastly, in support of the suspected causative role played by ICIs (according to reporters) in the onset of late toxicity, only a negligible proportion of cases reported the administration of other antineoplastic drugs during the period of discontinuation of ICI therapy, prior to the occurrence of late-onset irAEs.
From logistic regression, we found that ICI combination therapy, reporting of multiple irAEs, reporting of cutaneous irAEs, and melanoma were characteristics of ICSRs with ICIs associated with disproportionate reporting of late-onset irAEs. The association between reporting of multiple irAEs and disproportionate reporting of late-onset irAEs might suggest that ICI treatments that, for plausible reasons related to regimen,33 dose34 35 or molecular target(s),36 exhibit high-grade toxicity affecting a range of organs, are also more likely to be associated with the reporting of late-onset irAE. Consistently, we found that late-onset irAEs were more frequently reported with ICI combination therapy, which has a high incidence of multiple irAEs of all grades (26–40%).37 The melanoma/late-onset irAEs association could, on the one hand, reflect the fact that melanoma was the second most frequent type of cancer for which ICIs were administered in the study population and, on the other hand, could relate to ICI treatment, which, as aforementioned, because of dose, regimen, or molecular target, might have caused increased toxicity including late-onset irAEs. However, we did not carry out this evaluation, which was outside the scope of the study. Why reporting of cutaneous irAEs was associated with disproportionate reporting of late-onset irAEs is unclear and might be related to the fact that manifestations of skin toxicity could be more easily assessed on clinical examination than other organ toxicities and consequently might be reported more frequently by treating physicians. Consistently, most of the ICSRs from the study population were reported by physicians. When caused by anti-PD-1 or PD-L1 antibodies, cutaneous irAEs tend to have a delayed onset than when caused by anti-CTLA-4 monotherapies, either during a prolonged treatment or after treatment has been discontinued.38 Conversely, ICI combination therapy is associated with the development of earlier cutaneous irAEs compared with monotherapies.38 Since sex differences have been observed in the profile of organs and systems affected by ICI toxicity,4 5 we assessed whether the disproportionate reporting of late-onset irAEs in ICSRs reporting skin toxicity differed between females and males, without finding supporting evidence.
Strengths and limitations
By covering a wide (theoretically worldwide) geographical distribution through VigiBase, this pharmacovigilance study provided a broad overview of the current spontaneous reporting of irAEs with ICIs, notwithstanding the differences between countries in drug approvals and pharmacovigilance systems. These differences were confirmed by the observation that the selection criteria applied in the present study resulted in more than two-thirds of ICSRs coming from Europe and only a minority from North America, which, on the other hand, according to a 10-year overview of reporting with ICIs in VigiBase where all ICSRs with ICIs were considered regardless of the presence of co-suspect/interacting drugs, emerged as the main contributing region, just ahead of Europe.39 Furthermore, it collected the highest number of ICSRs of late-onset irAEs with ICIs to date, supporting the generalizability of the results. Since the collection of ICSRs in VigiBase takes place continuously without time restrictions, long-term surveillance of ADRs can be carried out even for late/delayed events, as in the case of this study.
This study suffers from the limitations of the data source used, VigiBase and ICSRs. First, reporting biases, including under-reporting—likely deriving from misrecognition of late-onset irAEs with ICIs and/or clinical reluctance at attributing late-onset irAEs to prior ICI treatments in favor of misattribution to more proximal events or treatments—and more frequent reporting of adverse events with serious consequences for the patients involved. Second, partial or missing data, which may affect the reliability of adjustment by regression modeling40 that is the reason why we excluded part of ICSRs from the study population. Third, the inability to prove causality, with late-onset toxicity with ICIs often remaining a diagnosis of exclusion in clinical practice.41 42 Fourth, the lack of clinical details including the exact reason why ICI treatment was discontinued, follow-up information on the management and course of ICI toxicity, long-term responders, patient’s quality of life and survival. Furthermore, the exclusion from the study population of ICSRs with other drugs besides ICIs as suspect/interacting (to minimize confounders and bias due to co-reported drugs), or with incomplete information for the variables considered (to run the regression models) is likely to have reduced the number of cases of potential interest. Therefore, it is important that clinicians, researchers and other healthcare professionals submit reports of suspected ICI-related late-onset irAEs with complete demographic information and detailed clinical data. For this purpose, similar to the SERIO registry recently established for the systematic analysis of immunotherapy-induced side effects,43 we would propose the creation of a tailor-made pharmacovigilance database gathering cases of late-onset irAEs with ICIs with specific information, not otherwise routinely collected, encompassing patient medical history, diagnostic and/or laboratory tests, management, course (including chronicity, eg, of late-onset endocrine irAEs), long-term sequelae (like impaired fertility and increased cardiovascular risk), outcome (especially when fatal), and impact of reported late-onset irAEs on patient’s quality of life and survival. This will allow a better understanding and evaluation of the actual spectrum of late-onset irAEs with ICIs and drug-related and patient-related characteristics associated with their reporting, thus finally supporting optimal management.
Conclusions
With this global pharmacovigilance study using VigiBase, we described the largest post-marketing case series of late-onset irAEs with ICIs and identified some drug-related and patient-related characteristics associated with disproportionate reporting: ICI combination therapy, reporting of multiple irAEs, reporting of cutaneous irAEs and melanoma.
We call clinicians for increased awareness on this potential distinct clinical entity. Future prospective observational studies should be planned to confirm whether the same characteristics associated with increased reporting may also be associated with the occurrence of late-onset irAE. In addition, translational research would be useful to establish the molecular basis of late-onset irAEs after ICI discontinuation.
supplementary material
Acknowledgements
The authors acknowledge the Uppsala Monitoring Center (UMC) for providing access to and use of the data from VigiBase for the purpose of the present study. In VigiBase, information comes from a variety of sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases. Moreover, the information of the present study does not represent the opinion of the UMC or the WHO. MedDRA trademark is registered by ICH.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: According to the Human Research Act (810.30, of September 30, 2011 - status as of September 1, 2023, Art. 2), from the Federal Assembly of the Swiss Confederation, ethical approval and written informed consents were not required.
Contributor Information
Roberta Noseda, Email: roberta.noseda@eoc.ch.
Francesca Bedussi, Email: francesca.bedussi@eoc.ch.
Valentina Giunchi, Email: valentina.giunchi2@unibo.it.
Michele Fusaroli, Email: michele.fusaroli2@unibo.it.
Emanuel Raschi, Email: emanuel.raschi@unibo.it.
Alessandro Ceschi, Email: alessandro.ceschi@eoc.ch.
Data availability statement
Data are available upon reasonable request.
References
- 1.Scott EC, Baines AC, Gong Y, et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat Rev Drug Discov. 2023;22:625–40. doi: 10.1038/s41573-023-00723-4. [DOI] [PubMed] [Google Scholar]
- 2.Delyon J, Michielin O. Adjuvant or neoadjuvant treatment with immune checkpoint inhibitors: re-assessing the risk-benefit ratio. Lancet Oncol. 2024;25:3–5. doi: 10.1016/S1470-2045(23)00575-2. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–68. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 4.Triggianese P, Novelli L, Galdiero MR, et al. Immune checkpoint inhibitors-induced autoimmunity: The impact of gender. Autoimmun Rev. 2020;19:102590. doi: 10.1016/j.autrev.2020.102590. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Zhang C, Jin Z, et al. Sex differences in immune-related adverse events with immune checkpoint inhibitors: data mining of the FDA adverse event reporting system. Int J Clin Pharm. 2022;44:689–97. doi: 10.1007/s11096-022-01395-7. [DOI] [PubMed] [Google Scholar]
- 6.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–80. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo J, Murphy C, Atkins MB, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J Immunother Cancer. 2023;11:e006398. doi: 10.1136/jitc-2022-006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suijkerbuijk KPM, van Eijs MJM, van Wijk F, et al. Clinical and translational attributes of immune-related adverse events. Nat Cancer . 2024;5:557–71. doi: 10.1038/s43018-024-00730-3. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–67. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen CN, Bai X, Quah T, et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann Oncol. 2021;32:917–25. doi: 10.1016/j.annonc.2021.03.204. [DOI] [PubMed] [Google Scholar]
- 11.Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:1465–77. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 12.Fukudo M, Sasaki T, Ohsaki Y. PD-1 Blockers: Staying Long in the Body and Delayed Toxicity Risks. J Thorac Oncol. 2020;15:e42–4. doi: 10.1016/j.jtho.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Brahmer JR, Drake CG, Wollner I, et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. JCO. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi: 10.1186/s40425-019-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alalawi M, Bakr AS, Reda R, et al. Late-onset toxicities of monoclonal antibodies in cancer patients. Immunotherapy (Los Angel) 2022;14:1067–83. doi: 10.2217/imt-2022-0042. [DOI] [PubMed] [Google Scholar]
- 16.Ghisoni E, Wicky A, Bouchaab H, et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur J Cancer. 2021;149:153–64. doi: 10.1016/j.ejca.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Mandalà M, Merelli B, Indriolo A, et al. Late-occurring toxicity induced by an immune checkpoint blockade in adjuvant treatment of a stage III melanoma patient. Eur J Cancer. 2018;95:130–2. doi: 10.1016/j.ejca.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Parakh S, Cebon J, Klein O. Delayed Autoimmune Toxicity Occurring Several Months After Cessation of Anti-PD-1 Therapy. Oncologist. 2018;23:849–51. doi: 10.1634/theoncologist.2017-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raschi E, Gatti M, Gelsomino F, et al. Lessons to be Learnt from Real-World Studies on Immune-Related Adverse Events with Checkpoint Inhibitors: A Clinical Perspective from Pharmacovigilance. Target Oncol. 2020;15:449–66. doi: 10.1007/s11523-020-00738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noseda R, Ruinelli L, Gaag LC van der, et al. Pre-Existing Cardiovascular Conditions as Clinical Predictors of Myocarditis Reporting with Immune Checkpoint Inhibitors: A VigiBase Study. Cancers (Basel) 2020;12:3480. doi: 10.3390/cancers12113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagerlund O, Strese S, Fladvad M, et al. WHODrug: A Global, Validated and Updated Dictionary for Medicinal Information. Ther Innov Regul Sci. 2020;54:1116–22. doi: 10.1007/s43441-020-00130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fusaroli M, Salvo F, Begaud B, et al. The Reporting of a Disproportionality Analysis for Drug Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Development and Statement. Drug Saf. 2024;47:575–84. doi: 10.1007/s40264-024-01421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusaroli M, Salvo F, Begaud B, et al. The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration. Drug Saf. 2024;47:585–99. doi: 10.1007/s40264-024-01423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen ST, Semenov YR, Alloo A, et al. Defining D-irAEs: consensus-based disease definitions for the diagnosis of dermatologic adverse events from immune checkpoint inhibitor therapy. J Immunother Cancer. 2024;12:e007675. doi: 10.1136/jitc-2023-007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raschi E, Rossi S, De Giglio A, et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: A Guide for Clinicians. Drug Saf. 2023;46:819–33. doi: 10.1007/s40264-023-01320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755–64. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation. 2017;136:2085–7. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 28.Moslehi JJ, Salem J-E, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391 doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolladille C, Ederhy S, Allouche S, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8:e000261. doi: 10.1136/jitc-2019-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, Zhao H, Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. 2020;12:1758835920937612. doi: 10.1177/1758835920937612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaddepally RK, Kharel P, Pandey R, et al. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers (Basel) 2020;12:738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016;375:1845–55. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 36.Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–85. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Pond G, McWhirter E. Multisystem Immune-Related Adverse Events from Dual-Agent Immunotherapy Use. Curr Oncol. 2024;31:425–35. doi: 10.3390/curroncol31010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. Am J Clin Dermatol. 2018;19:345–61. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 39.Gougis P, Jochum F, Abbar B, et al. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective. E Clin Med. 2024;70:102536. doi: 10.1016/j.eclinm.2024.102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fusaroli M, Mitchell J, Rudolph A, et al. Causal inference tools for pharmacovigilance: using causal graphs to systematize biases, plan disproportionality analyses, and reduce the risk of spin. OSF Preprints. 2024 doi: 10.31219/osf.io/h5w9u. [DOI] [Google Scholar]
- 41.Bihan K, Lebrun-Vignes B, Funck-Brentano C, et al. Uses of pharmacovigilance databases: An overview. Therapie. 2020;75:591–8. doi: 10.1016/j.therap.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Lindquist M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Information J. 2008;42:409–19. doi: 10.1177/009286150804200501. [DOI] [Google Scholar]
- 43.Ertl C, Ruf T, Mentzer D, et al. The side effect registry immuno-oncology (SERIO) - A tool for systematic analysis of immunotherapy-induced side effects. Eur J Cancer. 2024;199:113505. doi: 10.1016/j.ejca.2023.113505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.