Abstract
Background
Artificial urinary sphincter (AUS) is the gold standard for severe male stress urinary incontinence (SUI). This study aims to evaluate the interest of a new cutaneous preparation regarding the risk of early device infection.
Methods
A retrospective review of medical records has been built with all patients who underwent an AUS, implanted by experienced surgeons, between January 2010 and January 2023. Before January 2015, all AUS received a standard protocol (SP) of cutaneous cleansing with soap povidone iodine and disinfection with alcoholic povidone iodine. After January 2015, all AUS received the new protocol (NP) with two cleansings with soap povidone iodine and two disinfections with alcoholic povidone iodine. The primary focus was to compare the risk of early device infection between the two protocols. Multivariate analyses were done with several risk factors such as age, diabetes, underlying pathology (prostate cancer surgery, surgical treatment of benign prostatic hyperplasia or others), past history of pelvic radiation therapy and past AUS implantation.
Results
One hundred and fifty-six cases were enrolled, with 34 following the SP and 122 following the NP. In the univariate analysis, there were 15 explantations in the SP arm versus 8 for the NP arm due to infection (45.5% vs. 25%, P=0.09). The was no difference between the NP and the SP in multiparametric analysis [odds ratio (OR): 0.97; P=0.96]. No other risk factors were associated with increased risk of AUS removal.
Conclusions
Our study showed no correlation between the two types of skin preparation and the risk of AUS removal or revision. Future studies are needed to highlight the legitimate risk factors.
Keywords: Artificial sphincter, skin preparation, sphincter, urinary incontinence
Highlight box.
Key findings
• The study found no significant difference in early infection rates between the standard protocol (SP) and the new protocol (NP) following artificial urinary sphincter (AUS) surgery (SP: 45.5% vs. NP: 25%, P=0.09).
• Multiparametric analysis showed no significant association between skin preparation method and AUS removal due to infection (P=0.96).
What is known and what is new?
• Povidone-iodine has traditionally been used for skin preparation in genitourinary prosthetic surgeries, but its effectiveness in reducing infection rates has been debated.
• This manuscript is the first to compare two distinct skin preparation protocols for AUS implantation, revealing that the more intensive NP does not significantly reduce infection rates compared to the SP.
What is the implication, and what should change now?
• The findings suggest that different skin preparation protocols may not necessarily reduce the risk of infection in AUS surgeries.
• Future studies should focus on identifying other factors contributing to AUS infection, and the surgical community may reconsider the need for new skin preparation protocols based on these findings.
Introduction
Artificial urinary sphincter (AUS) is the gold standard for severe male stress urinary incontinence (SUI). Radical prostatectomy is the principal cause of SUI in a male population. This population is more likely to have a history of pelvic irradiation.
AUS implantation carries its risk with a rate of 75% to 83% (1,2) remaining free of removal. The risk of infection is important with a 5.5% infection rate appearing 3.7 months following the implantation (2). Several studies compared different technics to decrease the risk of explantation with no significant results: penoscrotal vs. transperineal (3) and transcorporal vs. bulbar (4).
A history of pelvic radiotherapy (5,6), history of urethral surgery (7) or history of AUS implantation (8) have been identified as risk factors for explantation.
To the authors knowledge, there is only one study by Yeung et al., that dealt with skin preparation before genito-urinary (GU) device implantation and demonstrated the superiority of chlorhexidine-alcohol in eradicating skin flora versus alcoholic povidone iodine; there does not appear to be any increased risk of urethral or genital skin irritation with the use of chlorhexidine compared to povidone-iodine (9).
This study aims to evaluate the interest of a new cutaneous preparation regarding the risk of early device infection. It relies on expert consensus that this protocol, which includes two rounds of skin cleansing with soap povidone iodine and two rounds of disinfection with alcoholic povidone iodine, offers enhanced infection control compared to the standard protocol (SP). We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-279/rc).
Methods
A retrospective review of medical records has been built with all patients who underwent an AUS, implanted by experienced surgeons, between January 2010 and January 2023.
All the AUS implanted before June 2015 received an SP of cutaneous preparation with skin cleansing with soap povidone iodine and one skin disinfection with alcoholic povidone iodine. All the AUS implanted after June 2015 received the new protocol (NP) which consisted of two skin cleansings with a soap povidone iodine using a soft brush with a 5-minute break in between, and then two cutaneous disinfections with alcoholic povidone iodine.
Throughout the study period, the used technique and device remained consistent, ensuring that any observed effects were attributable to the skin preparation protocols rather than variations in the surgical approach or equipment.
All patients received antibiotic prophylaxis with cefazolin, and in case of allergy Vancomycin was given according to Société Française d’anesthésie et réanimation (SFAR) guidelines. A urine bacterial culture was routinely performed 7 to 10 days before the surgery and had to be sterile. If positive, an antibiotic treatment was provided for more than 2 days before and 2 days after the surgery.
Collected data were as follows: age, diabetes, underlying pathology (prostate cancer surgery, surgical treatment of benign prostatic hyperplasia or others), history of pelvic radiation therapy and past AUS implantation.
Patients operated with an AUS other than AMS 800 (American Medical Systems, Minnetonka, MN, USA) were excluded from the study.
The objective of our study was to evaluate a potential relationship between skin preparation and device explantation due to infection.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee and the Commission Nationale de l’Informatique et des Libertés (CNIL, No. 2235445), and individual consent for this analysis was waived due to retrospective nature.
Statistical analysis
The data were analyzed using R Studio, 2024.04.0. A Chi-squared test was used to compare categorical variables between the two groups, while a multivariate logistic regression model was applied to assess the association between skin preparation protocols and the risk of AUS removal, controlling for potential confounders.
Results
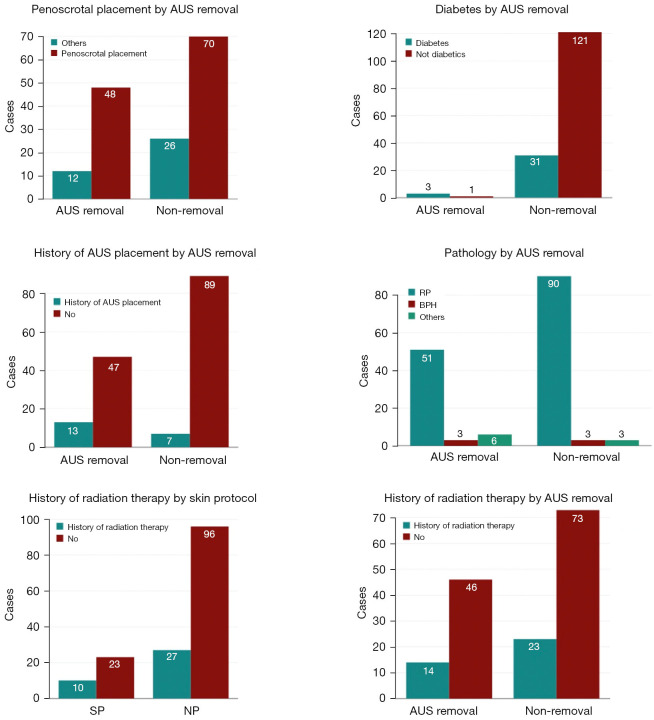
One hundred and fifty-six cases were enrolled, with 34 following the SP and 122 following the NP. Twenty patients had at least one history of AUS implantation (7 patients in SP, 13 patients in NP); 37 patients had a history of pelvic irradiation (10 patients in SP, 27 patients in NP) (Figures 1,2). All of the AUS had been implanted by two experienced surgeons.
Figure 1.
Descriptive analysis of multiple variables. AUS, artificial urinary sphincter; RP, radical prostatectomy; BPH, benign prostatic hypertrophy; SP, standard protocol; NP, new protocol.
Figure 2.
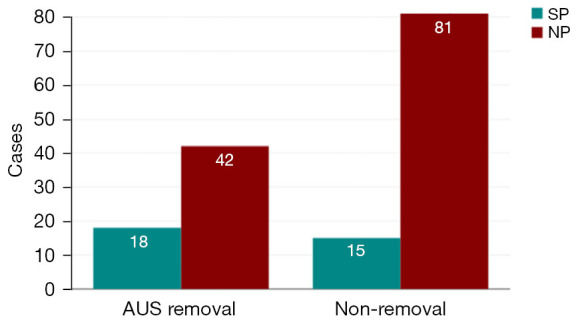
AUS removal according to the type of skin preparation. AUS, artificial urinary sphincter; SP, standard protocol; NP, new protocol.
A total of 60 AUS were explanted (38.5%), 18 in the group of SP (52.9%) and 42 in the group of NP (34.4%) as shown in Table 1.
Table 1. Patients characteristics and peri-operative outcomes.
| Variables | Numbers |
|---|---|
| Age (years), mean (SD) | 67.8 (10.8) |
| History of radiation therapy, n (%) | 37 (23.7) |
| History of AUS placement, n (%) | 20 (12.8) |
| Diabetes, n (%) | 34 (21.8) |
| Underlying pathology, n (%) | |
| Radical prostatectomy | 141 (90.4) |
| BPH surgery | 6 (3.8) |
| Others | 9 (5.8) |
| Cuff diameter (cm), mean (SD) | 4.2 (0.4) |
| Penoscrotal placement, n (%) | 118 (75.6) |
| Skin preparation protocol, n (%) | |
| Standard protocol | 33 (21.2) |
| New protocol | 123 (78.8) |
| Time before removal (months), mean (SD) | 44.4 (83.6) |
| AUS removal, n (%) | 60 (38.5) |
| Standard protocol | 18 (52.9) |
| New protocol | 42 (34.4) |
SD, standard deviation; AUS, artificial urinary sphincter; BPH, benign prostatic hypertrophy.
In the univariate analysis there was 15 infected devices that necessitated an AUS explantation for the SP and 8 AUS explantations for the NP (45.5% vs. 25%, P=0.09).
In the multiparametric analysis, no single variable was associated with an increased risk of AUS removal, with a P value for skin preparation of 0.96 which is not deemed statistically significant (Table 2).
Table 2. Multivariate analysis of the risk factors of AUS removal.
| Variables | Coefficient B | Standard error | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|
| Age | −0.02 | 0.02 | 0.98 | 0.94–1.03 | 0.46 |
| History of radiation therapy 1 | 0.11 | 0.41 | 1.11 | 0.5–2.48 | 0.80 |
| History of AUS placement | −1.16 | 0.76 | 0.31 | 0.07–1.38 | 0.13 |
| Diabetes | 1.91 | 1.19 | 6.78 | 0.66–69.16 | 0.11 |
| Pathology | |||||
| RP: reference | |||||
| BPH surgery | 0.48 | 0.92 | 1.62 | 0.27–9.74 | 0.60 |
| Others | 0.91 | 0.98 | 2.49 | 0.36–17.16 | 0.35 |
| Cuff diameter | −0.5 | 0.41 | 0.61 | 0.27–1.36 | 0.22 |
| Penoscrotal placement | −0.4 | 0.43 | 0.67 | 0.28–1.56 | 0.35 |
| Skin preparation protocol | −0.03 | 0.64 | 0.97 | 0.28–3.37 | 0.96 |
AUS, artificial urinary sphincter; CI, confidence interval; RP, radical prostatectomy; BPH, benign prostatic hypertrophy.
Discussion
In GU prosthetic surgery, especially in AUS, device infection is the most common complication, leading to additional operations including removal or revision, which results in significant patient morbidity. One of the most important steps in preventing device infection is the antisepsis of the surgical site.
Most of the time, device infection is caused by contamination of the prosthetic by microorganisms at the time of implantation, resulting in biofilm formation that hinders the host immune response and allows microorganisms to remain viable with reduced growth rates, which can promote resistance to antibiotics (10-12).
The antiseptic agent or skin preparation in this study’s protocols was mainly povidone-iodine that acts by damaging proteins and DNA via free iodine released from the solution after approximately 2 minutes of surface contact (13).
The recommended scrub time for povidone-iodine is 5 minutes, followed by a painting process, and then a dry time. This two-step process typically takes approximately ten minutes which is considered by experts of the GU prosthetic surgery as the necessary length of time needed to prevent infection (14).
In their study comparing two types of antiseptics, Yeung et al. proved the superiority of chlorhexidine-alcohol over iodine-iodine in term of eliminating skin flora at the surgical site before GU prosthetic procedures (9).
Mann et al. demonstrated that history of pelvic radiation and history of prior urethroplasty were predictive of early AUS erosion (15).
In their study, Lai et al. showed that the surgical learning curve of placing a virgin AUS was about 25 cases, as measured by complication and reoperation rates (16).
In their study about risk factors of AUS failure, Kretschmer et al. demonstrated that only perioperative anticoagulation and double-cuff placement were independent predictors of AUS failure in the multivariate analysis (17).
Prebay et al. evaluated the overall rates of AUS re-intervention, complication and infection that were 23.4%, 24.1% and 6.4%, respectively. They also showed median AUS survival of 10.6 years and a 20-year survival probability projection of 31.3%. History of smoking, urethroplasty, diabetes mellitus, history of pelvic radiation were considered risk factors of complications (18).
Our study did not find a significant difference in infection rates; however, we continue to use the NP, as it may provide other unmeasured benefits.
While the cited studies identified various risk factors for AUS infection or related complications, none specifically examined skin preparation protocols. This study, however, did not find any risk factors related to AUS infection including skin preparation protocols.
To our knowledge, no previous study compared the two skin preparation protocols for the AUS implantation. Nevertheless, our study limitations include a single center inclusion, limited number of patients, and the lack of follow up. A multicenter study or a randomized controlled trial comparing multiple types of skin preparation could be useful in the future.
Conclusions
Skin preparation is a mandatory step before any GU prosthesis surgery. It should be done thoroughly to limit the risk of surgical site and device infection. Our study showed no correlation between the two types of skin preparation and the risk of AUS removal or revision. Future studies are needed to highlight the legitimate risk factors.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee and the Commission Nationale de l’Informatique et des Libertés (CNIL, No. 2235445), and individual consent for this analysis was waived due to retrospective nature.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-279/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-279/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tau.amegroups.com/article/view/10.21037/tau-24-279/dss
References
- 1.Léon P, Chartier-Kastler E, Rouprêt M, et al. Long-term functional outcomes after artificial urinary sphincter implantation in men with stress urinary incontinence. BJU Int 2015;115:951-7. 10.1111/bju.12848 [DOI] [PubMed] [Google Scholar]
- 2.Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. 10.1016/j.juro.2006.10.062 [DOI] [PubMed] [Google Scholar]
- 3.Brierre T, Roumiguie M, Soulie M, et al. Comparison of penoscrotal and perineal approaches for implantation of an artificial urinary sphincter in man]. Prog Urol 2021;31:1182-91. 10.1016/j.purol.2021.08.238 [DOI] [PubMed] [Google Scholar]
- 4.El-Akri M, Bentellis I, Tricard T, et al. Transcorporal vs. bulbar artificial urinary sphincter implantation in male patients with fragile urethra. World J Urol 2021;39:4449-57. 10.1007/s00345-021-03783-6 [DOI] [PubMed] [Google Scholar]
- 5.Huang MM, Huffman P, Dani H, et al. Association between Previous Pelvic Radiation and All-Cause and Cause-Specific Failure of Replacement Artificial Urinary Sphincters. J Urol 2022;207:1268-75. 10.1097/JU.0000000000002433 [DOI] [PubMed] [Google Scholar]
- 6.Mamane J, Sanchez S, Lellouch AG, et al. Impact of radiation therapy on artificial urinary sphincter implantation in male patients: A multicenter study. Neurourol Urodyn 2022;41:332-9. 10.1002/nau.24825 [DOI] [PubMed] [Google Scholar]
- 7.Deruyver Y, Schillebeeckx C, Beels E, et al. Long-term outcomes and patient satisfaction after artificial urinary sphincter implantation. World J Urol 2022;40:497-503. 10.1007/s00345-021-03877-1 [DOI] [PubMed] [Google Scholar]
- 8.Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-8. 10.1016/j.urology.2014.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeung LL, Grewal S, Bullock A, et al. A comparison of chlorhexidine-alcohol versus povidone-iodine for eliminating skin flora before genitourinary prosthetic surgery: a randomized controlled trial. J Urol 2013;189:136-40. 10.1016/j.juro.2012.08.086 [DOI] [PubMed] [Google Scholar]
- 10.Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett 2004;236:163-73. 10.1111/j.1574-6968.2004.tb09643.x [DOI] [PubMed] [Google Scholar]
- 11.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis 1999;18:843-6. 10.1007/s100960050417 [DOI] [PubMed] [Google Scholar]
- 12.Costerton W, Veeh R, Shirtliff M, et al. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 2003;112:1466-77. 10.1172/JCI200320365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trostle SS, Hartmann FA. Surgical infection. In: Auer JA, Editor. Equine Surgery, 2nd ed. Philadelphia: WB Saunders; 1992:47. [Google Scholar]
- 14.Henry GD, Kansal NS, Callaway M, et al. Centers of excellence concept and penile prostheses: an outcome analysis. J Urol 2009;181:1264-8. 10.1016/j.juro.2008.10.157 [DOI] [PubMed] [Google Scholar]
- 15.Mann RA, Kasabwala K, Buckley JC, et al. The "Fragile" Urethra as a Predictor of Early Artificial Urinary Sphincter Erosion. Urology 2022;169:233-6. 10.1016/j.urology.2022.06.023 [DOI] [PubMed] [Google Scholar]
- 16.Lai HH, Boone TB. The surgical learning curve of artificial urinary sphincter implantation: implications for prosthetic training and referral. J Urol 2013;189:1437-43. 10.1016/j.juro.2012.10.116 [DOI] [PubMed] [Google Scholar]
- 17.Kretschmer A, Buchner A, Grabbert M, et al. Risk factors for artificial urinary sphincter failure. World J Urol 2016;34:595-602. 10.1007/s00345-015-1662-9 [DOI] [PubMed] [Google Scholar]
- 18.Prebay ZJ, Ebbott D, Foss H, et al. A global, propensity-score matched analysis of patients receiving artificial urinary sphincters and the risk of complications, infections, and re-interventions. Transl Androl Urol 2023;12:832-9. 10.21037/tau-22-631 [DOI] [PMC free article] [PubMed] [Google Scholar]