Abstract
Background
The prevalence of gestational diabetes mellitus (GDM) is increasing, particularly in low- and middle-income countries (LMICs) like Nepal. GDM self-management, including intensive dietary and lifestyle modifications and blood glucose monitoring, is critical to maintain glycemic control and prevent adverse outcomes. However, in resource-limited settings, several barriers hinder optimal self-management. Mobile health (mHealth) technology holds promise as a strategy to augment GDM treatment by promoting healthy behaviors and supporting self-management, but this approach has not yet been tested in any LMIC.
Objective
This report describes the protocol to develop a culturally tailored mHealth app that supports self-management and treatment of GDM (GDM–Dhulikhel Hospital [GDM-DH] app, phase 1) and test its usability and preliminary efficacy (phase 2) among patients with GDM in a periurban hospital setting in Nepal.
Methods
The study will be conducted at Dhulikhel Hospital in Dhulikhel, Nepal. In the development phase (phase 1), a prototype of the GDM-DH app will be developed based on expert reviews and a user-centered design approach. To understand facilitators and barriers to GDM self-management and to gather feedback on the prototype, focus groups and in-depth interviews will be conducted with patients with GDM (n=12), health care providers (n=5), and family members (n=3), with plans to recruit further if saturation is not achieved. Feedback will be used to build a minimum viable product, which will undergo user testing with 18 patients with GDM using a think-aloud protocol. The final GDM-DH app will be developed based on user feedback and following an iterative product design and user testing process. In the randomized controlled trial phase (phase 2), newly diagnosed patients with GDM (n=120) will be recruited and randomized to either standard care alone or standard care plus the GDM-DH app from 24-30 weeks gestation until delivery. In this proof-of-concept trial, feasibility outcomes will be app usage, self-monitoring adherence, and app usability and acceptability. Exploratory treatment outcomes will be maternal glycemic control at 6 weeks post partum, birth weight, and rates of labor induction and cesarean delivery. Qualitative data obtained from phase 1 will be analyzed using thematic analysis. In phase 2, independent 2-tailed t tests or chi-square analyses will examine differences in outcomes between the 2 treatment conditions.
Results
As of July 2024, we have completed phase 1. Phase 2 is underway. The first participant was enrolled in October 2021, with 99 participants enrolled as of July 2024. We anticipate completing recruitment by December 2024 and disseminating findings by December 2025.
Conclusions
App-based lifestyle interventions for GDM management are not common in LMICs, where GDM prevalence is rapidly increasing. This proof-of-concept trial will provide valuable insights into the potential of leveraging mHealth app–based platforms for GDM self-management in LMICs.
Trial Registration
ClinicalTrials.gov NCT04198857; https://clinicaltrials.gov/study/NCT04198857
International Registered Report Identifier (IRRID)
DERR1-10.2196/59423
Keywords: gestational diabetes mellitus, mobile health, mHealth, self-management, pregnancy, maternal and child health, South Asia, Nepal, low- and middle-income country, mobile phone
Introduction
Background
Gestational diabetes mellitus (GDM), characterized by hyperglycemia that develops during pregnancy, is one of the most common and increasingly prevalent pregnancy complications [1,2]. The International Diabetes Federation estimated that approximately 14% of pregnancies worldwide were affected by GDM in 2021 [2]. A significant proportion of these cases occur in low- and middle-income countries (LMICs), where over 90% of type 2 diabetes (T2D) cases in adults are also reported [3]. In Nepal, a low-income country in South Asia, the national prevalence of GDM is not documented, but regional estimates are high, ranging from 6.6% to over 20% [4-6].
GDM is associated with increased risks of adverse maternal and fetal outcomes, including preeclampsia, birth injuries, labor complications, cesarean delivery, and large for gestational-age babies [7-9]. Although GDM usually resolves after delivery, women with GDM are more likely to develop T2D in the future compared to women with normoglycemic pregnancies [10,11], and fetal exposure to maternal hyperglycemia may predispose offspring to obesity and T2D [12,13]. GDM is also a significant economic burden, with estimated costs from GDM and its downstream consequences ranging from £147 (US $196) million to US $1.6 billion in high-income countries [14,15].
Adequate management of GDM, including diet and lifestyle modifications and frequent self-monitoring of blood glucose, reduces maternal and neonatal complications [16,17]. However, in resource-limited settings like Nepal, GDM management constitutes a significant burden on both patients and the health care system [18,19]. Scalable and cost-effective strategies are thus needed to manage the growing burden of GDM and its consequences in low-resource countries such as Nepal. Barriers to the management of GDM in Nepal exist at multiple levels, including those at the individual (eg, health literacy, lack of knowledge or self-efficacy, and time constraints), interpersonal (eg, cultural practices and family influence on prenatal care decisions) [20-22], and health systems or structural (eg, access to health care, resources, transportation, availability of clinicians, and time for counseling) level [18,23-26]. Mobile health (mHealth) technology provides opportunities to address these multilevel barriers by enhancing self-efficacy and knowledge, promoting self-management behaviors relating to GDM, and facilitating communication between patients and their health care team [27,28]. Evidence from high-income countries shows that mHealth interventions for GDM can improve patient satisfaction and reduce costs [29,30], while achieving better or similar maternal glycemic levels and pregnancy outcomes compared to standard care alone [31]. Nepal has high rates of mobile service penetration among people who are pregnant [32], and prior studies in LMICs support the use of mHealth technology during pregnancy for health promotion, appointment reminders, and improved nutritional status [33-36].
Despite the evidence supporting the use of mHealth technology to improve the self-management of GDM, digital tools for GDM have not been designed to address the unique needs of people who are pregnant and living in LMICs. Innovative and culturally tailored approaches are needed to optimize self-management of GDM in low-resource settings like Nepal [37]. The aim of this paper is to describe the protocol to develop and test the usability, acceptability, and preliminary efficacy of a user-centered, culturally tailored mHealth platform (GDM–Dhulikhel Hospital [GDM-DH] app + web portal) designed to support self-management and treatment of GDM among patients in a periurban hospital setting in Nepal.
Objective and Hypothesis
In the first phase of the study, our objective is to develop an app prototype for the GDM-DH platform, gather user feedback on the proposed GDM-DH platform, and identify barriers and facilitators to its uptake. In the second phase, we will evaluate the preliminary efficacy of the GDM-DH platform alongside standard care, compared to standard care alone, on perinatal health outcomes, including maternal blood glucose levels, birth weight, and neonatal hypoglycemia. We hypothesize that the GDM-DH platform will show good usability and preliminary efficacy for improving perinatal health outcomes.
Methods
Study Overview
The study consists of two phases: (1) the design and development of the GDM-DH app platform and (2) a proof-of-concept randomized controlled trial (RCT) to assess its usability, acceptability, and preliminary efficacy (Figure 1). This study will be conducted at Dhulikhel Hospital, a community-based, flagship university hospital of Kathmandu University in Nepal. Dhulikhel Hospital has a catchment population of 1.9 million people and delivers approximately 3000 babies annually [38]. This trial is registered on ClinicalTrials.gov (NCT04198857).
Figure 1.
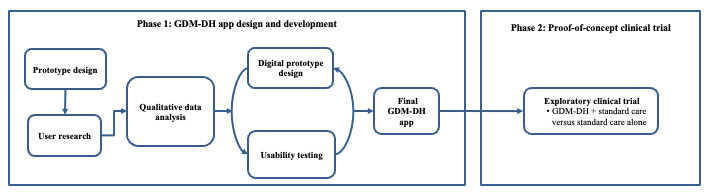
Overview of the study design. GDM-DH: gestational diabetes mellitus-Dhulikhel Hospital.
Ethical Considerations
Institutional approval for this study was granted by Rutgers University Newark Health Sciences institutional review board (Pro2019001883 [phase 1] and Pro2019002841 [phase 2]) and the ethical review board of the Nepal Health Research Council (registration 735/2019). All potential human participants will receive an explanation of the study at screening, including study rationale, procedures, and appointment schedules. Eligible participants will receive a detailed description of the study, including possible risks and benefits, measures to protect privacy, and the right to terminate involvement in the study at any time. Signed written informed consent will be obtained from each participant by a trained research assistant at Dhulikhel Hospital. Enrollment procedures will occur in a private room. To maintain participant confidentiality, documents and forms with identifying information will be stored separately in a locked filing cabinet and in a secure password-protected Box folder (Box Inc), a Health Insurance Portability and Accountability Act (HIPAA)–compliant file storage service. Only select study staff will have access to these files. In phase 1 of the study, participants, including women with GDM and their spouses, will receive a mobile recharge card worth Nepalese rupees 500 (approximately US $3.77) to compensate for their time in the interview, focus group, or usability testing. In phase 2, participants will be compensated with a US $10 prepaid internet data package.
Phase 1: GDM-DH App Design and Development
The goal of the GDM-DH app is to assist patients in the self-management of GDM by improving their efficacy in adhering to the recommended diet and physical activity regimens and facilitating desired clinical and social support. The app will also help clinicians by providing easily digestible visual displays of patient data and behaviors, which will in turn aid in their clinical decision-making and counseling.
We will take a user-centered approach to design and develop the GDM-DH app that matches the needs and technological sophistication of the target users. As outlined in Figure 1, we will form a multidisciplinary team of experts to design an app prototype, followed by a qualitative or requirement-gathering phase to collect user feedback on the app prototype features and functions. After incorporating and revising the app prototype, we will build a minimum viable product (MVP) and subsequently conduct usability testing. The final GDM-DH app will be developed following an iterative process of product design and user testing. Each step of the app design and development is detailed in the upcoming sections.
Phase 1A: Prototype Design
As part of the formative phase of the app development, we will first develop a prototype of the GDM-DH app, in collaboration with a multidisciplinary team including experts in GDM, mHealth, and behavior and implementation sciences, as well as health care providers at Dhulikhel Hospital. The content modules and features to be included in the app will be selected based on literature review, relevant theory-based behavioral strategies, and discussion with subject matter experts. The primary educational content will be information on GDM and associated risk factors and health outcomes, clinical and lifestyle management of GDM, dietary and physical activity recommendations for women with GDM, and the benefits of a healthy diet and active lifestyle during pregnancy. Other components will be guided by Bandura’s [39] Social Cognitive Theory (SCT), which postulates that behavior change is guided by several cognitive and social factors, including perceived self-efficacy, social support, outcome expectancies, and perceived barriers and facilitators to the behavior change [40]. Consistent with the SCT framework, we will include features that support self-management of GDM by (1) providing health education; (2) helping patients identify and set target health goals (for diet, physical activity, and glucose levels); (3) enhancing their self-efficacy to meet target goals; and (4) facilitating desired support from family members.
Key Domains of the GDM-DH App
Self-Management of GDM
The app will provide basic educational information about GDM and the importance of managing GDM for optimal maternal and infant health. The app will also include culturally tailored dietary and physical activity recommendations with relevant recipes and example meal plans. The core component of the app will allow participants to record and self-monitor their blood glucose levels, blood pressure, carbohydrate intake, physical activity, and gestational weight gain. To assist with estimating the carbohydrate intake of meals, the app will include typical portion-size images of common carbohydrate food sources in a staple Nepalese diet. Weight, blood pressure, and blood glucose levels can be manually entered by users either biweekly after clinic testing or daily if they have equipment at home.
Based on the user input data, the GDM-DH app will automatically generate tailored feedback and help users monitor their progress toward target health goals. The app will compare carbohydrate intake, physical activity, gestational weight gain, blood glucose, and blood pressure levels to existing evidence-based guidelines [41-45] via a feedback engine and will generate visual display charts. These charts will summarize diet, physical activity, gestational weight gain, blood glucose, and blood pressure patterns, allowing participants to monitor their alignment and progress toward target goals.
The GDM-DH app will enhance self-efficacy to adhere to recommendations and meet target goals with a variety of multimedia modules (eg, video lessons) that provide appropriate strategies and practice opportunities to problem-solve around barriers to health behaviors. These modules will consider specific cultural, social, and physical environmental challenges that patients with GDM face in adopting a healthy diet and lifestyle.
Facilitation of Health Exchange Between Patients and Providers
Providers will have access to a web-based administrative portal that syncs with the patient-facing GDM-DH app and allows them to register a new patient, as well as enter, update, or review clinical and other patient-related information (eg, glucose values, blood pressure, weight, diet entries, clinic history, and notes). This portal is intended to streamline the providers’ workflow, as they can quickly look at patient data visualizations to understand patient behaviors and progress and guide their treatment and counseling accordingly.
Family Member Support
The GDM-DH app will also facilitate involvement from family members or friends, who strongly influence prenatal care–related decisions and dietary behaviors in Nepal [20-22]. Via a social network “follow” feature, patients will be able to list 1 or more family members or friends as contacts in the app and give that contact permission to view their logged data and progress summary. This feature will be added to the GDM-DH app to garner social support and offer a source of accountability, motivation, and shared experience.
Phase 1B: Qualitative User Research
The objective of this qualitative phase is to gather user feedback on the GDM-DH app prototype and understand the perceived facilitators and barriers to GDM self-management. We will recruit patients diagnosed with GDM within the past year from Dhulikhel Hospital to participate in a focus group or semistructured in-depth interview [46]. Eligible patients will be pregnant women who (1) receive antenatal care at Dhulikhel Hospital, (2) receive a GDM diagnosis (within the preceding year), (3) own a smartphone, and (4) can understand and read Nepali. Patients with learning difficulties or vision or hearing impairments will be excluded. Patients with a confirmed GDM diagnosis will be recruited into the study with the help of a senior obstetrician-gynecologist (OB-GYN; coinvestigator in the study) and other staff in the OB-GYN Department at Dhulikhel Hospital. We will recruit 12 women with GDM but plan to recruit further if data saturation is not achieved [47-49]. We will also conduct key informant interviews with clinicians from Dhulikhel Hospital (n=5) and family members of patients with GDM (n=3) to collect feedback on the GDM-DH prototype. Eligibility criteria for family members include being a spouse or direct relative of a patient with GDM who was diagnosed with GDM within the past year at Dhulikhel Hospital. All participants will provide written informed consent.
Before the focus group or in-depth interview, current and previously diagnosed patients with GDM will complete a structured questionnaire assessing sociodemographics and pregnancy-related information. The focus group and in-depth interviews will be developed in Nepali with a set of questions and probes to thoroughly understand the perceived social, cultural, and environmental facilitators and barriers to GDM management, including participants’ views, opinions, and knowledge about GDM management, perceived self-efficacy, and strategies to enhance adherence to lifestyle modifications [50]. We will also collect feedback on the GDM-DH app prototype, including (1) the app dashboard, layout, and navigation; (2) the usefulness of app features; (3) the burden of data entry; (4) the usefulness of educational modules covered; (5) clarity of graphs and data visualizations; and (6) additional features and content. The focus group or interview guide will be developed in consultation with the study team with a study investigator (AS) who has prior experience conducting qualitative studies in this population taking the lead. A trained research coordinator will administer the focus group and in-depth interviews at Dhulikhel Hospital.
Given the clinical applications and setting of our study, the qualitative arm of this research takes a pragmatic worldview. In analyzing the qualitative data, our goal will be to generate practical and actionable insights that can directly inform and improve the GDM-DH platform with the ultimate goal of improving patient outcomes. The focus group and in-depth interview will be audio recorded and transcribed before analysis. Qualitative data will be uploaded onto the NVivo 12 software (Lumivero) for data management and analysis. We will follow Braun and Clarke’s [51] 6-phase approach to thematic analysis, including (1) familiarizing with the data, (2) generating initial codes that will be compiled into a codebook, (3) searching for themes, (4) reviewing themes, (5) defining and naming themes, and (6) producing the final report [51]. Our analysis will use both inductive and deductive approaches. First, we will develop deductive themes based on our interview guide. Next, we will read the transcripts to identify text related to the deductive themes and define new inductive themes that emerge from the data. A codebook will be developed by revisiting the text within the identified themes, defining codes, and reorganizing or grouping those not aligning with existing themes into new ones. A second research analyst will use this codebook to independently code the transcripts. After coding is completed, the 2 research assistants will compare the coding schemes and resolve any discrepancies through mutual agreement, with assistance from the study investigator. A third research analyst will calculate the intercoder reliability, achieving a level of 80%. Multiple investigators will review the data and provide insights based on their content knowledge and expertise.
Phase 1C: Usability Testing
Incorporating and revising the app prototype based on qualitative user research, we will build the MVP, the simplest possible version of the GDM-DH app, which will retain its most important features and functionalities. The MVP of the GDM-DH app will undergo usability testing with patients with GDM via think-aloud protocol [52]. The eligibility criteria to recruit patients with GDM for usability testing will be the same as the criteria used for phase 1B—qualitative user research. We will use a convenience sampling strategy to recruit 18 participants for the usability testing [53]. All patients will be required to provide written informed consent.
Individual one-on-one usability testing sessions will be conducted in a private space and overseen by 2 facilitators; 1 facilitator will lead the session while a designated note-taker will record users’ verbalizations. Usability testing will consist of a 2-step think-aloud protocol in which the participants will be asked to verbalize their thoughts as they navigate and complete various specified tasks (eg, profile setup, diet entry, review weight visualizations, and open video lesson) on the GDM-DH app [54]. Participants will also be asked to rate the difficulty of completing each task on a 5-point scale ranging from “very easy” to “difficult.” At the end of the usability testing session, participants will complete the System Usability Scale (SUS, scored 0-100), a reliable and widely used 10-item 5-point Likert scale questionnaire for global assessment of systems usability [54]. After the usability testing, we will ask open-ended questions to collect feedback on the app, such as how to improve upon the features and functions of the app. The final version of the GDM-DH app will be developed following an iterative process of product design and user testing and will be used in the proof-of-concept RCT in the second phase of the study.
Phase 2: Proof-of-Concept RCT
The objective of the RCT is to answer the research question—in women with GDM, does augmenting standard care with the GDM-DH platform, compared to standard care alone, show preliminary efficacy in improving clinical perinatal outcomes, including maternal blood glucose levels, birth weight, and neonatal hypoglycemia?
Participant Eligibility and Recruitment
Patients who are newly diagnosed with GDM will be recruited from the Obstetric Outpatient Department at Dhulikhel Hospital. Patients who are pregnant and receiving antenatal care at the Obstetric Outpatient Department undergo routine screening for GDM at 24 to 28 weeks of gestation and are diagnosed using the Carpenter-Coustan criteria [55]. Eligible patients must (1) receive GDM diagnosis based on Carpenter-Coustan criteria [55], (2) be aged 18 years or older, (3) be ≤30 weeks gestational age, (4) receive antenatal care at Dhulikhel Hospital, (5) own an Android smartphone, (6) have internet connectivity at home, and (7) understand and read Nepali. Patients with learning difficulties or vision or hearing impairments will be excluded.
Enrollment and Randomization
We will enroll 120 newly diagnosed patients with GDM from the Obstetric Outpatient Department at Dhulikhel Hospital between 24 and 30 weeks gestational age (baseline). At baseline, all participants will be briefed about the study and asked to provide written informed consent to agree to participate in the study. All participants will then complete a structured questionnaire to assess sociodemographics, prepregnancy weight, and lifestyle factors, such as smoking and alcohol use. Next, participants will be randomly assigned in a 1:1 fashion to 1 of the 2 treatment conditions—either GDM-DH app intervention + standard care or standard care only, for up to 16 weeks, starting from baseline until delivery (Figure 2). Random permuted blocks of sizes 4 or 6 will be used using a statistical software in order to prevent treatment imbalance and ensure that participants are allocated to each group equally. The allocation sequence will be concealed from the research assistant and research nurse using sequentially numbered opaque sealed envelopes. Due to the nature of the study, clinicians and participants will not be blinded to the group allocation.
Figure 2.
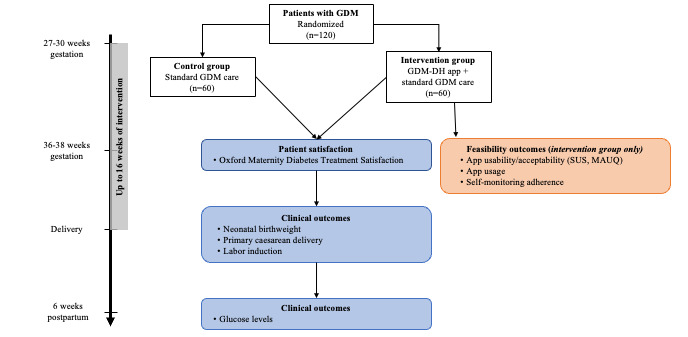
Overview of study design of phase 2 (proof-of-concept clinical trial) and measurement time points. Measures in blue are collected in both study groups, while measures in red are only collected in the intervention group. GDM: gestational diabetes mellitus; GDM-DH: gestational diabetes mellitus-Dhulikhel Hospital; MAUQ: mHealth App Usability Questionnaire; SUS: System Usability Scale.
Standard Care
Standard care for patients with GDM at Dhulikhel Hospital entails a counseling session with an OB-GYN physician, which covers basic disease information, dietary and physical activity recommendations for GDM management, target blood glucose levels before and after meals, and the course of treatment. The primary treatment for GDM includes modifications to diet and physical activity. Participants consult a dietitian and a physical therapist to develop a diet and physical activity plan based on prepregnancy weight and GDM disease severity. In addition to setting carbohydrate goals, major dietary recommendations include eating small meals throughout the day, lowering sugar and refined carbohydrate intake, and increasing the intake of vegetables and whole grains. Patients are provided with leaflets and brochures in addition to verbal information about managing GDM with diet and physical activity. As per the standard care protocol, patients with GDM are asked to visit the Obstetric Outpatient Department for glucose testing every 2 weeks, and after each testing, blood glucose levels are recorded in paper booklets assigned to each patient. If feasible, patients are also encouraged to buy a glucometer and perform daily blood glucose testing at home. The OB-GYN physicians monitor the blood glucose levels across testing and, if needed, may prescribe oral hypoglycemic medications or insulin to the patient over the course of treatment.
Study Intervention
In addition to standard care, participants in the intervention group will use the GDM-DH app to support self-management behaviors. At baseline, participants in the intervention group will have the GDM-DH app downloaded onto their mobile phones and receive a detailed orientation on its use from a trained research coordinator. Participants will be instructed to input their carbohydrate intake at meals, gestational weight gain, blood glucose levels, and blood pressure into the GDM-DH app, and the GDM-DH app will track the daily number of steps taken by the user. The GDM-DH app will automatically generate feedback through visual charts to help users understand how their health data relate to recommendations. Users can use this information to set goals and self-manage their GDM. The GDM-DH app will also send in-app notifications and reminders to input data and attend prenatal care appointments. During standard care prenatal visits, clinicians will review data from the app via a web portal to inform treatment decisions and recommendations. Approved family members can also be added to the app and can track patient progress.
Outcome Measures
Perinatal Health Outcomes
Exploratory maternal and neonatal health outcomes will be measured at delivery and 6 weeks post partum (Table 1). Primary exploratory treatment outcomes will be maternal glycemic control measures at 6 weeks post partum, neonatal birth weight, and rates of labor induction and cesarean delivery. As secondary exploratory treatment outcomes, we will also look at gestational weight gain, glucose levels above the glycemic targets and rates of insulin therapy, neonatal hypoglycemia, and admittance to the neonatal intensive care unit (NICU).
Table 1.
Study outcomes and covariates for phase 2, proof-of-concept randomized controlled trial.
| Outcome variable | Data source | Collection timepoint | ||||||||||
|
|
|
Baseline | 36-38 weeks gestation | Delivery | 6-weeks post partum | |||||||
| Maternal outcomes | ||||||||||||
|
|
Fasting and 2-hour blood glucose | Medical record | ✓ |
|
|
✓ | ||||||
|
|
Gestational weight gain | Medical record |
|
|
✓ |
|
||||||
|
|
Glucose readings during gestation | GDM-DHa app and medical record (intervention group); medical record (standard care group) |
|
|
✓ |
|
||||||
|
|
Initiation of insulin therapy | Medical record |
|
|
✓ |
|
||||||
|
|
Induction of labor | Medical record |
|
|
✓ |
|
||||||
|
|
Cesarean delivery | Medical record |
|
|
✓ |
|
||||||
| Neonatal outcomes | ||||||||||||
|
|
Birth weight | Medical record |
|
|
✓ |
|
||||||
|
|
Apgar score | Medical record |
|
|
✓ |
|
||||||
|
|
NICUb admission | Medical record |
|
|
✓ |
|
||||||
|
|
Neonatal hypoglycemia | Medical record |
|
|
✓ |
|
||||||
| Usability and acceptability outcomes | ||||||||||||
|
|
Oxford Maternity Diabetes Treatment Satisfaction Questionnaire [56] | Survey (self-report) |
|
✓ |
|
|
||||||
| Variables measured in the intervention group only | ||||||||||||
|
|
App usage | GDM-DH app |
|
|
|
|
||||||
|
|
|
Time spent on the app |
|
|
|
|
✓ | |||||
|
|
Self-monitoring compliance (observed vs expected number of entries) | GDM-DH app |
|
|
|
|
||||||
|
|
|
Carbohydrate intake |
|
|
|
|
✓ | |||||
|
|
|
Blood glucose entries |
|
|
|
|
|
|||||
|
|
|
Blood pressure entries |
|
|
|
|
|
|||||
|
|
|
Weight entries |
|
|
|
|
|
|||||
|
|
mHealthc App Usability Questionnaire [57] | Survey (self-report) |
|
✓ |
|
|
||||||
|
|
System Usability Scale [54] | Survey (self-report) |
|
✓ |
|
|
||||||
| Covariates | ||||||||||||
|
|
Sociodemographics | Survey (self-report) | ✓ |
|
|
|
||||||
|
|
Prepregnancy weight | Survey (self-report) | ✓ |
|
|
|
||||||
aGDM-DH: gestational diabetes mellitus-Dhulikhel Hospital.
bNICU: neonatal intensive care unit.
cmHealth: mobile health.
At 6 weeks (±5 days) post partum, all participants will undergo a standard 75 g oral glucose tolerance test. Fasting and 2-hour glucose levels will be measured in the hospital laboratory using standard laboratory methods, and we will abstract these measures from the medical record. Blood glucose measures from the routine screening for GDM at 24 to 28 weeks of gestation will also be abstracted from the medical record. Additional maternal outcome variables will be collected at delivery. Induction of labor (yes or no), cesarean delivery (yes or no), and initiation of insulin therapy (yes or no) will be abstracted from the medical records. Gestational weight gain measures will be abstracted from the medical record and calculated by subtracting the measured weight at or before 12 weeks gestation from the measured weight at delivery. Maternal gestational glucose readings will be extracted from the app and medical record (for the intervention group) or the medical record (standard care group), and the proportions and frequency of glucose levels above the glycemic targets (≤5.5 mmol/L preprandial and ≤6.6 mmol/L at 2 hours postprandial) will be calculated. Neonatal outcome measures will also be collected at delivery. Prior to discharge (<24 hours after delivery), neonatal birth weight will be abstracted from the medical record. Neonatal Apgar score, NICU admission (yes or no), and neonatal hypoglycemia (yes or no) will be abstracted from the medical record.
Usability and Acceptability
At 36 to 38 weeks gestation, we will use the Oxford Maternity Diabetes Treatment Satisfaction Questionnaire, a validated 9-item 7-point Likert scale questionnaire (+3= strongly agree to –3= strongly disagree; possible scores 0-27) that can be completed in under 5 minutes, to assess general satisfaction and acceptability of GDM care [56]. Good acceptability will be considered predominantly (>80%) neutral or positive scores on all 9 items. The questionnaire will also include space for free text responses, where participants will be encouraged to provide additional feedback or suggestions.
For participants randomized to the study intervention group, we will collect additional measures. Questionnaires, which can be completed in under 5 minutes, will be administered by a trained research assistant at 36 to 38 weeks gestation to assess app usability and acceptability. The SUS will be used to assess perceived app usability, with a score of 68 (out of 100) demonstrating good usability [54]. Usability will also be assessed through a secondary mHealth App Usability Questionnaire (MAUQ) [57]. The MAUQ is a 7-point, 21-item survey designed to assess patient feedback on mHealth apps and has been previously validated. The MAUQ comprises of 3 subscales—ease of use and satisfaction (8 items, questions 1-8); system information arrangement (6 items, questions 9-14); and usefulness (7 items, questions 15-21), with responses to questions ranging on a 7-point scale from 1 (strongly agree) to 7 (strongly disagree). Lower scores indicate superior performance or a more positive user experience compared to higher scores. In addition to app usability and acceptability, we will collect data about app usage and self-monitoring frequency (eg, the actual number of app entries or expected app entries multiplied by 100 for carbohydrate intake or blood glucose). These data will be collected post partum from the GDM-DH app.
Sample Size Estimation and Considerations
Treatment-related change in glycemic control is of primary interest to this study, and we will be adequately powered to examine whether mean change in fasting and 2-hour blood glucose levels from 24 to 28 weeks gestation (collected at enrollment) to 6 weeks post partum differed significantly by the treatment condition. Power analyses were estimated based on repeated measures ANOVA with in-between interaction using G*Power (version 3.1.9.2; Heinrich Heine University Düsseldorf). For an estimated medium effect size of 0.25 (partial eta squared =0.06), an α error level of .05, a nonsphericity correction of 1, and to test 2 groups with 2 repeated measurements with a correlation of 0.5, a sample size of 34 is required to achieve the power of 80%. We will recruit an additional 86 patients (total n=120) to account for possible attrition and to test other hypotheses with exploratory clinical outcomes. Prior RCTs evaluating mHealth solutions for GDM management detected significant differences in treatment arms with similar or smaller sample sizes [58-60]. In 2017, the number of live births in Dhulikhel Hospital was 2983. Using a conservative incidence rate of 5%, the expected number of newly diagnosed patients with GDM per month is 12. Anticipating a conservative recruitment rate of 50%, we expect it will take 20 months to recruit 120 patients with GDM for the study.
Statistical Analysis
Data analysis will be done using SAS (version 9.4; SAS Institute). Maternal and infant characteristics will be described overall and by treatment condition using descriptive statistics (mean, SDs for continuous variables or frequencies for categorical variables). Independent 2-tailed t tests or chi-square analyses will examine differences in birth weight or rates of labor induction or cesarean delivery, respectively, between the 2 treatment conditions [61,62]. Usability and acceptability measures reported by the study intervention group will be summarized using descriptive statistics. A repeated measures ANOVA will be used to investigate whether fasting and 2-hour blood glucose levels differed across the 2 timepoints (within effect), and more importantly, whether the mean changes in fasting and 2-hour blood glucose levels over time (24 to 28 weeks gestation to 6 weeks post partum) differed by the 2 treatment conditions (interaction effect) [63]. Two separate models will be tested for fasting and 2-hour blood glucose levels, respectively. Assumptions of sphericity will be evaluated using Mauchly’s [64] test statistic for all models. A P value of <.05 will be considered statistically significant for all analyses.
Results
As of July 2024, we have completed phase 1 of the study. Phase 2 is underway. The first participant was enrolled in the RCT in October 2021, with 99 participants enrolled as of July 2024. We anticipate completing recruitment by December 2024 and disseminating the findings by December 2025.
Discussion
Principal Findings
This protocol describes the study methodology to develop a user-centered and culturally tailored GDM-DH platform and to determine the usability, acceptability, and preliminary efficacy of the platform among patients with GDM in a university hospital setting in Nepal. The GDM-DH platform will be designed to support patients with GDM in adopting optimal self-management behaviors and assist providers with timely and informed clinical decision-making. We anticipate that the platform will show good usability and acceptability, as well as demonstrate preliminary efficacy for improving perinatal health outcomes, including maternal glycemic control measures at 6 weeks post partum, neonatal birth weight, and rates of labor induction and cesarean delivery. To our knowledge, this will be the first RCT evaluating an intervention that leverages mHealth for GDM treatment and management in an LMIC setting.
Prior research conducted in high-income countries demonstrates that mHealth interventions for GDM, including smartphone apps and web-based tools, promote self-management behaviors, improve glycemic control, and reduce the risks of adverse perinatal outcomes [65-67]. However, existing app-based interventions for GDM management primarily focus on remote glucose monitoring with manual feedback from health care providers [58,59,68,69], which is resource-intensive and burdensome for both providers and participants, thus limiting the potential for widespread dissemination and impact in resource-limited settings. Our platform addresses these challenges by providing automated feedback, which may help to overcome resource limitations and reduce provider burden. While personalized and culturally tailored digital tools are beneficial for enhancing GDM self-management and improving perinatal health [66], few app designs are culturally responsive [70]. The GDM-DH platform improves upon existing mobile interventions for GDM by integrating cultural practices and family influence on prenatal care, offering social support and culturally tailored dietary and physical activity recommendations, and providing automatically generated personalized feedback to optimize GDM management without the need for significant human resources.
Research and Practice Implications
The GDM-DH app has the potential to significantly improve standard GDM care in Nepal by supporting self-management practices, facilitating informed clinical decision-making, and potentially enhancing clinical outcomes. If our GDM-DH platform proves successful, our findings will be highly relevant to the broader South Asian population and will guide the future development, testing, and optimization of tailored mHealth GDM interventions for other patient groups with low technological sophistication and unique cultural needs. Additionally, the methodologies and lessons learned from designing and implementing the GDM-DH app may inform similar mHealth initiatives in other LMICs.
Strengths
This study has several strengths and innovative aspects, including the user-centered and culturally tailored design based on behavioral theory, a lifestyle-based self-management approach using technology to minimize user burden, family member involvement, clinician input, and potential to augment prenatal care in a resource-limited setting. The potential for the GDM-DH app to augment prenatal care in resource-limited settings may be particularly significant for countries like Nepal, given the increasing rates of T2D and its comorbidities that consume health care resources [71,72]. Moreover, adequate treatment and management of GDM are critical to disrupting the cycle of intergenerational obesity and diabetes [73], and mHealth may serve as a valuable early-stage intervention strategy to curb the burgeoning T2D epidemic in Nepal and other LMICs.
Limitations
There are also limitations to the GDM-DH app and study protocol. To minimize user burden, the app collects and provides feedback only on carbohydrate intake, so other aspects of diet (eg, energy intake and fat intake) are not collected. However, the Nepalese diet is characterized by frequent consumption of carbohydrate-rich food sources [74], and carbohydrate intake is a crucial component of GDM self-management [41]. Similarly, the app only records steps taken and does not collect any information about additional aspects of physical activity. Despite this limitation, we expect to capture the majority of physical activity as walking is the most commonly reported exercise during pregnancy [75]. Manual entry of diet, blood glucose, blood pressure, and weight may impact user engagement, as it is time and effort-consuming. However, manual entry may allow participants to be more aware of their behaviors and associated physiological effects, thereby enhancing self-efficacy [40]. Since the app was designed to address the cultural barriers and technological literacy of pregnant women in Nepal, findings may not be generalizable to other women with GDM. If the app is efficacious, a similar process to adapt and culturally tailor the app to other settings and populations may be followed. Finally, this study may not be adequately powered to test secondary clinical outcomes (eg, NICU admission). However, we will be able to explore the trends and treatment differences in secondary outcomes, enabling us to power a larger study.
Future Directions and Dissemination
In a future study, we will conduct a type 1 or type 2 effectiveness-implementation trial to test the clinical and cost-effectiveness of the GDM-DH platform in improving GDM treatment outcomes. We will make every effort to keep the technologies (GDM-DH platform) developed as a result of this research project, if any, widely available and accessible to the research community.
Conclusions
With increasing rates of GDM, particularly in resource-limited settings, there is a heightened interest in developing innovative approaches to augment the treatment and management of this common pregnancy complication. App-based lifestyle interventions for GDM management are not common, especially in LMICs where GDM prevalence is rapidly increasing. This protocol describes the study methodology for developing and testing an mHealth platform designed to manage and treat GDM in Nepal. This proof-of-concept trial will garner important information about leveraging mobile technology for GDM management in LMICs and holds important public health relevance for the broader South Asian population.
Acknowledgments
This work was supported by a research award from the Fogarty International Center (R21TW011377) for SR and a career development award from the National Institute on Drug Abuse (K01 DA051346) for RS.
Abbreviations
- GDM
gestational diabetes mellitus
- GDM-DH
gestational diabetes mellitus–Dhulikhel Hospital
- HIPAA
Health Insurance Portability and Accountability Act
- LMIC
low- and middle-income country
- MAUQ
Mobile Health App Usability Questionnaire
- mHealth
mobile health
- MVP
minimum viable product
- NICU
neonatal intensive care unit
- OB-GYN
obstetrician-gynecologist
- RCT
randomized controlled trial
- SCT
Social Cognitive Theory
- SUS
System Usability Scale
- T2D
type 2 diabetes
Peer-review report.
Data Availability
The data sets generated and analyzed during this study will be available from the corresponding author upon reasonable request.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Yuen L, Saeedi P, Riaz M, Karuranga S, Divakar H, Levitt N, Yang X, Simmons D. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019 Nov;157:107841. doi: 10.1016/j.diabres.2019.107841.S0168-8227(19)31245-8 [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. doi: 10.1016/j.diabres.2021.109050.S0168-8227(21)00409-5 [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, McClure EM, Harrison MS, Miodovnik M. Diabetes during pregnancy in low- and middle-income countries. Am J Perinatol. 2016;33(13):1227–1235. doi: 10.1055/s-0036-1584152. [DOI] [PubMed] [Google Scholar]
- 4.Thapa P, Shrestha S, Flora MS, Bhattarai MD, Thapa N, Mahat B, Pedersen BS. Gestational diabetes mellitus - a public health concern in rural communities of Nepal. J Nepal Health Res Counc. 2015;13(31):175–181. [PubMed] [Google Scholar]
- 5.Kampmann U, Madsen LR, Skajaa GO, Iversen DS, Moeller N, Ovesen P. Gestational diabetes: a clinical update. World J Diabetes. 2015;6(8):1065–1072. doi: 10.4239/wjd.v6.i8.1065. https://www.wjgnet.com/1948-9358/full/v6/i8/1065.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal F, Gupta S, Rimal B, Kafle D. Prevalence of gestational diabetes mellitus in National Medical College and Teaching Hospital, Birgunj, Nepal. Int Res J Pharm Appl Sci. 2013;3(6):1–3. https://www.cabidigitallibrary.org/doi/full/10.5555/20143108177 . [Google Scholar]
- 7.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJN, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943.358/19/1991 [DOI] [PubMed] [Google Scholar]
- 8.Yogev Y, Xenakis EMJ, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191(5):1655–1660. doi: 10.1016/j.ajog.2004.03.074.S0002937804003382 [DOI] [PubMed] [Google Scholar]
- 9.Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol. 2004;191(3):969–974. doi: 10.1016/j.ajog.2004.06.057.S000293780400657X [DOI] [PubMed] [Google Scholar]
- 10.England LJ, Dietz PM, Njoroge T, Callaghan WM, Bruce C, Buus RM, Williamson DF. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(4):365.e1–365.e8. doi: 10.1016/j.ajog.2008.06.031.S0002-9378(08)00642-X [DOI] [PubMed] [Google Scholar]
- 11.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 13.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94(7):2464–2470. doi: 10.1210/jc.2009-0305.jc.2009-0305 [DOI] [PubMed] [Google Scholar]
- 14.Hex N, MacDonald R, Pocock J, Uzdzinska B, Taylor M, Atkin M, Wild SH, Beba H, Jones R. Estimation of the direct health and indirect societal costs of diabetes in the UK using a cost of illness model. Diabet Med. 2024;41(9):e15326. doi: 10.1111/dme.15326. [DOI] [PubMed] [Google Scholar]
- 15.Dall TM, Yang W, Gillespie K, Mocarski M, Byrne E, Cintina I, Beronja K, Semilla AP, Iacobucci W, Hogan PF. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2019;42(9):1661–1668. doi: 10.2337/dc18-1226. https://europepmc.org/abstract/MED/30940641 .dc18-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian G. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. https://core.ac.uk/reader/192780825?utm_source=linkout .NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 17.Brown J, Alwan NA, West J, Brown S, McKinlay CJ, Farrar D, Crowther CA. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5(5):CD011970. doi: 10.1002/14651858.CD011970.pub2. https://europepmc.org/abstract/MED/28472859 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utz B, Kolsteren P, De Brouwere V. Screening for gestational diabetes mellitus: are guidelines from high-income settings applicable to poorer countries? Clin Diabetes. 2015;33(3):152–158. doi: 10.2337/diaclin.33.3.152. https://europepmc.org/abstract/MED/26203210 .152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utz B, Kolsteren P, De Brouwere V. A snapshot of current gestational diabetes management practices from 26 low-income and lower-middle-income countries. Int J Gynaecol Obstet. 2016;134(2):145–150. doi: 10.1016/j.ijgo.2016.01.020.S0020-7292(16)30141-2 [DOI] [PubMed] [Google Scholar]
- 20.Skordis J, Pace N, Vera-Hernandez M, Rasul I, Fitzsimons E, Osrin D, Manandhar D, Costello A. Family networks and healthy behaviour: evidence from Nepal. Health Econ Policy Law. 2019;14(2):231–248. doi: 10.1017/S1744133118000130.S1744133118000130 [DOI] [PubMed] [Google Scholar]
- 21.Simkhada B, Porter MA, van Teijlingen ER. The role of mothers-in-law in antenatal care decision-making in Nepal: a qualitative study. BMC Pregnancy Childbirth. 2010;10:34. doi: 10.1186/1471-2393-10-34. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-10-34 .1471-2393-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuta M, Salway S. Women's position within the household as a determinant of maternal health care use in Nepal. Int Fam Plan Perspect. 2006;32(1):17–27. doi: 10.1363/3201706. https://www.guttmacher.org/pubs/journals/3201706.html .3201706 [DOI] [PubMed] [Google Scholar]
- 23.Adhikari M, Devkota HR, Cesuroglu T. Barriers to and facilitators of diabetes self-management practices in Rupandehi, Nepal- multiple stakeholders' perspective. BMC Public Health. 2021;21(1):1269. doi: 10.1186/s12889-021-11308-4. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-021-11308-4 .10.1186/s12889-021-11308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhandari P, Kim M. Self-care behaviors of Nepalese adults with type 2 diabetes: a mixed methods analysis. Nurs Res. 2016;65(3):202–214. doi: 10.1097/NNR.0000000000000153.00006199-201605000-00005 [DOI] [PubMed] [Google Scholar]
- 25.Pokhrel S, Shrestha S, Timilsina A, Sapkota M, Bhatt MP, Pardhe BD. Self-care adherence and barriers to good glycaemic control in Nepalese type 2 diabetes mellitus patients: a hospital-based cross-sectional study. J Multidiscip Healthc. 2019;12:817–826. doi: 10.2147/JMDH.S216842. https://europepmc.org/abstract/MED/31632050 .216842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrestha A, Singh SB, Khanal VK, Bhattarai S, Maskey R, Pokharel PK. Health literacy and knowledge of chronic diseases in Nepal. Health Lit Res Pract. 2018;2(4):e221–e230. doi: 10.3928/24748307-20181025-01. https://journals.healio.com/doi/abs/10.3928/24748307-20181025-01?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care. 2016;39(11):2089–2095. doi: 10.2337/dc16-0346.39/11/2089 [DOI] [PubMed] [Google Scholar]
- 28.van den Heuvel JF, Groenhof TK, Veerbeek JH, van Solinge WW, Lely AT, Franx A, Bekker MN. eHealth as the next-generation perinatal care: an overview of the literature. J Med Internet Res. 2018;20(6):e202. doi: 10.2196/jmir.9262. https://www.jmir.org/2018/6/e202/ v20i6e202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie W, Dai P, Qin Y, Wu M, Yang B, Yu X. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: an updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth. 2020;20(1):198. doi: 10.1186/s12884-020-02892-1. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-020-02892-1 .10.1186/s12884-020-02892-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Carbone ET. Functionality, implementation, impact, and the role of health literacy in mobile phone apps for gestational diabetes: scoping review. JMIR Diabetes. 2017;2(2):e25. doi: 10.2196/diabetes.8045. https://diabetes.jmir.org/2017/2/e25/ v2i2e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastrogiannis DS, Igwe E, Homko CJ. The role of telemedicine in the management of the pregnancy complicated by diabetes. Curr Diab Rep. 2013;13(1):1–5. doi: 10.1007/s11892-012-0352-x. [DOI] [PubMed] [Google Scholar]
- 32.Thapa NR. Factors influencing the use of reproductive health services among young women in Nepal: analysis of the 2016 Nepal demographic and health survey. Reprod Health. 2020;17(1):102. doi: 10.1186/s12978-020-00954-3. https://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-020-00954-3 .10.1186/s12978-020-00954-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colaci D, Chaudhri S, Vasan A. mHealth interventions in low-income countries to address maternal health: a systematic review. Ann Glob Health. 2016;82(5):922–935. doi: 10.1016/j.aogh.2016.09.001. doi: 10.1016/j.aogh.2016.09.001.S2214-9996(16)30765-2 [DOI] [PubMed] [Google Scholar]
- 34.Dol J, Richardson B, Tomblin Murphy G, Aston M, McMillan D, Campbell-Yeo M. Impact of mobile health (mHealth) interventions during the perinatal period for mothers in low- and middle-income countries: a systematic review. JBI Database System Rev Implement Rep. 2019;17(8):1634–1667. doi: 10.11124/JBISRIR-2017-004022.01938924-201908000-00013 [DOI] [PubMed] [Google Scholar]
- 35.Sondaal SFV, Browne JL, Amoakoh-Coleman M, Borgstein A, Miltenburg AS, Verwijs M, Klipstein-Grobusch K. Assessing the effect of mHealth interventions in improving maternal and neonatal care in low- and middle-income countries: a systematic review. PLoS One. 2016;11(5):e0154664. doi: 10.1371/journal.pone.0154664. https://dx.plos.org/10.1371/journal.pone.0154664 .PONE-D-15-28893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saronga NJ, Burrows T, Collins CE, Ashman AM, Rollo ME. mHealth interventions targeting pregnancy intakes in low and lower-middle income countries: systematic review. Matern Child Nutr. 2019;15(2):e12777. doi: 10.1111/mcn.12777. https://europepmc.org/abstract/MED/30609297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American College of Obstetricians and Gynecologists ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–e64. doi: 10.1097/AOG.0000000000002501.00006250-201802000-00037 [DOI] [PubMed] [Google Scholar]
- 38.Martin K, Radler DR, Sackey J, Zhang C, Shrestha K, Shrestha A, Shrestha A, Barrett ES, Rawal S. Association between 1st trimester diet quality and gestational weight gain rate among pregnant women in Dhulikhel, Nepal. BMC Nutr. 2022;8(1):129. doi: 10.1186/s40795-022-00623-7. https://europepmc.org/abstract/MED/36369060 .10.1186/s40795-022-00623-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 40.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 41.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Jeffrie Seley J, Stanton RC, Gabbay RA. Management of diabetes in pregnancy: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S254–S266. doi: 10.2337/dc23-S015. https://europepmc.org/abstract/MED/36507645 .148052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Institute of Medicine (US) and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines . The National Academies collection: reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US) and National Academy of Sciences; 2009. [Google Scholar]
- 43.American College of Obstetricians and Gynecologists Physical activity and exercise during pregnancy and the postpartum period: ACOG committee opinion, number 804. Obstet Gynecol. 2020;135(4):e178–e188. doi: 10.1097/AOG.0000000000003772.00006250-202004000-00061 [DOI] [PubMed] [Google Scholar]
- 44.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26–e50. doi: 10.1097/AOG.0000000000003020.00006250-201901000-00050 [DOI] [PubMed] [Google Scholar]
- 45.American College of Obstetricians and Gynecologists ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1. doi: 10.1097/AOG.0000000000003018.00006250-201901000-00049 [DOI] [PubMed] [Google Scholar]
- 46.Creswell J. Qualitative inquiry and research design: choosing among five approaches. Thousand Oaks, CA: SAGE Publications, Inc; 2013. [Google Scholar]
- 47.Namey E, Guest G, McKenna K, Chen M. Evaluating bang for the buck: a cost-effectiveness comparison between individual interviews and focus groups based on thematic saturation levels. AJE. 2016;37(3):425–440. doi: 10.1177/1098214016630406. [DOI] [Google Scholar]
- 48.Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2017;27(4):591–608. doi: 10.1177/1049732316665344. https://europepmc.org/abstract/MED/27670770 .1049732316665344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guest G, Bunce A, Johnson L. How many interviews are enough?: An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82. doi: 10.1177/1525822X05279903. [DOI] [Google Scholar]
- 50.Shanmugavel A, Shakya PR, Shrestha A, Nepal J, Shrestha A, Daneault JF, Rawal S. Designing and developing a mobile app for management and treatment of gestational diabetes in Nepal: user-centered design study. JMIR Form Res. 2024;8:e50823. doi: 10.2196/50823. https://formative.jmir.org/2024//e50823/ v8i1e50823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 52.Ericsson KA, Simon HA. Protocol Analysis: Verbal Reports As Data. Cambridge, MA: The MIT Press; 1993. [Google Scholar]
- 53.Rubin J, Chisnell D, Spool J. Handbook of Usability Testing: How to Plan, Design, and Conduct Effective Tests, 2nd Edition. Indianapolis, IN: Wiley Publishing, Inc; 2008. [Google Scholar]
- 54.Brooke J. Usability Evaluation In Industry. London, United Kingdom: CRC Press; 1996. SUS: a 'quick and dirty' usability scale. [Google Scholar]
- 55.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. doi: 10.1016/0002-9378(82)90349-0.0002-9378(82)90349-0 [DOI] [PubMed] [Google Scholar]
- 56.Hirst JE, Mackillop L, Loerup L, Kevat DA, Bartlett K, Gibson O, Kenworthy Y, Levy JC, Tarassenko L, Farmer A. Acceptability and user satisfaction of a smartphone-based, interactive blood glucose management system in women with gestational diabetes mellitus. J Diabetes Sci Technol. 2015;9(1):111–115. doi: 10.1177/1932296814556506. https://europepmc.org/abstract/MED/25361643 .1932296814556506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, Bao J, Setiawan IMA, Saptono A, Parmanto B. The mHealth app usability questionnaire (MAUQ): development and validation study. JMIR Mhealth Uhealth. 2019;7(4):e11500. doi: 10.2196/11500. https://mhealth.jmir.org/2019/4/e11500/ v7i4e11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo H, Zhang Y, Li P, Zhou P, Chen LM, Li SY. Evaluating the effects of mobile health intervention on weight management, glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus. J Endocrinol Invest. 2019;42(6):709–714. doi: 10.1007/s40618-018-0975-0.10.1007/s40618-018-0975-0 [DOI] [PubMed] [Google Scholar]
- 59.Miremberg H, Ben-Ari T, Betzer T, Raphaeli H, Gasnier R, Barda G, Bar J, Weiner E. The impact of a daily smartphone-based feedback system among women with gestational diabetes on compliance, glycemic control, satisfaction, and pregnancy outcome: a randomized controlled trial. Am J Obstet Gynecol. 2018;218(4):453.e1–453.e7. doi: 10.1016/j.ajog.2018.01.044.S0002-9378(18)30133-9 [DOI] [PubMed] [Google Scholar]
- 60.Al-Ofi EA, Mosli HH, Ghamri KA, Ghazali SM. Management of postprandial hyperglycaemia and weight gain in women with gestational diabetes mellitus using a novel telemonitoring system. J Int Med Res. 2019;47(2):754–764. doi: 10.1177/0300060518809872. https://journals.sagepub.com/doi/10.1177/0300060518809872?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Student The probable error of a mean. Biometrika. 1908;6(1):1–25. doi: 10.1093/biomet/6.1.1. [DOI] [Google Scholar]
- 62.Pearson KX. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond Edinb Dubl Phil Mag. 1900;50(302):157–175. doi: 10.1080/14786440009463897. [DOI] [Google Scholar]
- 63.Girden ER. ANOVA: Repeated Measures. Thousand Oaks, CA: SAGE Publications, Inc; 1992. [Google Scholar]
- 64.Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Ann Math Stat. 1940;11(2):204–209. doi: 10.1214/aoms/1177731915. [DOI] [Google Scholar]
- 65.Adesina N, Dogan H, Green S, Tsofliou F. Effectiveness and usability of digital tools to support dietary self-management of gestational diabetes mellitus: a systematic review. Nutrients. 2021;14(1):10. doi: 10.3390/nu14010010. https://www.mdpi.com/resolver?pii=nu14010010 .nu14010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo P, Chen D, Xu P, Wang X, Zhang W, Mao M, Zheng Q, Jin Y, Feng S. Web-based interventions for pregnant women with gestational diabetes mellitus: systematic review and meta-analysis. J Med Internet Res. 2023;25:e36922. doi: 10.2196/36922. https://www.jmir.org/2023//e36922/ v25i1e36922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei HX, Yang YL, Luo TY, Chen WQ. Effectiveness of mobile health interventions for pregnant women with gestational diabetes mellitus: a systematic review and meta-analysis. J Obstet Gynaecol. 2023;43(2):2245906. doi: 10.1080/01443615.2023.2245906. https://www.tandfonline.com/doi/full/10.1080/01443615.2023.2245906 . [DOI] [PubMed] [Google Scholar]
- 68.Varnfield M, Redd C, Stoney RM, Higgins L, Scolari N, Warwick R, Iedema J, Rundle J, Dutton W. M♡THer, an mHealth system to support women with gestational diabetes mellitus: feasibility and acceptability study. Diabetes Technol Ther. 2021;23(5):358–366. doi: 10.1089/dia.2020.0509. https://europepmc.org/abstract/MED/33210954 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackillop L, Hirst JE, Bartlett KJ, Birks JS, Clifton L, Farmer AJ, Gibson O, Kenworthy Y, Levy JC, Loerup L, Rivero-Arias O, Ming WK, Velardo C, Tarassenko L. Comparing the efficacy of a mobile phone-based blood glucose management system with standard clinic care in women with gestational diabetes: randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(3):e71. doi: 10.2196/mhealth.9512. https://mhealth.jmir.org/2018/3/e71/ v6i3e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birati Y, Yefet E, Perlitz Y, Shehadeh N, Spitzer S. Cultural and digital health literacy appropriateness of app- and web-based systems designed for pregnant women with gestational diabetes mellitus: scoping review. J Med Internet Res. 2022;24(10):e37844. doi: 10.2196/37844. https://www.jmir.org/2022/10/e37844/ v24i10e37844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gyawali B, Sharma R, Neupane D, Mishra SR, van Teijlingen E, Kallestrup P. Prevalence of type 2 diabetes in Nepal: a systematic review and meta-analysis from 2000 to 2014. Glob Health Action. 2015;8:29088. doi: 10.3402/gha.v8.29088. https://europepmc.org/abstract/MED/26613684 .29088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaidya A. Tackling cardiovascular health and disease in Nepal: epidemiology, strategies and implementation. Heart Asia. 2011;3(1):87–91. doi: 10.1136/heartasia-2011-010000. https://europepmc.org/abstract/MED/27326001 .heartasia-2011-010000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma RCW, Tutino GE, Lillycrop KA, Hanson MA, Tam WH. Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog Biophys Mol Biol. 2015;118(1-2):55–68. doi: 10.1016/j.pbiomolbio.2015.02.010.S0079-6107(15)00035-8 [DOI] [PubMed] [Google Scholar]
- 74.Bhandari S, Sayami JT, Thapa P, Sayami M, Kandel BP, Banjara MR. Dietary intake patterns and nutritional status of women of reproductive age in Nepal: findings from a health survey. Arch Public Health. 2016;74:2. doi: 10.1186/s13690-016-0114-3. https://archpublichealth.biomedcentral.com/articles/10.1186/s13690-016-0114-3 .114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forczek W, Curyło M, Forczek B. Physical activity assessment during gestation and its outcomes: a review. Obstet Gynecol Surv. 2017;72(7):425–444. doi: 10.1097/OGX.0000000000000458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer-review report.
Data Availability Statement
The data sets generated and analyzed during this study will be available from the corresponding author upon reasonable request.


