Abstract
Background
The prognosis of patients with lung cancer and malignant pleural effusion (MPE) caused by carcinomatous pleurisy is poor. Chemical pleurodesis is commonly performed clinically, however, often has a high failure rate. Furthermore, prolonged sustained drainage and delayed introduction of systemic chemotherapy could increase the risk of worsening the Eastern Cooperative Oncology Group Performance Status (ECOG PS) in the treatment of patients with non-small cell lung cancer (NSCLC). Therefore, both systemic and local treatments are crucial to control MPE. Ramucirumab, an antibody targeting vascular endothelial growth factor receptor 2, is expected to be effective for treatment of MPE. However, there are no data supporting this hypothesis. Herein, we performed a prospective phase II study to evaluate the efficacy and safety of ramucirumab plus docetaxel in NSCLC patients with MPE.
Methods
A single-arm phase II study was conducted to elucidate the efficacy and safety of ramucirumab plus docetaxel as a combined treatment for patients NSCLC and MPE previously treated with platinum-based chemotherapy. The primary endpoint was the MPE control proportion at eight weeks after protocol treatment initiation. The secondary endpoints of the study were objective response rate (ORR), progression-free survival (PFS), one-year survival rate, overall survival (OS), and toxicity profile.
Results
Between September 2019 and March 2022, 15 patients were enrolled. The pleural effusion control proportion at eight weeks was 100% [90% confidence interval (CI): 84.0–100%, and 95% CI: 78.4–100%], and the primary endpoint of this study was met. The ORR was 6.7% (95% CI: 0.2–32.0%), the median PFS was 6.3 months (95% CI: 1.9–6.9), and the median OS was 10.4 months (95% CI: 3.2–16.5). No Grade 5 or unexpected adverse events were observed.
Conclusions
Ramucirumab plus docetaxel is a promising and safe treatment option for previously treated patients with NSCLC and MPE, showing a high pleural effusion control rate.
Keywords: Non-small cell lung cancer (NSCLC), malignant pleural effusion (MPE), ramucirumab, docetaxel
Highlight box.
Key findings
• Ramucirumab plus docetaxel is a promising and safe treatment option for previously treated patients with non-small cell lung cancer (NSCLC) and malignant pleural effusion (MPE), showing a high pleural effusion control rate.
What is known and what is new?
• Lung cancer with MPE has a poor prognosis.
• A single-arm phase II study was conducted in platinum-treated NSCLC and MPE cases. The efficacy of ramucirumab plus docetaxel in NSCLC with MPE was assessed in this study.
What is the implication, and what should change now?
• The ramucirumab plus docetaxel treatment demonstrated systemic antitumor effect and the potential to avoid local treatments such as pleural drainage for NSCLC patients with MPE, thereby reducing the risk of unnecessary worsening in performance status.
Introduction
Lung cancer is one of the leading causes of death both worldwide and in Japan, and many patients suffer from lung cancer (1,2). Since lung cancer metastasizes to various sites such as the brain, pleura, and bones, many patients require appropriate local treatments.
The prognosis of patients with lung cancer and malignant pleural effusion (MPE) caused by carcinomatous pleurisy is poor (3). An investigation into the clinical influence of MPE accumulation in 490 patients with lung cancer reported that 40% of the patients had MPE (3). The median overall survival (OS) was 5.49 months [95% confidence interval (CI): 3.62–7.35] in the MPE group and 12.65 months (95% CI: 10.41–14.89) in the group without MPE (3). Furthermore, 79 of 94 patients (84%) with MPE received palliative MPE treatment to relieve their symptoms when MPE volume covered more than half of the thoracic cavity volume (3).
Chemical pleurodesis is the most common method for relieving MPE symptoms in clinical practice. However, the failure rate of pleurodesis is 22–31.4%, and several complications, such as fever and chest pain, sometimes occur (4,5). Furthermore, prolonged sustained drainage and delayed introduction of systemic chemotherapy could increase the risk of worsening the Eastern Cooperative Oncology Group Performance Status (ECOG PS). Therefore, systemic chemotherapy that is effective for MPE is necessary to avoid worsening of PS due to thoracic drainage.
Two randomized clinical studies in Japan revealed that targeting vascular endothelial growth factor (VEGF) pathway with bevacizumab, an anti-VEGF antibody, showed the antitumor effect in patients with non-small cell lung cancer (NSCLC) and MPE (6,7). The newly developed VEGF pathway inhibitor ramucirumab, a fully humanized IgG1 monoclonal antibody targeting VEGF receptor 2, is widely used in combination with docetaxel for NSCLC after the development of platinum-based chemotherapy resistance (8). Because its mechanism of action is similar to that of bevacizumab, ramucirumab is also expected to be effective in treating MPE. However, there are no data supporting the efficacy of ramucirumab in MPE treatment. Additionally, a safety concern is associated with treating patients with NSCLC and MPE using ramucirumab and docetaxel because no prospective interventional trials have been reported. Therefore, this single-arm phase II study was conducted to examine the effect of ramucirumab in combination with docetaxel in patients with NSCLC and MPE along with its clinical outcomes. We present this article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-508/rc).
Methods
Objective
The primary endpoint of the current study was to evaluate the MPE control proportion at eight weeks after initiating treatment with ramucirumab in combination with docetaxel in patients with NSCLC and MPE, who were previously treated with platinum-based chemotherapy. The secondary endpoints were to evaluate the efficacy and toxicity profile of ramucirumab in combination with docetaxel in terms of the objective response rate (ORR), progression-free survival (PFS), 1-year survival rates, OS, and toxicity profile. For post-hoc analysis, the disease control rate (DCR) was calculated. DCR was defined as the proportion of patients for whom the best overall effect was a complete response, partial response, or stable disease.
Study design
This single-arm phase II study examined the efficacy and safety of administering ramucirumab and docetaxel as a combined treatment to patients with NSCLC with MPE. Between September 2019 and March 2022, patients from five hospitals in Japan were recruited.
Clinical hypothesis and phase setting of the current study
The clinical hypothesis of the current study was that combined treatment using ramucirumab and docetaxel is safe even for patients with NSCLC with MPE and is expected to control MPE.
Key inclusion criteria
The key inclusion criteria were as follows: (I) the patient was 20 years old or older on the day of enrollment; (II) the patient had histologically or cytologically confirmed NSCLC; (III) the patient’s disease has a stage IV clinical presentation; (IV) the patient showed disease progression during or after only one prior platinum-based chemotherapy with or without maintenance therapy for advanced/metastatic disease, or in combination with immune checkpoint inhibitors (ICIs); (V) the patient had clinical MPE and did not undergo pleurodesis after discontinuation of prior treatment; (VI) the patient had no invasion or narrowing of major blood vessels due to cancer based on radiologically documented evidence; (VII) the patient had an ECOG PS of 0 or 1 at the time of enrollment; (VIII) the patient had adequate organ function.
Key exclusion criteria
The key exclusion criteria were as follows: (I) the patient required treatments for MPE, including urgent and continuous pleural effusion drainage for mediastinum displacement owing to MPE accumulation (for reference, SpO2 <90%, unbearable respiratory difficulty, and pleural effusion 3 L or more); (II) the patient was unsuitable for ramucirumab administration; (III) the patient had preexisting interstitial lung disease, as evidenced by a chest computed tomography (CT) scan and/or radiography at the baseline; (IV) the patient had undergone prior therapy with ramucirumab and/or docetaxel.
Interventions
The ramucirumab (10 mg/kg) and docetaxel (60 mg/m2) combination was administered intravenously every 3 weeks. Treatment was continued until the criteria for discontinuation were met. Ramucirumab was not administered as a monotherapy.
Outcomes
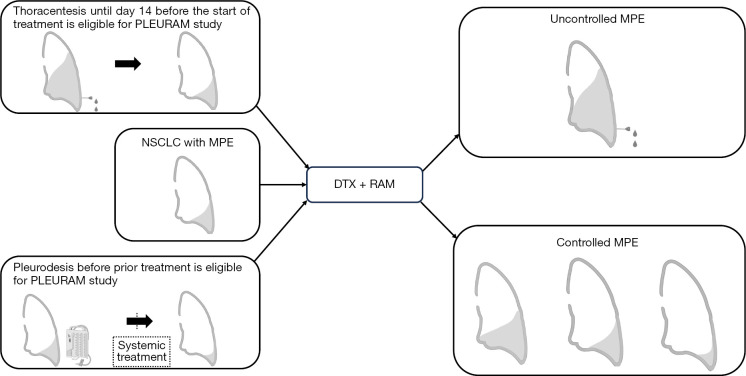
The study scheme regarding the eligibility and assessment of MPE is shown in Figure 1. The revised Response Evaluation Criteria for Solid Tumors (RECIST) version 1.1 (9) was used for evaluation. MPE control proportion at eight weeks was defined as the proportion of cases that did not require pleural effusion puncture and drainage for eight weeks after treatment initiation. The investigators determined whether pleural effusion puncture and drainage were required based on clinical findings and simple chest radiography. Chest radiography on day 56 was mandatory to examine changes in pleural fluid volume. The percentage of patients who required local treatment, such as continuous pleural effusion drainage by day 21 after the start of treatment, was defined as the safety endpoint of ramucirumab plus docetaxel treatment. Rate of adverse events (AEs) was defined that the frequency of the worst grade throughout all courses of treatment using NCI-CTCAE v4.0 for each of the subsequent AEs.
Figure 1.
Scheme of the study. Eligibility and assessment of MPE. Uncontrolled MPE: drainage for pleural effusion was required within 8 weeks. Controlled MPE: no drainage for pleural effusion was required within 8 weeks. NSCLC, non-small cell lung cancer; DTX, docetaxel; RAM, ramucirumab; MPE, malignant pleural effusion.
Statistical analysis
Based on the results from two phase II studies on NSCLC with MPE, sample size ware calculated. The MPE control proportion of bevacizumab in combination with carboplatin and paclitaxel was 91.8% (7) and the proportion of MPE controlled without pleurodesis under bevacizumab, carboplatin, and pemetrexed was 93.0% (6). Since these studies were conducted in the setting of the first treatment, the treatment in the current study could be less effective because it was conducted in a later setting. Based on this information, we calculated the required sample size. With an expected proportion of pleural effusion control at 50% and a threshold proportion of pleural effusion control at 20%, calculations using a one-sided exact test with α=0.05 and β=0.20, showed that 13 subjects were needed for the study. In anticipation of dropout, the expected number of enrolled subjects was determined to be 15. For the primary analysis, we estimated the MPE control proportion within eight weeks with 90% and 95% CI using Wilson’s method. In addition to the primary analysis, ORR, DCR, and two-sided 95% CI were calculated using Wilson’s method. Pleural effusion PFS (pePFS) was estimated as a post-hoc analysis. pePFS was defined as the time from treatment to the evidence of grade 3 pleural effusion. In the PFS, OS, DCR, and pePFS analyses, survival functions and 1-year survival rates were calculated using the Kaplan-Meier method. The CI for the median survival time was estimated using the method of Brookmeyer and Crowley, and the CI for 1-year survival rates was estimated using Greenwood’s method. The precise study protocol has been described previously (10). Erectrical data collection system [REDCap (11)] was used to collect data. GraphPadPrism version 9.5.0® was used for the calculations and figure creation.
Ethical statement
All methods were performed in accordance with relevant guidelines and regulations. The study protocol was reviewed and approved by the Nagasaki University Clinical Research Review Committee [Certified Review Board (CRB)] (approval No. CRB7180001) (registration No. jRCTs071190013). All patients signed an informed consent form prior to inclusion. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Patient’s characteristics
Fifteen patients were enrolled in this study between September 2019 and March 2022. The median observation period was 10.4 months (range, 1.1–18.0 months). All patients enrolled in this study received protocol treatment. The patient characteristics are shown in Table 1. The median patient age was 70 years, and 86.6% of the patients were male. The percentages of patients who received ICIs, bevacizumab, and pleurodesis before participation in the study were 33.3%, 13.3%, and 6.6%, respectively. All patients were stage IV at the time of registration in the current study, including 4 (26.6%) stage III patients at diagnosis. Two patients (13.3%) harbored EGFR mutations.
Table 1. Patient characteristics.
| Characteristics | Value (N=15) |
|---|---|
| Age (years), median [range] | 70 [47–80] |
| Sex, n (%) | |
| Male | 13 (86.7) |
| Female | 2 (13.3) |
| Performance status, n (%) | |
| 0 | 3 (20.0) |
| 1 | 12 (80.0) |
| Number of prior treatment, n (%) | |
| 1 | 10 (66.7) |
| 2 | 5 (33.3) |
| Prior immune checkpoint inhibitors, n (%) | |
| No | 10 (66.7) |
| Yes | 5 (33.3) |
| Prior bevacizumab, n (%) | |
| No | 13 (86.7) |
| Yes | 2 (13.3) |
| Stage (at diagnosis), n (%) | |
| III | 4 (26.7) |
| IV | 7 (46.7) |
| Relapse, n (%) | 4 (26.7) |
| Smoking, n (%) | |
| Never | 4 (26.7) |
| Past/current | 11 (73.3) |
| Prior pleurodesis, n (%) | |
| No | 14 (93.3) |
| Yes | 1 (6.7) |
| Pathology, n (%) | |
| Adeno | 13 (86.7) |
| Squamous | 1 (6.7) |
| Pleomorphic | 1 (6.7) |
| EGFR mutation, n (%) | |
| No | 13 (86.7) |
| Yes | 2 (13.3) |
EGFR, epidermal growth factor receptor.
Treatment delivery
The median number of protocol treatment was 4 (range, 1–13). The reasons for protocol treatment discontinuation were disease progression (n=7), toxicities (grade 3 edema, n=1; long-lasting grade 2 anolexia, n=1; long-lasting grade 2 edema, n=1), patient refusal (n=4), physician’s decision (n=1). One patient refused the protocol treatment on day 29 of the first course and did not visit our hospital after that, therefore, we could not evaluate MPE control of the patient until 8 weeks. Survival data of the patient were included as cut-off data and the AEs were also included in the analysis.
Efficacy regarding MPE
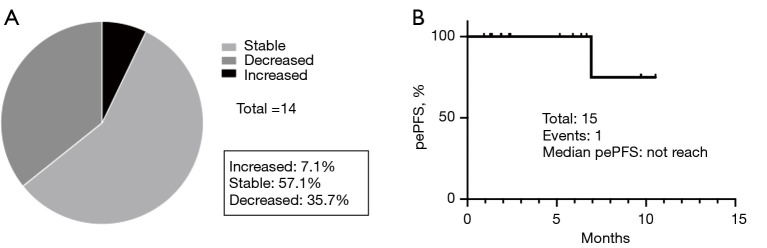
The MPE control proportion at eight weeks was 100% (90% CI: 84.0–100%, 95% CI: 76.8–100%), therefore the primary endpoint of the current study was met (Figure 2). The percentage of patients who required local treatment, such as continuous pleural effusion drainage, by day 21 after the start of treatment was 0%. In the post hoc analysis, 35.7% (95% CI: 8.4–58.1%) of the patients had decreased pleural effusion as determined by chest radiography or CT (Figure 3A). To estimate the period during which MPE controlled the benefits of ramucirumab and docetaxel, pePFS was analyzed. Only one progression event occurred during the observation period (Figure 3B).
Figure 2.
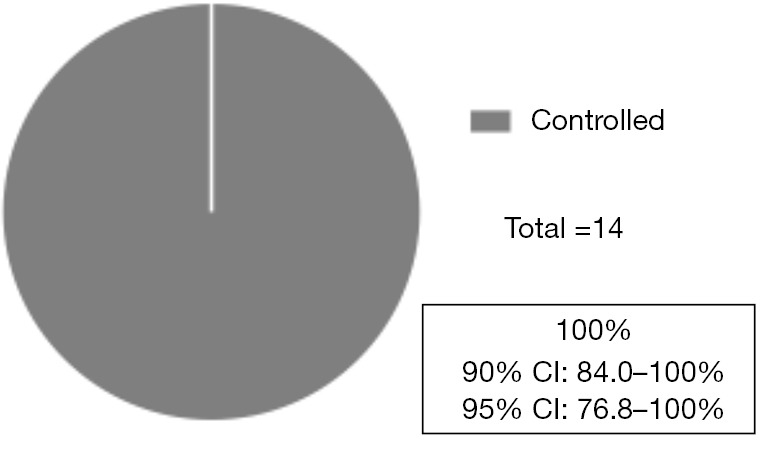
Pleural effusion control proportion at 8 weeks. The pleural effusion control proportion at 8 weeks was 100% [90% CI: 84.0–100%, 95% CI: 76.8–100%, n=14]. One patient refused the protocol treatment on day 29 and was excluded from the analysis. CI, confidence interval.
Figure 3.
Post hoc analysis of MPE. (A) Changes in pleural effusion 8 weeks after registration in this study. One patient refused the protocol treatment on day 29 and was excluded from the analysis. MPE in one patient was increased within 8 weeks after ramucirumab plus docetaxel without requiring drainage assessed by the investigator with chest radiography. One patient discontinued the protocol treatment on day 29 because of treatment refusal and was excluded from the analysis. (B) pePFS. pePFS was defined as the time from treatment to the evidence of grade 3 pleural effusion. MPE, malignant pleural effusion; pePFS, pleural effusion progression-free survival.
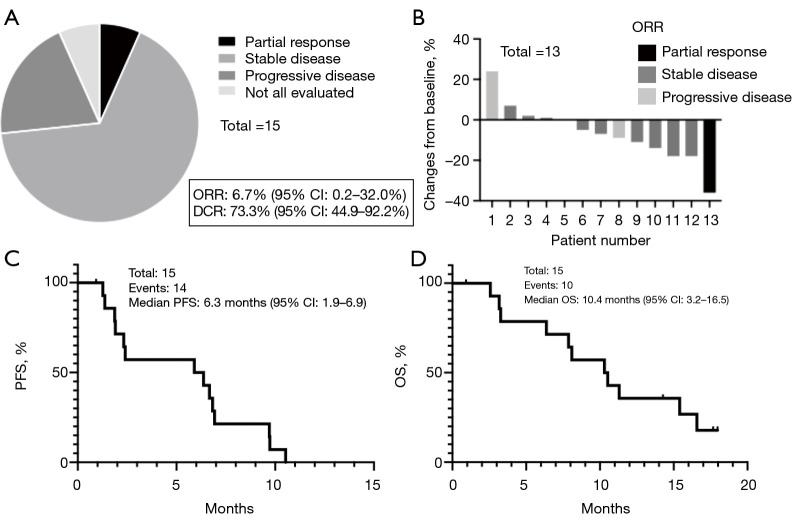
Efficacy regarding response rate and survival
The ORR was 6.7% (95% CI: 0.2–32.0%) and DCR was 73.3% (95% CI: 44.9–92.2%) (Figure 4A). The waterfall plot showed the best response of the target lesions (Figure 4B). The median PFS was 6.3 months (95% CI: 1.9–6.9), and the median OS was 10.4 months (95% CI: 3.2–16.5) (Figure 4C,4D). The 1-year survival rate was 35.7% (95% CI: 13.0–59.4%). Although sample size was small, we compared PFS and OS between patients who had received ICIs prior to this protocol treatment (ICI group) and those who had never received ICIs (no ICI group). The median PFS was 5.9 months in the ICI group and 6.8 months in the No ICI group [hazard ratio (HR): 2.00, 95% CI: 0.56–7.16; P=0.16]. The median OS was 7.8 months in the ICI group vs. 11.3 months in the No ICI group (HR: 2.58, 95% CI: 0.69–9.66; P=0.15) (Figure S1). Details about the treatments administered after the protocol treatment are provided in Tables S1,S2.
Figure 4.
Systemic efficacy of ramucirumab plus docetaxel for NSCLC. (A) ORR and DCR. (B) Waterfall plot of target lesions. One patient was not evaluated for the target lesion because symptomatic brain metastasis occurred prior to the planned assessment. One patient discontinued the protocol treatment on day 29 because of treatment refusal and was excluded from the analysis. (C) PFS. In total, 14 events occurred during the observation period. The median PFS was 6.3 months (95% CI: 1.9–6.9). (D) OS. Ten events occurred. The median OS was 10.4 months (95% CI: 3.2–16.5). NSCLC, non-small cell lung cancer; CI, confidence interval; OS, overall survival; PFS, progression-free survival; ORR, overall response rate; DCR, disease-control rate.
Toxicities
AEs are shown in Table 2. Severe AEs were reported as follows: Grade 3 pneumonitis (n=2, 13.3%), Grade 3 edema of the limbs (n=1, 6.7%), and Grade 3 lung infection (n=1, 6.7%), Grade 3 febrile neutropenia (n=3, 20.0%), Grade 4 neutropenia (n=4, 26.7%), and Grade 4 leukopenia (n=3, 20%). Polyethylene glycol granulocyte colony-stimulating factor (PEG-G-CSF) was administered to all patients to prevent febrile neutropenia. No Grade 5 or unexpected AEs were observed.
Table 2. Number of adverse events (n=15).
| Adverse events | G1, n | G2, n | G3, n | G4, n | Total, n (%) | ≥ G3, n (%) |
|---|---|---|---|---|---|---|
| Hematological | ||||||
| Anemia | 9 | 1 | 0 | 0 | 10 (66.7) | 0 |
| Neutropenia | 0 | 0 | 2 | 4 | 6 (40.0) | 6 (40.0) |
| Febrile neutropenia | 0 | 0 | 3 | 0 | 3 (20.0) | 3 (20.0) |
| Leukopenia | 0 | 0 | 2 | 3 | 5 (33.3) | 5 (33.3) |
| Thrombocytopenia | 3 | 0 | 0 | 0 | 3 (20.0) | 0 |
| Non-hematological | ||||||
| Edema of the limbs | 2 | 2 | 1 | 0 | 5 (33.3) | 1 (6.7) |
| Anorexia | 3 | 2 | 0 | 0 | 5 (33.3) | 0 |
| Malaise | 4 | 1 | 0 | 0 | 5 (33.3) | 0 |
| Hiccups | 3 | 0 | 0 | 0 | 3 (20.0) | 0 |
| Eruption | 2 | 1 | 0 | 0 | 3 (20.0) | 0 |
| Pneumonitis | 0 | 1 | 2 | 0 | 3 (20.0) | 2 (13.3) |
| Fatigue | 3 | 0 | 0 | 0 | 3 (20.0) | 0 |
| Constipation | 1 | 1 | 0 | 0 | 2 (13.3) | 0 |
| Dyspnea | 2 | 0 | 0 | 0 | 2 (13.3) | 0 |
| Watering eyes | 2 | 0 | 0 | 0 | 2 (13.3) | 0 |
| Insomnia | 2 | 0 | 0 | 0 | 2 (13.3) | 0 |
| Paronychia | 1 | 1 | 0 | 0 | 2 (13.3) | 0 |
| Oral hemorrhage | 2 | 0 | 0 | 0 | 2 (13.3) | 0 |
| Mucositis oral | 1 | 1 | 0 | 0 | 2 (13.3) | 0 |
| Nausea | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Dehydration | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Oral pain | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| ALT increased | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| AST increased | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Hyponatremia | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Creatinine increased | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Sinus tachycardia | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Hoarseness | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Herpes infection | 0 | 1 | 0 | 0 | 1 (6.7) | 0 |
| Lung infection | 0 | 0 | 1 | 0 | 1 (6.7) | 1 (6.7) |
| Perianal abscesses | 0 | 1 | 0 | 0 | 1 (6.7) | 0 |
| Epistaxis | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
| Bronchopulmonary hemorrhage | 1 | 0 | 0 | 0 | 1 (6.7) | 0 |
G, grade (grading by Common Terminology Criteria for Adverse Events, version 4); ALT, aspartate aminotransferase; AST, alanine aminotransferase.
Discussion
In the current study, the efficacy of ramucirumab and docetaxel against MPE was assessed, and 100% MPE control was achieved with ramucirumab and docetaxel in patients with NSCLC and MPE after platinum therapy. This study revealed that systemic treatment with ramucirumab plus docetaxel could be a promising treatment option for patients with NSCLC and MPE, circumventing local therapies, such as pleural drainage.
Regarding the effect of VEGF inhibitors against MPE, two phase II trials of VEGF inhibitors for the treatment of patients with NSCLC and MPE have been reported previously. Tamiya et al. reported a single-arm phase II study of chemotherapy-naïve patients with advanced non-squamous NSCLC and MPE treated with carboplatin, paclitaxel, and bevacizumab. In this study, chest radiography and physical examinations for MPE were performed every 2 weeks, and the MPE control rate was 91.8% (7). Usui et al. reported a single-arm phase II study of a similar population treated with carboplatin, pemetrexed, and bevacizumab, calculated the percentage of patients who did not require pleurodesis after 8 weeks of treatment, and reported an MPE control rate of 93% (95% CI: 77–99%) (6). Our study included patients with NSCLC with a history of prior treatment with platinum-based chemotherapy, and the efficacy of VEGF inhibitors for MPE in this population has not been previously reported. We revealed that the rate of MPE control at 8 weeks after the start of treatment was 100% (95% CI: 76.8–100%), which was comparable to the rates reported in previous studies. In addition, MPE decreased in 35.7% of the cases, and the median pePFS was not reached during the observation period. The MPE control was maintained for 8 weeks or longer with ramucirumab plus docetaxel. Considering these novel findings, treatment with a VEGF inhibitor has proven to be effective as a treatment for MPE in NSCLC.
The systemic efficacy of ramucirumab plus docetaxel in controlling conditions other than MPE was also evaluated in the current study. In a phase III study comparing placebo plus docetaxel with ramucirumab plus docetaxel for NSCLC after disease progression on platinum-based therapy, the ORR was 23% and the odds ratio was 1.89 (compared with that of placebo plus docetaxel, 95% CI: 1.41–2.54; P<0.0001) (8). The current study showed that DCR and PFS demonstrated remarkable outcomes in addition to the MPE control proportion, confirming its effect as systemic chemotherapy even in patients with MPE compared with other studies (Table S3). On the other hand, although no severe adverse events characteristic of VEGF pathway inhibitors such as serious bleeding, thrombosis, hypertension, or proteinuria were observed in this study, adequate measures to prevent adverse effects are also necessary (8,12).
In the current study, PEG-G-CSF was used in all patients, which may have contributed to the prevention of febrile neutropenia. In a previous randomized phase II study comparing placebo plus docetaxel with ramucirumab plus docetaxel in Japanese patients with NSCLC after disease progression on platinum-based therapy, the incidence rates of febrile neutropenia in the placebo plus docetaxel and ramucirumab plus docetaxel groups were 19.8% (16 of 81) and 34.2% (26 of 76), respectively (13). In the current study, febrile neutropenia occurred in 20% of the patients, which was lower than that reported in a previous study (13). With adequate supportive treatment, ramucirumab plus docetaxel was well-tolerated in patients with NSCLC and MPE in our study.
The future direction of our study will be to identify biomarkers that predict the effect of ramucirumab plus docetaxel treatment in patients with NSCLC and MPE. A previous study reported that low VEGF-A levels in plasma were associated with a longer PFS (7.4 versus 6.1 months; HR: 1.57; 95% CI: 1.17–2.09, P=0.002) and OS (19.8 vs. 11.1 months; HR: 1.57; 95% CI: 1.15–2.13, P=0.004) in combination with bevacizumab and carboplatin-based chemotherapy for nonsquamous NSCLC (14). Other studies reported that VEGF-D (15,16) and Tie2 (17) were promising biomarkers for VEGF inhibition by ramucirumab in patients with colorectal cancer. It would be beneficial to investigate useful biomarkers for providing ramucirumab and docetaxel treatment to the appropriate patients with NSCLC and MPE.
This study has several limitations. First, the number of patients was small and there was no control group because of the single-arm design. Further validation in larger clinical cohort will be required. Second, because the assessment of pleural effusion volume was subjective, there may have been some bias in the current study. Third, a mild discrepancy from the current real-world clinical practice may be present regarding treatment prior to ramucirumab plus docetaxel. In clinical practice, ICIs are commonly administered prior to ramucirumab plus docetaxel treatment. A previous single-arm phase II trial showed favorable ORR of docetaxel plus ramucirumab for patients who have progressed on front-line ICIs (18). This raises concerns regarding the efficacy of this treatment in patients with NSCLC with MPE who have been treated with ICIs although the current study did not show any significant differences between ICI group and No ICI group. In addition, we cannot rule out the possibility that the enrolled cases might not have experienced an increase in pleural effusion even without the treatment. The other outstanding question is whether ramucirumab plus docetaxel treatment, when applied to a cohort consisting only of patients who required prior pleural interventions, is effective for MPE. Further validation through other clinical trials is needed to resolve these questions. Also, there were no registrations from January to August 2020 due to the coronavirus disease 2019 pandemic, therefore, the registration period was extended by 6 months.
Conclusions
Ramucirumab plus docetaxel is a promising and safe treatment option for previously treated patients with NSCLC and MPE.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing, Shuntaro Sato (Clinical Research Center, Nagasaki University Hospital, Nagasaki, Japan) for statistical analysis, and Nagasaki Thoracic Oncology Group for support. Images for Figure 1 were created using BioRender.com.
Funding: This study was sponsored by Eli Lilly Japan K.K.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All methods were performed in accordance with relevant guidelines and regulations. The study protocol was reviewed and approved by the Nagasaki University Clinical Research Review Committee [Certified Review Board (CRB)] (approval No. CRB7180001) (Registration No. jRCTs071190013). All patients signed an informed consent form prior to inclusion. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-508/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-508/coif). S.T. reports research funding from Eli Lilly Japan KK to Nagasaki University Hospital. H.M. reports honoraria for lectures from Eli Lilly Japan KK and Sanofi KK. The other authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-508/dss
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Mortality Data (1958-2022) in Japan. Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare). Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html
- 3.Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology 2015;20:654-9. 10.1111/resp.12496 [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020;323:60-9. 10.1001/jama.2019.19997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida K, Sugiura T, Takifuji N, et al. Randomized phase II trial of three intrapleural therapy regimens for the management of malignant pleural effusion in previously untreated non-small cell lung cancer: JCOG 9515. Lung Cancer 2007;58:362-8. 10.1016/j.lungcan.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 6.Usui K, Sugawara S, Nishitsuji M, et al. A phase II study of bevacizumab with carboplatin-pemetrexed in non-squamous non-small cell lung carcinoma patients with malignant pleural effusions: North East Japan Study Group Trial NEJ013A. Lung Cancer 2016;99:131-6. 10.1016/j.lungcan.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Tamiya M, Tamiya A, Yamadori T, et al. Phase2 study of bevacizumab with carboplatin-paclitaxel for non-small cell lung cancer with malignant pleural effusion. Med Oncol 2013;30:676. 10.1007/s12032-013-0676-7 [DOI] [PubMed] [Google Scholar]
- 8.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 10.Takemoto S, Fukuda M, Yamaguchi H, et al. Phase II study of ramucirumab and docetaxel for previously treated non-small cell lung cancer patients with malignant pleural effusion: Protocol of PLEURAM study. Thorac Cancer 2020;11:389-93. 10.1111/1759-7714.13279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinner RG, Obasaju CK, Spigel DR, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 2015;10:134-42. 10.1097/JTO.0000000000000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoh K, Hosomi Y, Kasahara K, et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer 2016;99:186-93. 10.1016/j.lungcan.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 14.Mok T, Gorbunova V, Juhasz E, et al. A correlative biomarker analysis of the combination of bevacizumab and carboplatin-based chemotherapy for advanced nonsquamous non-small-cell lung cancer: results of the phase II randomized ABIGAIL study (BO21015). J Thorac Oncol 2014;9:848-55. 10.1097/JTO.0000000000000160 [DOI] [PubMed] [Google Scholar]
- 15.Tabernero J, Hozak RR, Yoshino T, et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann Oncol 2018;29:602-9. 10.1093/annonc/mdx767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katayama Y, Yamada T, Sawada R, et al. Prospective Observational Study of Ramucirumab Plus Docetaxel After Combined Chemoimmunotherapy in Patients With Non-Small-Cell Lung Cancer. Oncologist 2024;29:e681-9. 10.1093/oncolo/oyae001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayson GC, Zhou C, Backen A, et al. Plasma Tie2 is a tumor vascular response biomarker for VEGF inhibitors in metastatic colorectal cancer. Nat Commun 2018;9:4672. 10.1038/s41467-018-07174-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzawa R, Morise M, Ito K, et al. Efficacy and safety of second-line therapy of docetaxel plus ramucirumab after first-line platinum-based chemotherapy plus immune checkpoint inhibitors in non-small cell lung cancer (SCORPION): a multicenter, open-label, single-arm, phase 2 trial. EClinicalMedicine 2023;66:102303. 10.1016/j.eclinm.2023.102303 [DOI] [PMC free article] [PubMed] [Google Scholar]