Abstract
Background
The appreciation of sex differences is substantial for precise cancer management. Surgery is the main treatment for non-small cell lung cancer (NSCLC). We aimed to identify sex differences on perioperative outcomes in NSCLC patients and to uncover the origins of sex effect in outcomes using a Chinese cohort.
Methods
We retrospectively enrolled patients undergoing NSCLC surgery in the Western China Lung Cancer Database from January 2014 to April 2021. We compared baseline characteristics and perioperative outcomes between male and female. Multivariable analyses were performed. We conducted causal mediation analysis to identify drivers to sex differences in perioperative outcomes.
Results
Altogether, data of 10,181 patients (5,738 women and 4,443 men) were analyzed. Women had lower incidence of complications (5.05% vs. 12.15%), shorter postoperative length of stays (4.92 vs. 6.41 days), and less hospitalization cost (50,713.69 vs. 54,580.85, Chinese Yuan). Multivariable regression analysis identified sex as an independent factor of perioperative complications [odds ratio (OR), 1.843, 95% confidence interval (CI): 1.476–2.294], as well as of postoperative length of hospital stays (beta 0.123, 95% CI: 0.099–0.148), and hospitalization cost (beta 0.026, 95% CI: 0.026–0.026). Mediation analysis revealed that age, body mass index, prevalence of chronic obstructive pulmonary disease, predicted diffusion capacity for carbon monoxide, tumor size, pleural adhesion, and surgery duration were identified as mediators for sex differences in outcomes, while smoking status, surgery type, and resection extent were not.
Conclusions
Female NSCLC patients demonstrated lower incidence of complications, shorter postoperative length of stays, and less hospitalization cost after surgery. Those differences between men and women could be explained by their inherent biological differences and baseline health status. Perioperative management strategies for NSCLC should prioritize recognizing the potentially poorer outcomes among male patients and implementing tailored precautions accordingly.
Keywords: Lung cancer, sex difference, short-term outcomes, surgery, mediation analysis
Highlight box.
Key findings
• Female had better perioperative outcomes after non-small cell lung cancer (NSCLC) surgery.
• Sex differences in outcomes were partly attributed to clinicopathologic differences.
• Treatment differences were not drivers to sex differences in perioperative outcomes.
What is known and what is new?
• Sex significantly influences the outcomes of NSCLC, with existing research highlighting better survival for female after NSCLC surgery compared to male, attributed to biological and clinical differences.
• This study demonstrated sex differences in perioperative outcomes in NSCLC surgery. It found sex as an independent predictor of less postoperative complications and hospital stays. The mediation analysis identified that clinicopathologic factors like age, body mass index (BMI), and lung function drive sex differences in perioperative outcomes.
What is the implication, and what should change now?
• Clinicians should recognize that male patients may face higher risks of complications and longer hospital stays and should prompt enhanced monitoring and targeted interventions.
• Inherent biological differences and baseline health status play significant roles in influencing surgical outcomes between male and female.
• The perioperative management might consider prioritize comprehensive preoperative assessments that account for sex-specific risk factors such as age, BMI, and lung function to optimize surgical outcomes.
Introduction
Lung cancer remains the leading cause of cancer-related mortality globally (1). Treatment outcomes in lung cancer patients vary due to numerous factors, with sex being a significant biological and genetic determinant of treatment response (2,3). Females have shown greater responsiveness to targeted therapies such as epidermal growth factor receptor inhibitors and immune checkpoint inhibitors in non-small cell lung cancer (NSCLC) treatment (3-5). These disparities may stem from gender-related behavioral factors, including higher rates of smoking and alcohol consumption among males (2,6), as well as sex-specific biological influences such as immunity and metabolism regulated by sex hormones and chromosomes (3,7). Understanding these sex differences in treatment response is essential for tailoring therapies to individual male and female NSCLC patients.
Surgery is the mainstay treatment regimen for NSCLC. Sex differences have been reported in long-term survival following lung cancer surgery (8,9). As surgical practices advance towards enhanced recovery protocols, early postoperative outcomes hold particular significance for patient recovery. Tong et al.’s study (10) on lung cancer patients from Society of Thoracic Surgeons (STS) Database indicated lower rates of postoperative complications and reduced in-hospital mortality among females. Conversely, Nelson et al.’s study (11) found that sex differences in perioperative outcomes diminished after propensity score matching, suggesting underlying factors influencing these disparities. Despite these findings, there remains limited evidence on how sex specifically influences perioperative outcomes in NSCLC patients. Identifying the origins of sex differences in perioperative outcomes could aid in developing optimal management strategies for male and female NSCLC patients undergoing surgery.
In this study, we hypothesized that sex differences existed in perioperative outcomes after NSCLC lung cancer. Furthermore, we would like to uncover the origins of sex-specific variations in perioperative outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-336/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethic approval has been obtained from the Institutional Ethic Committee for Clinical Research of West China Hospital, Sichuan University [No. 2022(227)]. Individual consent for this retrospective analysis was waived.
Patient selection and data collection
We retrospectively enrolled patients who underwent surgical resection for NSCLC from January 2014 to April 2021 in the Western China Lung Cancer Database, a prospectively maintained database at the Department of Thoracic Surgery, West China Hospital. Inclusion criteria included: (I) older than 18 years old; (II) receiving pulmonary resection; (III) pathological diagnosed with NSCLC. Exclusion criteria included: (I) lack of detailed sex information; (II) emergency surgery; (III) pathologically confirmed as stage 0 (see Figure 1). We extracted data including baseline characteristics [demographic characteristics, smoking status, high blood pressure (HBP), coronary artery disease, chronic heart failure, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), asthma, tuberculosis history], tumor features, operation details, postoperative management, outcomes, and complications.
Figure 1.
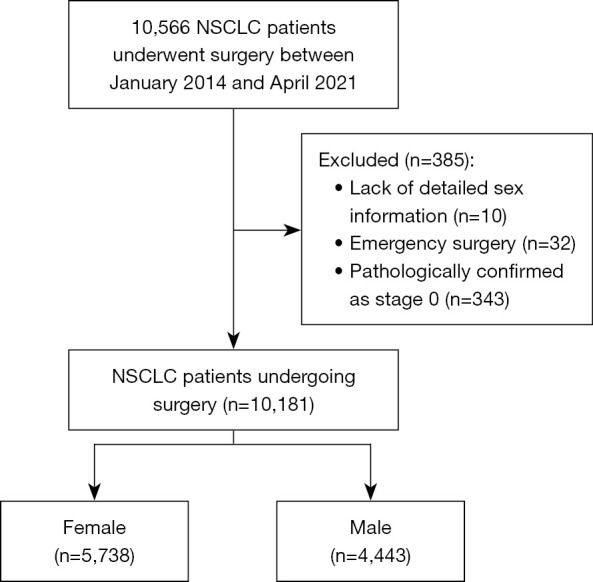
The flowchart of patient inclusion and exclusion. NSCLC, non-small cell lung cancer.
Perioperative outcomes
Perioperative outcomes included incidence of postoperative complications, postoperative drainage volume in the first 3 days, postoperative drainage volume in total, length of postoperative hospital stays, and hospitalization cost, which were partly in consistent with previous studies on perioperative management (10-12). Postoperative complications included pulmonary complications [prolonged air leak (PAL), pulmonary infection, chylothorax, atelectasis, respiratory failure, pulmonary embolism], and surgical site infection. PAL was defined as air leak lasting >5 days. The definition and assessment of other postoperative complications were in line with the standardized variable definitions established by the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases (13).
Sample size estimation
We chose incidence of PAL as the outcome for sample size estimation based on its clinical significance and prevalence in NSCLC patients undergoing surgery (14,15). We calculated sample size by taking PAL as the outcome which was reported in the previous study on sex differences in postoperative outcomes (10). The incidence of PAL was 7.9% and 10.0% in the female and male patients in that study. The effect size was estimated as 0.036. We set the type I error as 5% and statistical power as 95%. The sample size was calculated to be 9,922 using R package ‘pwr’.
Statistical analysis
Continuous variables were described as mean (standard difference), while categorical variable as number of cases (proportion). We first checked the distribution of the continuous variables using Kolmogorov-Smirnov test and confirmed their normal distribution. Baseline characteristics and perioperative outcomes between male and female were compared with Student’s t-test and χ2 test when appropriate (see Tables 1,2). Two-sided P<0.05 was determined to be significant. We first performed univariable regression analysis on each outcome to examine covariates for multivariable analysis. The covariates included age, body mass index (BMI), smoking history, COPD, forced expiratory volume in 1 second (FEV1%), predicted percentage of diffusion capacity for carbon monoxide (DLCO%), neoadjuvant therapy, clinical AJCC stage, tumor size, video-assisted thoracoscopic surgery (VATS), resection extent, pleural adhesion, and surgery duration. The significant covariates in univariable analysis were then adjusted during multivariable analysis (see Figure 2). We conducted variance inflation factors analysis to assess collinearity among the independent variables included in the model, while the results indicating no significant collinearity issues among the variables.
Table 1. Baseline characteristics between female and male patients.
| Variables | Female (n=5,738) | Male (n=4,443) | P |
|---|---|---|---|
| Age, years, mean (SD) | 55.76 (10.95) | 58.71 (10.78) | <0.001 |
| BMI, kg/m2, mean (SD) | 22.67 (2.99) | 23.78 (2.90) | <0.001 |
| Smoking history, n (%) | <0.001 | ||
| Current | 36 (0.62) | 699 (15.7) | |
| Ever | 60 (1.05) | 2,256 (50.8) | |
| Never | 5,597 (97.5) | 1,433 (32.3) | |
| Missing | 45 (0.78) | 55 (1.23) | |
| HBP, n (%) | 969 (16.89) | 947 (21.31) | <0.001 |
| Coronary artery disease, n (%) | 80 (1.39) | 117 (2.63) | <0.001 |
| Chronic heart failure, n (%) | 4 (0.07) | 3 (0.07) | >0.99 |
| DM, n (%) | 303 (5.28) | 413 (9.30) | <0.001 |
| COPD, n (%) | 59 (1.03) | 271 (6.10) | <0.001 |
| Asthma, n (%) | 49 (0.85) | 17 (0.38) | 0.005 |
| Tuberculosis history, n (%) | 18 (0.31) | 14 (0.32) | >0.99 |
| FEV1%, mean (SD) | 108.71 (17.17) | 100.23 (19.35) | <0.001 |
| DLCO%, mean (SD) | 99.31 (15.50) | 101.16 (19.65) | <0.001 |
| Clinical AJCC stage, n (%) | <0.001 | ||
| I | 5,109 (89.04) | 3,178 (71.53) | |
| II | 291 (5.07) | 561 (12.63) | |
| III | 333 (5.80) | 691 (15.55) | |
| IV | 5 (0.09) | 13 (0.29) | |
| Neoadjuvant therapy, n (%) | 29 (0.51) | 98 (2.21) | <0.001 |
| Preoperative chemotherapy | 13 (0.23) | 89 (2.00) | <0.001 |
| Preoperative radiotherapy | 4 (0.07) | 6 (0.14) | 0.47 |
| Preoperative targeted therapy | 17 (0.30) | 10 (0.23) | 0.62 |
| VATS, n (%) | 5,580 (97.25) | 4,015 (90.37) | <0.001 |
| Conversion to open thoracotomy (%) | 69 (1.20) | 151 (3.40) | <0.001 |
| Resection extent, n (%) | <0.001 | ||
| Lobectomy | 3,491 (60.84) | 3,307 (74.43) | |
| Segmentectomy | 1,850 (32.24) | 824 (18.55) | |
| Wedge resection | 379 (6.61) | 261 (5.87) | |
| Pneumonectomy | 18 (0.31) | 51 (1.15) | |
| Tumor size, cm, mean (SD) | 1.79 (1.15) | 2.51 (1.60) | <0.001 |
| Histology, n (%) | <0.001 | ||
| Adenocarcinoma | 5,458 (95.12) | 3,322 (74.77) | |
| Squamous carcinoma | 81 (1.41) | 830 (18.68) | |
| Others* | 199 (3.47) | 291 (6.55) | |
| Pleural adhesion, n (%) | <0.001 | ||
| No | 2,429 (42.70) | 1,823 (41.39) | |
| Moderate | 2,870 (50.45) | 2,126 (48.27) | |
| Complete | 390 (6.86) | 455 (10.33) | |
| Missing | 49 (0.85) | 39 (0.87) | |
| Uncompleted intralobular fissure, n (%) | 2,724 (47.47) | 2,105 (47.38) | 0.001 |
| Surgery duration, min, mean (SD) | 108.43 (43.23) | 127.78 (55.80) | <0.001 |
P values are calculated using the Chi-squared test for categorical variables and the Student’s t-test for continuous variables. *, others included adenosquamous carcinoma, large cell carcinoma, undifferentiated cancers, neuroendocrine tumor, and salivary gland tumor. SD, standard difference; BMI, body mass index; HBP, high blood pressure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; FEV1%, predicted percentage of forced expiratory volume in 1 second; DLCO%, predicted percentage of diffusing capacity for carbon monoxide; AJCC, American Joint Committee on Cancer; VATS, video-assisted thoracoscopic surgery.
Table 2. Perioperative outcomes of female and male patients.
| Outcomes | Female (n=5,738) | Male (n=4,443) | P |
|---|---|---|---|
| Complications, n (%) | 290 (5.05) | 540 (12.15) | <0.001 |
| PAL | 177 (3.08) | 337 (7.58) | <0.001 |
| Pulmonary infection | 50 (0.87) | 118 (2.66) | <0.001 |
| Chylothorax | 40 (0.70) | 60 (1.35) | 0.001 |
| Atelectasis | 7 (0.12) | 14 (0.32) | 0.06 |
| Respiratory failure | 6 (0.10) | 17 (0.38) | 0.007 |
| Pulmonary embolism | 6 (0.10) | 4 (0.09) | >0.99 |
| Arrhythmia | 4 (0.07) | 8 (0.18) | 0.19 |
| Surgical site infection | 3 (0.05) | 2 (0.05) | >0.99 |
| Gastrointestinal complications | 2 (0.03) | 13 (0.29) | 0.002 |
| Urinary tract infection | 3 (0.05) | 2 (0.05) | >0.99 |
| Length of postoperative hospital stays, days, mean (SD) | 4.92 (2.81) | 6.41 (3.99) | <0.001 |
| Chest drainage volume in the first 3 days, mL, mean (SD) | 482.23 (336.82) | 541.91 (372.79) | <0.001 |
| Hospitalization cost, CNY, mean (SD) | 50,713.69 (11,159.19) | 54,580.85 (12,701.56) | <0.001 |
| Death in hospital, n (%) | 3 (0.05) | 10 (0.23) | 0.03 |
P values are calculated using the Student’s t-test for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables as appropriate. PAL, prolonged air leak; SD, standard difference; CNY, Chinese Yuan.
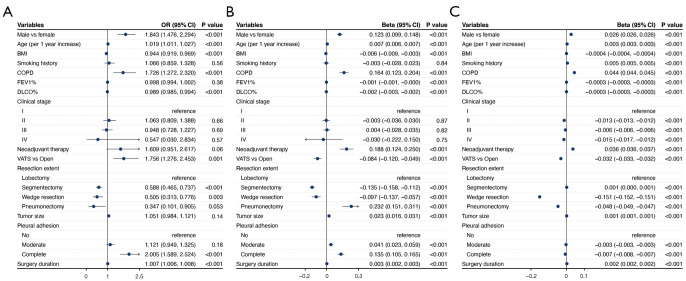
Figure 2.
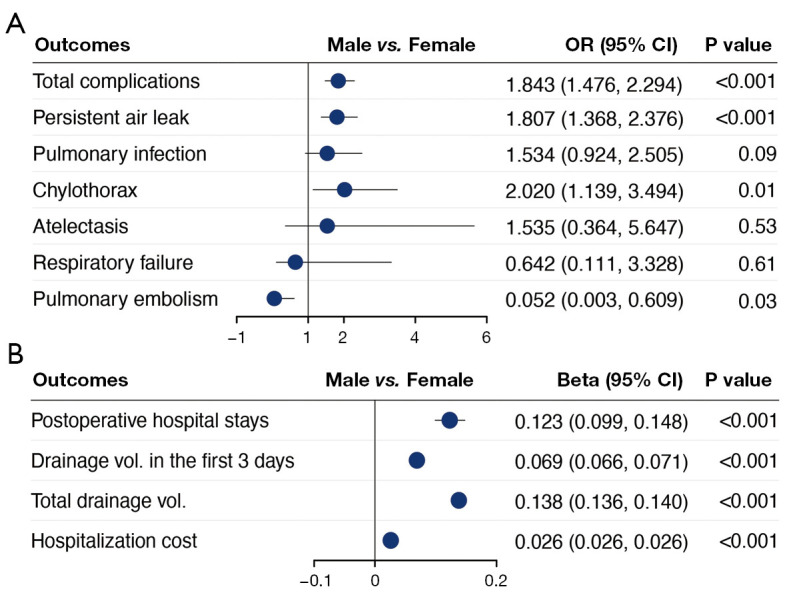
Multivariable analysis on the association between sex and perioperative outcomes. (A) Categorical outcomes; (B) continuous outcomes. Multivariable logistic regression analyses were conducted for categorical outcomes and multivariable linear regression analysis for continuous outcomes. OR, odds ratio; CI, confidence interval.
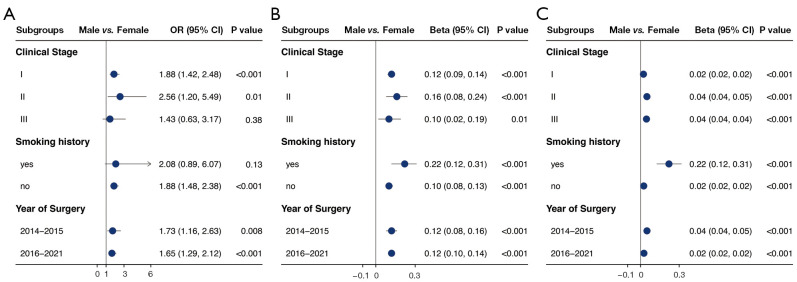
We then performed subgroup analysis regarding stage, smoking history, and year of surgery. We divided the year of surgery into 2015–2016 and 2017–2021, due to the beginning of enhanced recovery after surgery in 2016 in our department. Multivariable analysis of sex impact on complications, length of stays, and hospitalization cost were performed in each subgroup (see Figure 3). In the regression models, male sex was used as the reference group for the sex variable. Two-sided P<0.05 was determined to be significant. We conducted multivariable logistic regression analysis for categorical outcomes and multivariable linear regression analysis for continuous outcomes using R package ‘glm’. We then performed stratified multivariable regression analyses of male and female for each of the perioperative outcomes.
Figure 3.
Details of multivariable analysis on the association between sex and (A) incidence of complications, (B) postoperative hospital stays, and (C) hospitalization cost. Multivariable logistic regression analyses were conducted for categorical outcomes and multivariable linear regression analysis for continuous outcomes. OR, odds ratio; CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1%, predicted percentage of forced expiratory volume in 1 second; DLCO%, predicted percentage of diffusing capacity for carbon monoxide; VATS, video-assisted thoracoscopic surgery.
We included all available data in the statistical analysis, regardless of missing values. Specifically, we employed complete case analysis, where cases with missing data were included as they were. The baseline characteristics had minimal missing data (see Table 1). The perioperative outcomes had some percentage of missing data as follows: postoperative drainage volume in the first 3 days (1.1%), postoperative drainage volume in total (1.2%), and hospitalization cost (3.7%). We also conducted sensitivity analyses by excluding cases with missing data.
To identify drivers to sex differences in perioperative outcomes, we performed causal mediation analysis (16,17). Factors like age, BMI, could function as covariables to the association between sex and outcomes. On the other hand, they could act as intervening variables. Sex influenced the intervening variables, which in turn influenced the outcomes. Mediation analysis could identify those intervening variables, or mediators. The factors adjusted in multivariable regression analysis were also considered as potential mediators. We used structured equation models for analysis, since it is convenient to deal with binary mediators and to perform mediation analysis with moderated covariates.
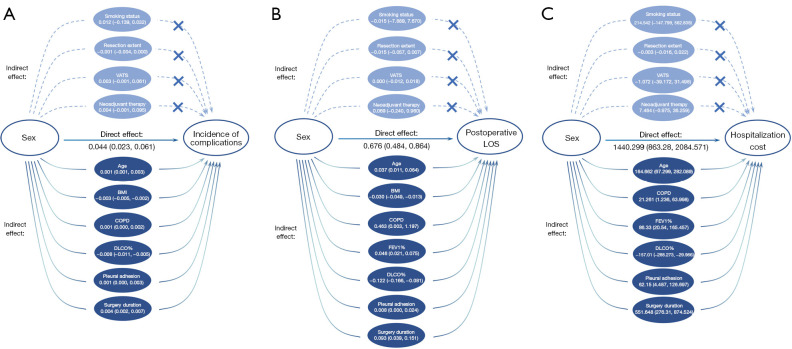
We evaluated individual effect of each mediator (see Figure 4). The causal mediation analysis required strict assumption that there was no unmeasured exposure-mediator, mediator-outcome, and exposure-outcome confounders. In each analysis on individual mediator, we adjusted for potential confounders including all other baseline characteristics that were not set to be mediator in the same analysis. The direct effect, indirect effect, and proportion of indirect effect on total effect were calculated. The bias-corrected and accelerated (BCa) bootstrap interval was used to evaluate the estimates. The upper and lower bounds of intervals on the same side of zero were considered significant. In the mediation models, sex was treated as a categorical variable, while males were used as the reference group. All analyses were performed with R (4.0.1, R Development Core Team, Vienna, Austria).
Figure 4.
Subgroup analysis of sex impact on perioperative outcomes: (A) incidence of complications, (B) postoperative hospital stays, and (C) hospitalization cost. Multivariable logistic regression analyses were conducted for categorical outcomes and multivariable linear regression analysis for continuous outcomes. OR, odds ratio; CI, confidence interval.
Results
Baseline characteristics and perioperative outcomes
We included 10,181 patients in the analysis, including 5,738 (56.4%) female and 4,443 (43.6%) male (Table 1, Figure 1). Female were presented with younger age (55.76 vs. 58.71, P<0.001), less comorbidities (HBP, 16.89% vs. 21.31%, P<0.001, COPD, 1.03% vs. 6.10%, P<0.001). The distribution of age was in consistent with our previous study (12). Regarding to therapy, female received less neoadjuvant therapy (0.51% vs. 2.21%, P<0.001), greater proportion of VATS (97.25% vs. 90.37%, P<0.001), and greater proportion of sublobar resection including segmentectomy and wedge resection (Table 1).
Sex differences in perioperative outcomes are shown in Table 2. Female arose less complications both in total (5.05% vs. 12.15%, P<0.001) and in specific, such as the PAL (3.08% vs. 7.58%, P<0.001). Female also presented with shorter length of postoperative hospital stays (4.92 vs. 6.41 days, P<0.001), less chest drainage volume, and lower hospitalization cost (50,713.69 vs. 54,580.85, Chinese Yuan, P<0.001). Sensitivity analysis on by excluding patients with missing data indicated that the inclusion of missing data did not significantly impact our primary findings.
Multivariable regression identified that male had independent association with increased incidence of complications, and increased postoperative hospital stays, drainage volume, and hospitalization cost (Figure 2). Age, BMI, COPD, VATS, and resection extent were also identified to be independently associated with incidence of complications, postoperative hospital stays, and hospitalization cost by regression analysis (Figure 3). The subgroup analysis also found male as an independent risk factor of increased hospital stays and hospitalization cost in different group of stages, smoking history, and year of surgery. But regarding incidence of complications, male sex was failed to be identified as independent risk factor in the group of stage III and positive smoking history (Figure 4). The additional stratified analysis on male and female patients showed that COPD, smoking history, and VATS was risk factor for incidence of complications only in males rather than females (Table S1).
Mediation effects
As shown in Figure 5A, in the mediation analysis regarding sex effect on the incidence of complications, age showed the indirect effects (95% confidence interval) of 0.001 (0.001 to 0.003, P=0.002), while −0.003 (−0.005 to −0.002) for BMI, −0.008 (−0.011 to −0.005) for DLCO%, 0.001 (0.000 to 0.002) for COPD, 0.001 (0.000 to 0.003) for pleural adhesion, and 0.004 (0.002 to 0.007) for surgery duration. Smoking status, neoadjuvant therapy, VATS, and resection extent were not identified as mediators.
Figure 5.
Indirect effect of each mediator on sex differences in perioperative outcomes. (A) Incidence of complications, (B) postoperative hospital stays, (C) hospitalization cost. The data presented direct effects of sex and indirect effects of each variable on outcomes, followed by the corresponding bias-corrected and accelerated bootstrap interval. The effects were calculated through causal mediation analysis using structure equation models. VATS, video-assisted thoracoscopic surgery; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO%, predicted percentage of diffusing capacity for carbon monoxide; FEV1%, predicted percentage of forced expiratory volume in 1 second; LOS, length of stays.
Regarding to the sex effect on postoperative hospital stays (Figure 5B), age, BMI, COPD, FEV1%, DLCO% were identified to have significant mediation effect, while smoking status, neoadjuvant therapy, VATS, nor resection extent were not identified as mediators. Similar results appeared in sex effect on the hospitalization cost (Figure 5C). Age, FEV1%, DLCO%, COPD, pleural adhesion, and surgery duration were identified to mediate sex differences in hospitalization cost, while smoking status, neoadjuvant therapy, VATS, and resection extent were not identified as mediators. The proportion of the indirect effect on total effect for each individual mediators are shown in Table S2.
Discussion
We illustrated sex differences among baseline characteristics and perioperative outcomes in patients undergoing NSCLC surgery. Female had less incidence of complications, shorter postoperative hospital stays, and less hospitalization cost. We identified that sex differences in perioperative outcomes were mediated by differences in age, BMI, FEV1%, DLCO%, COPD, tumor size, pleural adhesion, and surgery duration. The findings denied the contribution of smoking status, resection extent, or neoadjuvant therapy differences to sex differences in perioperative outcomes.
Female patients undergoing NSCLC surgery performed better during perioperative period. Our study showed female patients had shorter length of hospital stays and less incidence of complications. We identified female sex as an independent predictor of reduced postoperative morbidity. A study on lung cancer patients after surgery from STS Database noted similar results (10). A study on patients from Surveillance Epidemiology and End Results (SEER) database identified male sex as an independent predictor for postoperative pulmonary complications (18). Another study including both SEER and single-institution database found female had higher rates of psychological disorders than male after general treatment for NSCLC, while female showed more urinary tract infection after surgery (19). The incidence of urinary tract infection was low in our cohort and did not show statistical significance. The study on patients from STS database also noted higher rate urinary tract infection in female patients after surgery. Female might have higher risk for urinary tract infection due to the different anatomy from male. It seemed female still performed worse in some aspects due to the specific characteristics, although female showed better outcomes in general outcomes like hospital stays.
Situations are different in difference type of surgery. Female were noted to have increased length of stay and higher rates of 30-day readmission after surgical myectomy for hypertrophic cardiomyopathy (20). Female experienced higher risk for morbidity and mortality after coronary artery bypassing grafting (21). After surgery for aortic aneurysm, female showed higher risk for adverse events than male did (22). There were etiologies proposed to explain sex differences after vascular surgery, like more complex aneurysms with smaller vessels, and aortic size index in female patients (22). It seemed to have specific drivers under each type of surgery to explain sex impact on perioperative outcomes. The origin for sex difference after lung cancer surgery remains unknown. A study performed propensity-score matched analysis using clinicopathologic variables and showed no sex differences regarding length of stay and pulmonary complications (11). Although it did not show results in unmatched cohorts, we hypothesized that clinicopathologic variables played a role in sex differences of perioperative outcomes.
Mediation analysis could identify intervening variables that drive the association between exposures and outcomes. It has been largely applying in psychological studies (16,23). Herein, we would like to uncover any observable factors that drive sex differences in outcomes. It seemed that female benefited from their good baseline status. We found younger age, better FEV1%, less prevalence of COPD, less tumor size, less pleural adhesion, and shorter surgery duration were responsible for female’s less incidence of complications and shorter postoperative hospital stays. The comorbidity of COPD has been reported to be associated with increased postoperative complications after lung cancer surgery (24). Studies on lung cancer have shown that shorter surgery duration was related to reduced postoperative morbidities and length of hospital stays (25). The shorter surgery duration of females might attribute to their less pleural adhesion. We found male presented greater proportion of complete pleural adhesion, which requires more technically demanding surgery. Severe pleural adhesion might also indicate the increased risk for PAL after lung cancer surgery (14), directly contributing to the incidence of complications.
The estimates of indirect effect of BMI and DLCO% were negative values, which indicated their suppressing effect on sex differences in outcomes (14,26). Lower BMI is related to greater risk for PAL (14). One of the hypotheses is that lower BMI indicates poorer nutritional status, which delays the recovery of air leak (15). Lower DLCO% is a well-known predictor for postoperative complications (27). In our cohort, male had significant higher BMI and DLCO% than female. Thus, female and male’s differences in BMI and DLCO% functioned to narrow sex differences in outcomes driven by other factors.
It is acknowledged that male and female have different smoking status. In our cohort, most patients with smoking history were male, in line with prior studies (8,28). The smoking rates among females are inconsistent with previous studies. It might be partly attributed to differences in study periods. The study included patients undergoing lung cancer surgery in 1999, whereas our study covers patients from 2014 to 2021. This temporal difference could reflect evolving trends in female smoking habits over time. Additionally, according to recent studies, the overall smoking rate among Chinese females is 1.85% (29), which aligns closely with the findings of our study. We found difference in smoking status not responsible for sex differences in outcomes. One explanation was that smoking was disproportionately deleterious on female and male. A prior study found that despite lower cigarettes consumption among female, lung cancer incidence is higher in young female than young male (30). There were hypotheses that female may be more susceptible to tobacco carcinogens (31,32), but results from observational studies have been debating (32,33).
Although treatment approaches are associated with perioperative outcomes, we found that all the three treatment factors were not responsible for sex differences in outcomes, while sex differences did exist in treatment. Treatment decision depends on patient baseline status like frailty (34) and disease diagnosis like tumour stage and histology (35,36). In our cohort, more male patients received neoadjuvant therapy than female did, consistent with the higher disease stage of male patients. Female patients received more sublobar resection, in line with the prior studies using STS database (10) and among the National Lung Cancer Trial (37). Neither of the three treatment factors were identified as the mediator of sex differences in outcomes. Sex effect on outcomes seemed mostly from baseline status of male and female, without interference from their treatment differences. It should be noted that the lack of a mediating effect of treatment factors does not mean that treatment is not important in influencing perioperative outcomes. Treatment factors still had direct effects on perioperative outcomes, which were shown in our multivariable regression.
Since the drivers to sex differences in outcomes are mostly clinicopathologic factors, essential efforts should be made to prevent the negative effect of poor baseline status. A prospective randomized study proved that preoperative pulmonary rehabilitation could improve short-term outcomes after lung cancer surgery (38). A meta-analysis reviewed that preoperative exercise training could benefit short-term outcomes regarding to less complications and shorter hospital stays (39). Individualized management strategies that minimize the negative impact of inferior clinicopathologic status may help reduce or eliminate sex differences in perioperative outcomes. By doing so, healthcare providers can optimize patient outcomes and improve overall quality of care for NSCLC patients.
This study revealed that male had higher incidence of complications, longer postoperative and hospital stays. These findings emphasize the need for increased attention to perioperative management in male NSCLC patients. Furthermore, in the development of perioperative management strategies, some factors should be taken into consideration holistically, such as age, BMI, FEV1%, DLCO%, COPD, tumor size, pleural adhesion, and surgery duration.
Our results should be interpreted in consideration of some limitations. Firstly, it is important to note that this study is retrospective in nature, which inevitably introduces certain biases. Secondly, this study was conducted at a single institution, which may limit the generalizability of the findings to a broader population. Thirdly, our study is the constraint imposed by sample size regarding some variables, particularly evident in the analysis of patients with stage IV lung cancer and rare postoperative complications such as gastrointestinal issues and arrhythmias. Finally, our analysis might not cover all potential mediators. But we have found the apparent trend that the origins of sex differences were mostly clinicopathologic factors rather than treatment factors. We look forward to analyses covering more mediators and analysis on contributors for sex differences in survival outcomes.
Conclusions
We found female patients presented with reduced incidence of complications, shorter postoperative hospital stays, and less hospitalization cost after NSCLC surgery. Sex differences in perioperative outcomes could be partly explained by clinicopathologic differences between female and male NSCLC patients. Our findings underscored sex when tailoring management strategies. Minimizing the negative impact of inferior clinicopathological status might help to reduce or eliminate sex differences in perioperative outcomes of NSCLC patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (82102968 to J.Z.), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21002 to L.L.).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethic approval has been obtained from the Institutional Ethic Committee for Clinical Research of West China Hospital, Sichuan University [No. 2022(227)]. Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-336/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-336/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-336/dss
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229-63. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020;396:565-82. 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haupt S, Caramia F, Klein SL, et al. Sex disparities matter in cancer development and therapy. Nat Rev Cancer 2021;21:393-407. 10.1038/s41568-021-00348-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto JA, Vallejos CS, Raez LE, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018;3:e000344. 10.1136/esmoopen-2018-000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Y, Jing Y, Li L, et al. Sex-associated molecular differences for cancer immunotherapy. Nat Commun 2020;11:1779. 10.1038/s41467-020-15679-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCartney G, Mahmood L, Leyland AH, et al. Contribution of smoking-related and alcohol-related deaths to the gender gap in mortality: evidence from 30 European countries. Tob Control 2011;20:166-8. 10.1136/tc.2010.037929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clocchiatti A, Cora E, Zhang Y, et al. Sexual dimorphism in cancer. Nat Rev Cancer 2016;16:330-9. 10.1038/nrc.2016.30 [DOI] [PubMed] [Google Scholar]
- 8.Sakurai H, Asamura H, Goya T, et al. Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol 2010;5:1594-601. 10.1097/JTO.0b013e3181f1923b [DOI] [PubMed] [Google Scholar]
- 9.Yu XQ, Yap ML, Cheng ES, et al. Evaluating Prognostic Factors for Sex Differences in Lung Cancer Survival: Findings From a Large Australian Cohort. J Thorac Oncol 2022;17:688-99. 10.1016/j.jtho.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 10.Tong BC, Kosinski AS, Burfeind WR, Jr, et al. Sex differences in early outcomes after lung cancer resection: analysis of the Society of Thoracic Surgeons General Thoracic Database. J Thorac Cardiovasc Surg 2014;148:13-8. 10.1016/j.jtcvs.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson DB, Lapid DJ, Mitchell KG, et al. Perioperative Outcomes for Stage I Non-Small Cell Lung Cancer: Differences Between Men and Women. Ann Thorac Surg 2018;106:1499-503. 10.1016/j.athoracsur.2018.06.070 [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Zheng Q, Pu Q, et al. Perioperative and oncological outcomes of uniportal versus three-port thoracoscopic segmentectomy for lung cancer: a propensity score matching analysis. Transl Lung Cancer Res 2023;12:446-59. 10.21037/tlcr-22-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. 10.1016/j.athoracsur.2014.05.104 [DOI] [PubMed] [Google Scholar]
- 14.Zheng Q, Ge L, Zhou J, et al. Risk factors for prolonged air leak after pulmonary surgery: A systematic review and meta-analysis. Asian J Surg 2022;45:2159-67. 10.1016/j.asjsur.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690-699.e2. 10.1016/j.jtcvs.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 17.Gunzler D, Morris N, Tu XM. Causal Mediation Analysis Using Structure Equation Models. In: He H, Wu P, Chen DG, editors. Statistical Causal Inferences and Their Applications in Public Health Research. Cham: Springer International Publishing; 2016. p. 295-314. [Google Scholar]
- 18.Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. 10.1016/j.jtcvs.2011.09.072 [DOI] [PubMed] [Google Scholar]
- 19.Stabellini N, Bruno DS, Dmukauskas M, et al. Sex Differences in Lung Cancer Treatment and Outcomes at a Large Hybrid Academic-Community Practice. JTO Clin Res Rep 2022;3:100307. 10.1016/j.jtocrr.2022.100307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osman M, Syed M, Osman K, et al. Sex-based outcomes of surgical myectomy for hypertrophic cardiomyopathy: An analysis from the National Readmission Database. J Thorac Cardiovasc Surg 2023;166:504-511.e1. 10.1016/j.jtcvs.2021.11.043 [DOI] [PubMed] [Google Scholar]
- 21.Shi D, Zhang B, Motamed M, et al. Higher Mortality in Women After Coronary Artery Bypass: Meta-analysis and Bias Analysis of Confounding. Ann Thorac Surg 2022;113:674-80. 10.1016/j.athoracsur.2020.11.039 [DOI] [PubMed] [Google Scholar]
- 22.Deery SE, Soden PA, Zettervall SL, et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg 2017;65:1006-13. 10.1016/j.jvs.2016.08.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards JR, Lambert LS. Methods for integrating moderation and mediation: a general analytical framework using moderated path analysis. Psychol Methods 2007;12:1-22. 10.1037/1082-989X.12.1.1 [DOI] [PubMed] [Google Scholar]
- 24.Irie M, Nakanishi R, Yasuda M, et al. Risk factors for short-term outcomes after thoracoscopic lobectomy for lung cancer. Eur Respir J 2016;48:495-503. 10.1183/13993003.01939-2015 [DOI] [PubMed] [Google Scholar]
- 25.Dexter E, Attwood K, Demmy T, et al. Does Operative Duration of Lobectomy for Early Lung Cancer Increase Perioperative Morbidity? Ann Thorac Surg 2022;114:941-7. 10.1016/j.athoracsur.2022.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci 2000;1:173-81. 10.1023/A:1026595011371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerfolio RJ, Bryant AS. Different diffusing capacity of the lung for carbon monoxide as predictors of respiratory morbidity. Ann Thorac Surg 2009;88:405-10; discussion 410-1. 10.1016/j.athoracsur.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 28.Visbal AL, Williams BA, Nichols FC, 3rd, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg 2004;78:209-15; discussion 215. 10.1016/j.athoracsur.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 29.Xia X, Li YH, Liu Y, et al. Prevalence of cigarette use and addiction among Chinese females by age and province: Findings from nationwide China Health Literacy Survey during 2018-19. Drug Alcohol Depend 2024;258:111258. 10.1016/j.drugalcdep.2024.111258 [DOI] [PubMed] [Google Scholar]
- 30.Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med 2018;378:1999-2009. 10.1056/NEJMoa1715907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Q, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst 2000;92:1764-72. 10.1093/jnci/92.21.1764 [DOI] [PubMed] [Google Scholar]
- 32.Henschke CI, Yip R, Miettinen OS. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 2006;296:180-4. 10.1001/jama.296.2.180 [DOI] [PubMed] [Google Scholar]
- 33.Freedman ND, Leitzmann MF, Hollenbeck AR, et al. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008;9:649-56. 10.1016/S1470-2045(08)70154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunne MJ, Abah U, Scarci M. Frailty assessment in thoracic surgery. Interact Cardiovasc Thorac Surg 2014;18:667-70. 10.1093/icvts/ivt542 [DOI] [PubMed] [Google Scholar]
- 35.Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. 10.1200/JCO.2015.64.6729 [DOI] [PubMed] [Google Scholar]
- 36.Raman V, Jawitz OK, Voigt SL, et al. The Effect of Tumor Size and Histologic Findings on Outcomes After Segmentectomy vs Lobectomy for Clinically Node-Negative Non-Small Cell Lung Cancer. Chest 2021;159:390-400. 10.1016/j.chest.2020.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balekian AA, Wisnivesky JP, Gould MK. Surgical Disparities Among Patients With Stage I Lung Cancer in the National Lung Screening Trial. Chest 2019;155:44-52. 10.1016/j.chest.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 38.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer 2011;74:441-5. 10.1016/j.lungcan.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev 2017;6:CD012020. 10.1002/14651858.CD012020.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]