Abstract
Background
The B-Raf proto-oncogene, serine/threonine kinase (BRAF) V600E mutation is responsible for approximately 3% of acquired resistance mechanisms to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) in advanced EGFR-mutant non-small cell lung cancer (NSCLC). This study investigated the efficacy and safety of a triple-targeted therapy combining EGFR/BRAF/mitogen-activated protein kinase kinase (MEK) inhibitors of dabrafenib, trametinib, and osimertinib in NSCLC patients with acquired BRAF V600E mutation after EGFR-TKI treatment.
Methods
A multi-center retrospective review of medical records was performed to analyze EGFR-mutated advanced Chinese NSCLC patients who acquired the BRAF V600E mutation following EGFR-TKI treatment. All patients subsequently received dabrafenib, trametinib, and osimertinib. The clinical characteristics, progression-free survival (PFS), and adverse events (AEs) were documented. The in-vivo drug response of patient-derived organoids (PDOs) was observed. Next-generation sequencing (NGS) was performed upon progression to triple-targeted therapy.
Results
Thirteen patients with BRAF V600E mutations were included. Following triple-targeted therapy, the corresponding objective response rate and disease control rate were 61.5% and 92.3%, respectively. The median PFS was 13.5 months (95% confidence interval: 6.6–20.4). PDOs derived from one patient’s tumor sample were established, revealing that the triple-targeted therapy had a significantly lower half-maximal inhibitory concentration (IC50) value compared to other regimens. The tumor growth inhibitory rate was 99.36% for dabrafenib, trametinib, and osimertinib; 99.25% for osimertinib plus vemurafenib; 98.92% for osimertinib, encorafenib, and cetuximab; and 62.83% for pemetrexed plus carboplatin. NGS analysis identified major resistance mechanisms following the triple-targeted therapy, including the EGFR-dependent pathway, EGFR and BRAF V600E-dependent pathway, and an off-target mechanism.
Conclusions
EGFR/BRAF/MEK triple-targeted therapy is an effective and safe approach for treating EGFR-mutated NSCLC patients resistant to EGFR-TKIs with acquired BRAF V600E mutations.
Keywords: Non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), resistance, B-Raf proto-oncogene, serine/threonine kinase V600E (BRAF V600E), treatment strategy
Highlight box.
Key findings
• Triple-targeted therapy [epidermal growth factor receptor (EGFR)/B-Raf proto-oncogene, serine/threonine kinase (BRAF)/mitogen-activated protein kinase (MEK)] exhibited significant efficacy and safety in treating non-small-cell lung cancer (NSCLC) patients with acquired BRAF V600E mutations post-EGFR-tyrosine kinase inhibitor (TKI) treatment. Notably, it achieved an objective response rate of 61.5%, a disease control rate of 92.3%, and a median progression-free survival (PFS) of 13.5 months.
What is known and what is new?
• The BRAF V600E mutation arises in approximately 3% of EGFR-TKI-treated patients. Prior research has indicated the potential of targeted therapies against EGFR and BRAF or MEK mutations to enhance outcomes in resistant cases.
• This study with real-world evidence affirmed the efficacy of triple-targeted therapy for this specific patient subgroup. Additionally, it validated the therapy's superior efficacy over standard chemotherapy through patient-derived organoids. Furthermore, it identified major resistance mechanisms.
What is the implication, and what should change now?
• Triple-targeted therapy is a treatment option for EGFR-mutated NSCLC patients with acquired BRAF V600E mutations post-EGFR-TKI resistance.
• Further research into resistance mechanisms is warranted to refine treatment strategies. Updating clinical guidelines and practices to acknowledge the efficacy of triple-targeted therapy may lead to improved patient outcomes.
Introduction
Lung cancer remains a leading cause of cancer-related mortality globally, with non-small cell lung cancer (NSCLC) accounting for 85% of cases (1). The epidermal growth factor receptor (EGFR) gene mutation is a predominant driver mutation, particularly in lung adenocarcinoma, the most common NSCLC subtype. Notably prevalent in East Asian populations, EGFR mutations occur in approximately 50% of lung adenocarcinoma cases, prompting the adoption of EGFR-tyrosine kinase inhibitor (EGFR-TKI) as the primary first-line treatment for EGFR-mutated NSCLC patients (2,3). Despite initial success of EGFR-TKIs, acquired resistance (AR) inevitably develops in most advanced NSCLC patients.
Overcoming resistance to EGFR-TKI treatment poses an unmet challenge. While various on-target and off-target AR mechanisms have been identified, 30–50% of resistance mechanisms remain unknown, and chemotherapy is currently a practical and standard therapeutic strategy (4,5). One notable off-target downstream pathway contributing to AR in EGFR-mutated lung cancer is the presence of the B-Raf proto-oncogene, serine/threonine kinase (BRAF) V600E mutation (6,7). The BRAF V600E mutation acts as an oncogenic driver via mitogen-activated protein kinase (MAPK), affecting approximately 3% of patients treated with EGFR-TKIs (8). Preclinical models suggest potential success in overcoming AR mediated by the BRAF V600E mutation through the combination of targeted therapies inhibiting EGFR and BRAF or mitogen-activated protein kinase kinase (MEK) simultaneously (7,9). Sporadic cases also have demonstrated clinical improvement and good tolerance with the triple combination of osimertinib, dabrafenib, and trametinib (10-12).
There have been no large-scale multicenter trials to compare the efficacy of the triple-targeted therapy (combination of EGFR/BRAF/MEK inhibitors) with dual-targeted therapy (combination of BRAF/MEK inhibitors) and clarify the safety of triple-targeted therapy. In this multicenter retrospective study, we investigated the real-world efficacy and safety of a triple-targeted therapy involving dabrafenib, trametinib, and osimertinib for NSCLC patients with an acquired BRAF V600E mutation after EGFR-TKI treatment (13). Additionally, in-vitro experiments using patient-derived organoids (PDOs) were conducted to gain deeper insights into the drug response dynamics potential resistance mechanisms associated with this triple-targeted therapy (13). We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-358/rc).
Methods
Study design and patients
In this real-world, multicenter study, we retrospectively reviewed the medical records of advanced Chinese NSCLC patients who acquired BRAF V600E mutations following EGFR-TKI treatment. These patients were admitted to seven participating hospitals (Guangdong Provincial People’s Hospital, Dongguan People’s Hospital, Peking University Shenzhen Hospital, Shenzhen People’s Hospital, The First Affiliated Hospital of Guangzhou Medical University, West China Hospital of Sichuan University, The First Affiliated Hospital of Sun Yat-sen University) between December 15, 2018, and October 30, 2023. The inclusion criteria were as follows: (I) pathologically confirmed stage IIIB/IIIC/IV NSCLC according to the 8th edition of the American Joint Committee on Cancer staging manual; (II) presence of EGFR mutations and receipt of EGFR-TKIs as a first- or later-line therapy; (III) development of an acquired BRAF V600E mutation upon progression with EGFR-TKI treatment and subsequent treatment with osimertinib, dabrafenib, and trametinib. Patients without complete medical records or follow-up information were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Research Ethics Committee of Guangdong Provincial People’s Hospital approved the study (approval No. GDREC2019217H). Informed consent was waived due to the retrospective nature of the present study. All participating hospitals/institutions were informed and agreed to the study.
Data collection and treatment procedures
The baseline demographic and clinicopathological data were extracted from the medical record system. EGFR and BRAF genotyping were performed using polymerase chain reaction or next-generation sequencing (NGS).
Patients initially received osimertinib at a daily oral dose of 80 mg, dabrafenib at 150 mg twice daily orally, and trametinib at 2 mg once daily orally. The treatment efficacy was assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1). Progression-free survival (PFS) was measured from the start of triple-therapy to the occurrence of progressive disease or the last follow-up. Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events, version 5.0.
Upon progression during triple-targeted therapy, NGS analysis and fluorescence in situ hybridization (FISH) testing were performed to detect resistance mechanisms (14).
PDO cultures and drug sensitivity tests
Malignant effusion samples were collected at the Guangdong Provincial People’s Hospital for the PDO experiments. The PDO cultures and sensitivity tests involving anticancer drugs were performed according to established procedures (15), the detailed information and concentrations of drug compounds are presented in Table S1. Cell viability was assessed using a CellTiter-Glo 3D assay (Promega, Madison, WI, USA). The half-maximal inhibitory concentration (IC50) values were calculated using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation or median and interquartile range. Categorical variables were reported as counts and percentages. PFS was analyzed by Kaplan-Meier survival curves. Statistical analyses were performed using SPSS 25.0 software.
Results
Baseline characteristics
Thirteen patients with an acquired BRAF V600E mutation receiving the triple-targeted therapy were included in this study. The median age of the patients was 53.0 years old, and 84.6% were female. The triple-targeted therapy was initiated as the second-line treatment for 2 (15.4%), as the third-line treatment for 5 (38.5%) patients and as treatment beyond the third line for 6 (46.2%) patients (Table 1). The median follow-up time was 19.8 months [95% confidence interval (CI): 10.2–29.5].
Table 1. Baseline demographic information and characteristics of patients with an acquired BRAF V600E mutation after EGFR-TKI resistance.
| Clinical characteristics | Value (n=13) |
|---|---|
| Age (years) | 53.0 (39.5–66.5) |
| ≥60 | 4 (30.8) |
| <60 | 9 (69.2) |
| Gender | |
| Female | 11 (84.6) |
| Male | 2 (15.4) |
| Smoking history | |
| Non-smoker | 11 (84.6) |
| Smoker | 2 (15.4) |
| Clinical stage | |
| IVA | 3 (23.1) |
| IVB | 10 (76.9) |
| ECOG PS | |
| 0 | 2 (15.4) |
| 1 | 9 (69.2) |
| ≥2 | 2 (15.4) |
| Metastatic site | |
| Bone | 7 (53.8) |
| Anterior mediastinal lymph nodes | 5 (38.4) |
| Liver | 2 (15.4) |
| Lung | 5 (38.4) |
| Brain | 4 (30.8) |
| Others | 6 (46.2) |
| Histology | |
| Adenocarcinoma | 13 (100.0) |
| EGFR mutation | |
| Exon 19 del | 9 (69.2) |
| Exon 21 L858 | 4 (30.8) |
| Prior treatment line | |
| 1 | 2 (15.4) |
| 2 | 5 (38.5) |
| 3 | 3 (23.1) |
| ≥4 | 3 (23.1) |
| Genetic test specimen | |
| Tissue | 7 (53.8) |
| Plasma | 4 (30.8) |
| Pleural effusion | 2 (15.4) |
Data are presented as n (%) or median (interquartile range). Stages IVA and IVB are classified according to the eighth edition of the TNM staging system for lung cancer. ECOG PS, Eastern Cooperative Oncology Group Performance Status; BRAF, B-Raf proto-oncogene, serine/threonine kinase; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Clinical outcomes
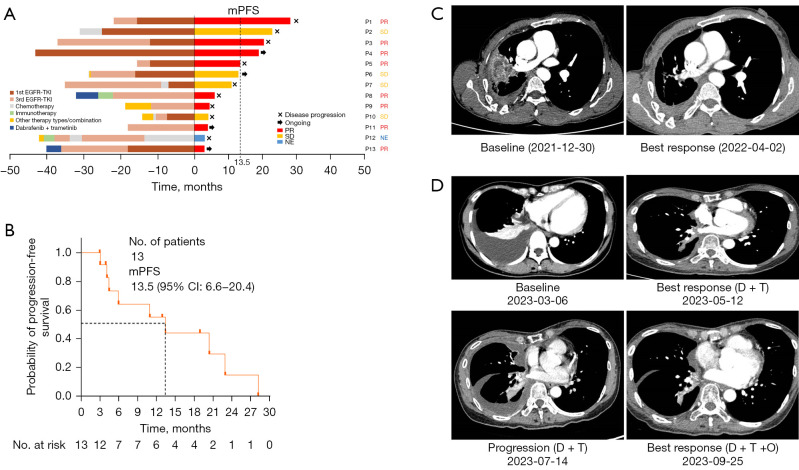
As of October 30, 2023, twelve patients were evaluated for having the best response. Eight patients (61.5%) achieved a partial response (PR) and 4 (30.8%) achieved stable disease, resulting in an objective response rate (ORR) of 61.5% and a disease control rate of 92.3%. The median PFS was 13.5 months (95% CI: 6.6–20.4) (Figure 1A,1B).
Figure 1.
Efficacy and patient cases of triple-targeted therapy. (A) Swimmer plot. The x-axis at 0 represents the start of triple-targeted therapy. The left side of the y-axis indicates the treatment timelines before triple-targeted therapy, and the right side of the y-axis represents the treatment assessment after triple-targeted therapy. (B) Kaplan-Meier survival curve for progression-free survival. (C) Patient case—thoracic lesion changes before and after triple-targeted therapy. Patient No. 5 achieved a partial response after triple-targeted therapy. The thoracic lesions exhibited a significant reduction (75%) following the therapy. The triple-targeted therapy maintained its efficacy for a duration of 15 months. (D) Patient case—changes of thoracic lesions at baseline, after dual therapy, at progression, and after triple-targeted therapy. Patient No. 13 initially received dabrafenib and trametinib after experiencing progression on EGFR-TKI therapy upon the emergence of a BRAF V600E mutation. Although a partial response was initially achieved with the dual therapy, the disease progressed after 3 months. Subsequently, triple-targeted therapy was started, resulting in a PR after 2 months. mPFS, median progression-free survival; PR, partial response; SD, stable disease; NE, non-evaluable; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; 1st EGFR-TKI, first generation EGFR-TKI; 3rd EGFR-TKI, third generation EGFR-TKI; BRAF, B-Raf proto-oncogene, serine/threonine kinase; D + T, dabrafenib + trametinib; D + T + O, dabrafenib, trametinib and osimertinib.
Patient No. 5 achieved PR of triple-targeted therapy. Thoracic lesion exhibited significant reduction (75%) following the therapy. The triple-targeted therapy maintained its efficacy for a duration of 15 months (Figure 1C).
Patient No. 13 initially received dabrafenib and trametinib after experiencing progression of EGFR-TKI therapy upon the emergence of BRAF V600E. Although a PR was initially achieved with the dual therapy, the disease progressed after 3 months. Subsequently, triple-targeted therapy was started, resulting a PR after 2 months (Figure 1D).
PDOs for drug response
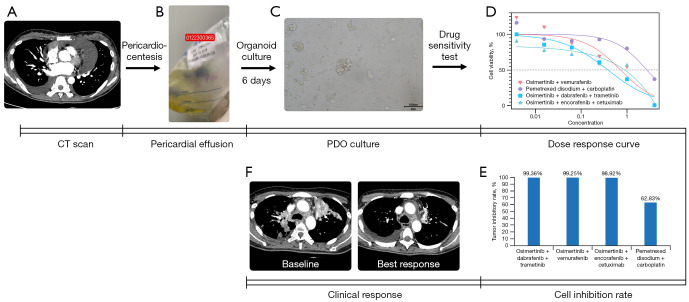
PDOs were successfully established using a malignant effusion sample from patient No. 10 who had received osimertinib as first-line therapy and osimertinib plus bevacizumab as second-line therapy. The drug sensitivity test showed that the triple-targeted therapy had a significantly lower IC50 value compared to the other treatment regimens. The tumor growth inhibitory rate was 99.36% for dabrafenib, trametinib, and osimertinib; 99.25% for osimertinib plus vemurafenib; 98.92% for osimertinib, encorafenib, and cetuximab; and 62.83% for pemetrexed plus carboplatin (Figure 2A-2D). The thoracic lesions of the corresponding patient exhibited a significant reduction in size after the triple-targeted therapy (Figure 2E).
Figure 2.
The procedure of the PDO experiment. (A) Chest CT image depicting malignant epicardial effusion sampled for the PDO model. (B) Pericardial effusion for PDO sampling. (C) PDO culture: on the 6th day of cultivation, the organoids displayed an average diameter of 63.67 µm (with a range of approximately 21.03–126.22 µm), and a total count of around 3,000 organoids, as illustrated in the light microscope image. (D) Cell proliferation inhibitory rates. (E) IC50 values. (F) Efficacy of the triple-targeted therapy shown in the chest CT images from patient No. 9. PDO, patient-derived organoid; CT, computed tomography; IC50, half-maximal inhibitory concentration.
Safety profiles
AE related to triple-targeted therapy included diarrhea (n=6, 46.2%), pyrexia (n=3, 23.1%), anemia (n=3, 23.1%), nausea (16.7%), mouth ulcer (15.4%), decreased appetite (7.7%), rash (7.7%) and edema (7.7%), among which, ≥ Grade 3 anemia were observed in two cases (15.4%) (Table 2).
Table 2. Triple-targeted TRAEs.
| TRAEs | All grades, n (%) | Grade 3–4, n (%) |
|---|---|---|
| Diarrhea | 6 (46.2) | 1 (7.7) |
| Pyrexia | 3 (23.1) | 0 |
| Anemia | 3 (23.1) | 2 (15.4) |
| Mouth ulcer | 2 (15.4) | 0 |
| Nausea | 1 (7.7) | 0 |
| Decreased appetite | 1 (7.7) | 0 |
| Rash | 1 (7.7) | 0 |
| Edema | 1 (7.7) | 0 |
| Unknown | 4 (33.3) | 0 |
TRAE, treatment-related adverse event.
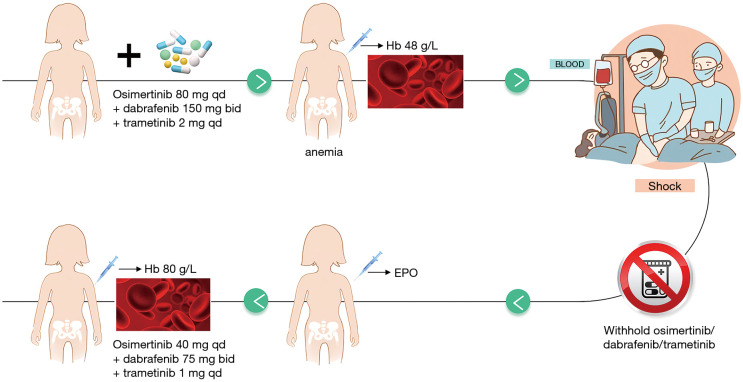
Patient No. 5 developed Grade 4 anemia after the triple-targeted therapy. This situation was alleviated after drug withdrawal, red blood cell transfusions and erythropoietin treatment. Dosing is resumed at a half dose (osimertinib at 80 mg Qd, dabrafenib at 75 mg bid, and trametinib at 1 mg qd) after hemoglobin rose to 80 g/L and patient status improved (Figure 3), and the patient did not experience any AEs after resuming dosing. Patient No. 5 experienced PFS for 1 month after resuming dosing.
Figure 3.
The clinical management of grade 4 anemia. EPO, erythropoietin; Hb, hemoglobin.
Exploration of AR mechanisms following triple-targeted therapy
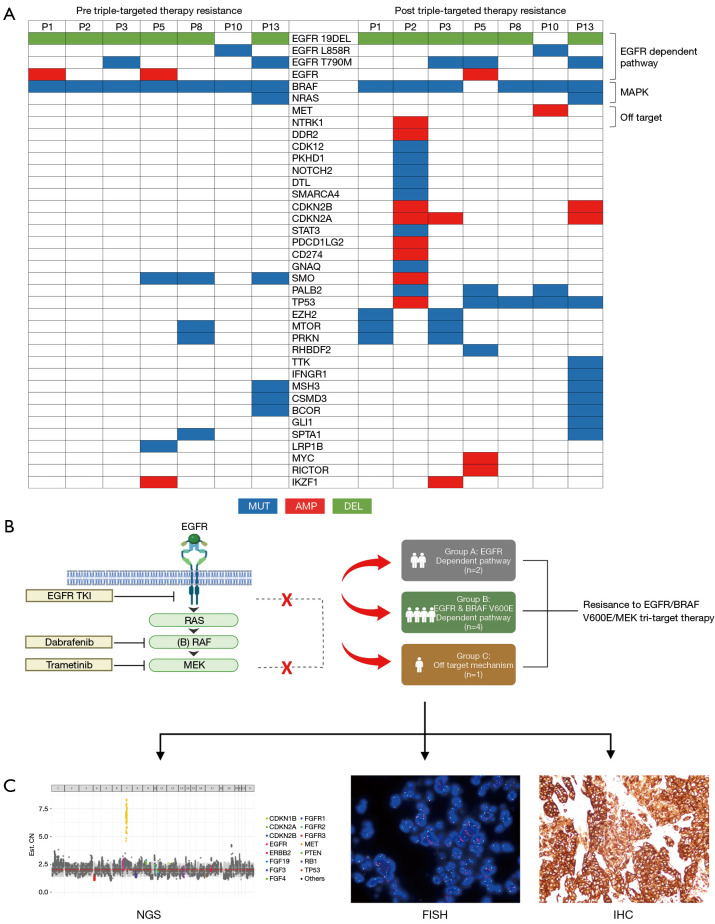
Seven patients underwent NGS analysis to identify the primary resistance mechanism after receiving the triple-targeted therapy. The heatmap of NGS-detected genetic alterations exhibited significant differences between before and after triple-targeted therapy resistance (Figure 4A). The gene profiles became more intricate post-resistance. The predominant changes post-resistance included BRAF loss, reactivation of the EGFR pathway, and the emergence of bypass activation events involving MET proto-oncogene, receptor tyrosine kinase (MET)/neurotrophic tyrosine receptor kinase (NTRK). In most cases of post-triple-targeted therapy resistance, TP53 mutations (5 out of 7) appeared, often accompanied by multiple gene amplifications. These resistance mechanisms can be categorized into three types, namely the EGFR-dependent pathway (n=2), the EGFR and BRAF V600E-dependent pathway (n=4), and an off-target mechanism (n=1). Among the patients categorized into the EGFR-dependent pathway, neither exhibited BRAF mutations, yet in one patient, EGFR C797S emerged upon progression (Figure 4B). Furthermore, one patient demonstrated MET amplification as further confirmed by NGS (CN =7.0), FISH (c-Met/CSP7 ratio: 8.6), and immunohistochemical (IHC) staining (70%+++, 30%++), indicating an off-target resistance mechanism to the triple-targeted therapy (Figure 4C).
Figure 4.
Exploration of resistance mechanisms to triple-targeted therapy. (A) The genetic alterations detected by NGS prior to and upon progression following the triple-targeted therapy. (B) Resistance mechanism following the triple-targeted treatment. (C) Detection of MET amplification by NGS/FISH (1,000×)/IHC (200×) as one of the resistance mechanisms observed in patient No. 9. NGS, next-generation sequencing; MET, MET proto-oncogene, receptor tyrosine kinase; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; MEK, mitogen-activated protein kinase kinase; BRAF, B-Raf proto-oncogene, serine/threonine kinase; EGFR, epidermal growth factor receptor; MUT, mutation; AMP, amplification; DEL, deletion; RAS, Ras protein; RAF, Raf protein; TKI, tyrosine kinase inhibitor.
Discussion
This study evaluated the efficacy of a novel triple-targeted therapy involving the EGFR/BRAF/MEK inhibitors dabrafenib, trametinib, and osimertinib in a real-world setting. We specifically examined its potential in NSCLC patients who acquired BRAF V600E mutations after prior EGFR-TKI treatment. Our findings highlight a robust clinical response to this triple regimen, along with manageable AEs and a promising PFS of 13.5 months. Utilizing PDOs, we not only validated the clinical effectiveness and but also identified the molecular resistance mechanisms, primarily categorized into the EGFR-dependent pathway, EGFR and BRAF V600E-dependent pathway, and off-target mechanisms, thereby providing valuable directions for subsequent therapeutic strategies.
BRAF, a serine-threonine kinase within the RAS/RAF/MEK/ERK signaling cascade, plays a pivotal role in regulating cell proliferation, differentiation, and survival by activating downstream targets MEK1 and MEK2 (16). The BRAF V600E mutation, a prominent BRAF mutation subtype in NSCLC, presents a therapeutic challenge. Immunotherapy has demonstrated limited clinical efficacy in this cohort. The IMMUNOTARGET study reported an ORR of 24% and a PFS of 3.1 months with immune checkpoint inhibitors monotherapy in advanced NSCLC patients with BRAF alterations (17). The AcSé vemurafenib monotherapy for BRAF V600E-mutated NSCLC displayed an ORR of 37.1–44.9% and a PFS of 5.2–6.5 months (5,18). Notably, trials have validated the efficacy of BRAF/MEK inhibitors, specifically dabrafenib and trametinib, in managing advanced NSCLC patients with BRAF V600E mutations (19). The BRF113928 trial reported an ORR of 64%, a median PFS of 14.6 months and a median OS of 24.6 months (20). As a second-line treatment, dabrafenib plus trametinib yielded encouraging outcomes, with a median PFS of 10.2 months and a median OS of 18.2 months (21). For Chinese patients with BRAF V600E-mutated NSCLC, the combined regimen of dabrafenib plus trametinib achieved an impressive ORR of 75% (22). The National Comprehensive Cancer Network, European Society of Medical Oncology, and Chinese Society of Clinical Oncology guidelines all recommend dabrafenib plus trametinib as a preferred treatment of BRAF V600E-mutated NSCLC.
EGFR downstream signaling pathways include PI3K/AKT, RAS/MAPK/ERK, and JAK/STAT pathways (5). BRAF V600E mutation observed in 3% of patients post-EGFR-TKI treatment is a primary alteration in the MAPK pathway triggering EGFR-TKI resistance (8,9,16,23). Dual inhibition of EGFR and MEK has emerged as a potential approach against RAS mutation-mediated osimertinib resistance in EGFR-mutated NSCLC (24,25). Preliminary studies support combining osimertinib with an MEK inhibitor to overcome resistance (26-28). For NSCLC patients developing BRAF V600E mutations following EGFR-TKI treatment, case reports have highlighted the feasibility of EGFR/BRAF/MEK co-inhibitors, yielding an ORR of 50–71.4%, and a median PFS of up to 11 months (12,29,30). In this study, the majority received triple-targeted therapy as a third- or subsequent-line treatment. Interestingly, EGFR mutations persisted alongside BRAF V600E mutations upon EGFR-TKI resistance, emphasizing the need for combination therapies (16,30). Patient No. 9, progressing after dabrafenib and trametinib treatment, exhibited improvement with the triple-targeted therapy.
Standard chemotherapy post-EGFR-TKI failure in EGFR-mutated NSCLC results in a median PFS of approximately five months (4). Trials exploring combinations of immunotherapy with chemotherapy post-EGFR-TKI progression (ORIENT-31, Keynote 789), reported inconsistent findings (14,31). Preclinical studies revealed increased tumor sensitivity when co-treating tumor cells expressing both EGFR T790M and BRAF V600E mutations with osimertinib and a BRAF V600E inhibitor (9). Our study, through the use of PDOs (32), observed superior efficacy of dabrafenib, trametinib, and osimertinib compared to current standard chemotherapy.
Adverse reactions to triple-targeted therapy typically include fever, rash, nausea, fatigue, reduced appetite, diarrhea, and pain, manageable through symptomatic treatment or by dosage adjustment (30). Consistent with previously reported safety profiles, our study found most AEs, including gastrointestinal symptoms, fever, oral mucositis, and rash, to be mild and controllable. Grade 3–4 anemia in two patients, one progressing to shock, was swiftly resolved with transfusions and erythropoietin treatment (33). Temporary dosage reduction, later resumed at half the initial, maintained efficacy.
Molecular profiling is a prerequisite for precision medicine in NSCLC. Liquid biopsy has emerged as a valuable alternative to nucleic acid testing (16). The loss of sensitivity to EGFR-TKIs can primarily be categorized into three groups: mutations acquired within the target gene (EGFR), such as the T790M or C797S mutation; activation of bypass signaling pathways, represented by RAS mutations or MET amplification; and histologic transformation into small cell lung cancer (34). BRAF/MEK inhibition resistance in BRAF-mutated NSCLC is mainly due to the recurrent MAPK pathway activation (35). The resistance mechanism with triple-targeted therapy involves EGFR-dependent pathways, or EGFR and BRAF V600E-dependent pathway. The mechanisms of resistance to dual-targeted and triple-targeted therapies differ notably, particularly with the emergence of BRAF loss, reoccurrence of the EGFR pathway with the C797S mutation appearing post-resistance, and in some cases MET amplification.
This retrospective study, albeit with a small sample size, highlights the potential efficacy and safety of triple-targeted therapy involving dabrafenib, trametinib, and osimertinib in treating acquired BRAF V600E mutation after progression on EGFR-TKIs. However, further confirmation is necessary through large-sample, multi-center randomized controlled trials. Additionally, the different sample sources for NGS detection after triple-targeted therapy resistance may impact the determination of resistance mechanisms.
Conclusions
This is the largest cohort study to date demonstrating that EGFR/BRAF/MEK triple-targeted therapy may be an effective and safe approach for acquired BRAF V600E mutations in EGFR-mutated NSCLC post-EGFR-TKIs resistance. The PDO model confirms its superior efficacy over chemotherapy, serving as a promising predictive tool. A multifaceted resistance mechanism involves alterations in EGFR/BRAF-dependent pathways and off-target mechanisms.
Supplementary
The article’s supplementary files as
Acknowledgments
A preliminary version of this work was presented at ESMO ASIA as poster presentation in 2023. We thank all patients who participated in this study as well as their families. We also thank Burning Rock Biotech, Berry Oncology, and Nanjing Geneseeq Technology Inc. for their help with NGS data analysis.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81972164 to J.J.Y.) and Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (No. 2017B030314120).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Research Ethics Committee of Guangdong Provincial People’s Hospital approved the study (approval No. GDREC2019217H). Informed consent was waived due to the retrospective nature of the present study. All participating hospitals/institutions were informed and agreed the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-358/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-358/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-358/dss
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Hanna NH, Robinson AG, Temin S, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2021;39:1040-91. 10.1200/JCO.20.03570 [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 4.Steendam CMJ, Ernst SM, Badrising SK, et al. Chemotherapy for patients with EGFR-mutated NSCLC after progression on EGFR-TKI's: Exploration of efficacy of unselected treatment in a multicenter cohort study. Lung Cancer 2023;181:107248. 10.1016/j.lungcan.2023.107248 [DOI] [PubMed] [Google Scholar]
- 5.Fu K, Xie F, Wang F, et al. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol 2022;15:173. 10.1186/s13045-022-01391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. 10.1038/nrc.2017.84 [DOI] [PubMed] [Google Scholar]
- 7.Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A 2012;109:E2127-33. 10.1073/pnas.1203530109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. 10.1038/s41416-019-0573-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho CC, Liao WY, Lin CA, et al. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J Thorac Oncol 2017;12:567-72. 10.1016/j.jtho.2016.11.2231 [DOI] [PubMed] [Google Scholar]
- 10.Wei XW, Deng JY, Xu CR, et al. Characteristics of and Treatment Strategies for Advanced EGFR-Mutant NSCLC With Concomitant BRAF Variations. JTO Clin Res Rep 2022;3:100348. 10.1016/j.jtocrr.2022.100348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro MFSA, Knebel FH, Bettoni F, et al. Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient. NPJ Precis Oncol 2021;5:5. 10.1038/s41698-021-00149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng P, Koopman B, Kok K, et al. Combined osimertinib, dabrafenib and trametinib treatment for advanced non-small-cell lung cancer patients with an osimertinib-induced BRAF V600E mutation. Lung Cancer 2020;146:358-61. 10.1016/j.lungcan.2020.05.036 [DOI] [PubMed] [Google Scholar]
- 13.Weng CD, Tang KJ, Jin S, et al. 560P-Triple-targeted therapy of dabrafenib, trametinib and osimertinib for the treatment of acquired BRAF V600E mutation after progression on EGFR-TKIs in advanced EGFR-mutant NSCLC. Ann Oncol 2023;34:S1668. [Google Scholar]
- 14.Lu S, Wu L, Jian H, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med 2023;11:624-36. 10.1016/S2213-2600(23)00135-2 [DOI] [PubMed] [Google Scholar]
- 15.Ding RB, Chen P, Rajendran BK, et al. Molecular landscape and subtype-specific therapeutic response of nasopharyngeal carcinoma revealed by integrative pharmacogenomics. Nat Commun 2021;12:3046. 10.1038/s41467-021-23379-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chmielecki J, Mok T, Wu YL, et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat Commun 2023;14:1071. 10.1038/s41467-023-35962-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbiah V, Gervais R, Riely G, et al. Efficacy of Vemurafenib in Patients With Non-Small-Cell Lung Cancer With BRAF V600 Mutation: An Open-Label, Single-Arm Cohort of the Histology-Independent VE-BASKET Study. JCO Precis Oncol 2019;3:PO.18.00266. [DOI] [PMC free article] [PubMed]
- 19.Pratilas CA, Hanrahan AJ, Halilovic E, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res 2008;68:9375-83. 10.1158/0008-5472.CAN-08-2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. 10.1016/S1470-2045(16)30146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schadendorf D, Robert C, Dummer R, et al. Pyrexia in patients treated with dabrafenib plus trametinib across clinical trials in BRAF-mutant cancers. Eur J Cancer 2021;153:234-41. 10.1016/j.ejca.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 22.Fan Y, Jianying Z, Yuanyuan Z, et al. EP08.02-052 Safety and Efficacy of Dabrafenib Plus Trametinib in Chinese Patients With BRAF V600E- Mutation Positive Metastatic NSCLC. J Thorac Oncol 2022;17:S423. [Google Scholar]
- 23.Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. 10.1001/jamaoncol.2018.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi P, Oh YT, Deng L, et al. Overcoming Acquired Resistance to AZD9291, A Third-Generation EGFR Inhibitor, through Modulation of MEK/ERK-Dependent Bim and Mcl-1 Degradation. Clin Cancer Res 2017;23:6567-79. 10.1158/1078-0432.CCR-17-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu J, Yao W, Shi P, et al. MEK or ERK inhibition effectively abrogates emergence of acquired osimertinib resistance in the treatment of epidermal growth factor receptor-mutant lung cancers. Cancer 2020;126:3788-99. 10.1002/cncr.32996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol 2020;31:507-16. 10.1016/j.annonc.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Fukuda K, Otani S, Takeuchi S, et al. Trametinib overcomes KRAS-G12V-induced osimertinib resistance in a leptomeningeal carcinomatosis model of EGFR-mutant lung cancer. Cancer Sci 2021;112:3784-95. 10.1111/cas.15035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberlein CA, Stetson D, Markovets AA, et al. Acquired Resistance to the Mutant-Selective EGFR Inhibitor AZD9291 Is Associated with Increased Dependence on RAS Signaling in Preclinical Models. Cancer Res 2015;75:2489-500. 10.1158/0008-5472.CAN-14-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Gan J, Guo K, et al. Acquired BRAF V600E Mutation Mediated Resistance to Osimertinib and Responded to Osimertinib, Dabrafenib, and Trametinib Combination Therapy. J Thorac Oncol 2019;14:e236-7. 10.1016/j.jtho.2019.05.040 [DOI] [PubMed] [Google Scholar]
- 30.Chimbangu CT, Ya Z, Xi L, et al. Promising response of dabrafenib, trametinib, and osimertinib combination therapy for concomitant BRAF and EGFR-TKI resistance mutations. Anticancer Drugs 2024;35:109-15. 10.1097/CAD.0000000000001537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang JCH, Lee DH, Lee JS, et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study. J Clin Oncol 2023;41:LBA9000. 10.1200/JCO.23.02747 [DOI] [PubMed] [Google Scholar]
- 32.Wang HM, Zhang CY, Peng KC, et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: A real-world study. Cell Rep Med 2023;4:100911. 10.1016/j.xcrm.2022.100911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Society of Chemotherapy China Anti-cancer Association, Committee of Neoplastic Supportive-Care (CONS) China Anti-cancer Association . A consensus on the clinical diagnosis, treatment, and prevention of cancer- and chemotherapy-related anemia in China (2019 edition). Chinese Journal of Clinical Oncology 2019;46:869-75. [Google Scholar]
- 34.Feldt SL, Bestvina CM. The Role of MET in Resistance to EGFR Inhibition in NSCLC: A Review of Mechanisms and Treatment Implications. Cancers (Basel) 2023;15:2998. 10.3390/cancers15112998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchinetti F, Lacroix L, Mezquita L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAF(V600E) non-small cell lung cancer. Eur J Cancer 2020;132:211-23. 10.1016/j.ejca.2020.03.025 [DOI] [PubMed] [Google Scholar]