Abstract
Study objective
To evaluate the impact of a medication optimization clinic (MOC) on GDMT and outcomes for patients with HFrEF versus usual care.
Design
Retrospective evaluation of a multi-site MOC was conducted.
Setting
Large health system with academic and community hospitals.
Participants
Patients with HFrEF referred to MOC by their cardiologist versus usual care.
Interventions
GDMT use managed by an advanced practice provider or clinical pharmacist through weekly telemedicine visits.
Main outcome measures
The primary outcome was HF hospitalization. Cardiovascular hospitalization and all-cause mortality were also assessed. Kaplan−Meier Curve, Cumulative Incidence Function, and competing risk analysis with regression models were conducted.
Results
1419 patients in MOC group were compared to 5116 control patients. GDMT use was significantly higher in MOC: quadruple therapy (49 % vs. 19 %; p < 0.0001), angiotensin-receptor neprilysin inhibitor (62 % vs. 45 %; p < 0.0001), beta blocker (92 % vs. 88 %; p < 0.0001), mineralocorticoid receptor antagonist (69 % vs. 45 %; p < 0.0001), and sodium glucose cotransporter-2 inhibitor (68 % vs. 35 %; p < 0.0001). Competing risk analyses showed that HF and CV hospitalizations were significantly lower at all times points (3, 6, and 12 months) for MOC vs. control (p < 0.001). All-cause mortality was significantly lower at 6 months (p = 0.006) and 12 months (p < 0.001), but did not differ at 3 months (p = 0.35), for MOC vs. control.
Conclusions
MOC was associated with improved GDMT and lower risks of hospitalizations due to HF and any cardiovascular cause, and all-cause mortality in patients with HFrEF.
Keywords: Heart failure, Medication optimization, Readmissions, Guideline-directed medical therapy
Highlights
-
•
Patients with HFrEF derive significant clinical benefit from GDMT.
-
•
Implementation of GDMT is suboptimal and varying approaches have been described.
-
•
Our MOC uses a team-based model in an outpatient telemedicine clinic with a nurse practitioner and clinical pharmacist to improve GMDT.
-
•
The MOC demonstrated significant reductions in HF and cardiovascular hospitalizations, and mortality, compared to usual care.
1. Introduction
The contemporary goal of HFrEF medical management is to achieve target, or maximally tolerated, doses of foundational evidence-based therapies as efficiently as possible [1,2]. Multiple studies have described various approaches to GDMT optimization, however, most of the interventions were conducted in the acute care or transitional setting and the impact on GDMT rates were variable [[3], [4], [5], [6], [7], [8]]. Regardless of approach, GDMT implementation is fraught with numerous barriers, including therapeutic inertia, inefficient medication sequencing strategies, adverse effects, and costs leading to suboptimal therapy [9,10].. Additionally, evidence demonstrating improvements in both morbidity and mortality from these interventions are limited [5]. Our multidisciplinary medication optimization clinic (MOC) manages outpatients with HFrEF, across a large academic health system, who are not receiving optimal GDMT. Through this multidisciplinary collaboration, our MOC has previously demonstrated significant improvements in GDMT use and reductions in HF hospitalizations compared to control [11]. Since the initial report of our experience at a single site, we have scaled to nine sites across our health system. Therefore, the goal of this study was to determine whether our pilot study results could be replicated in a larger, multi-site, cohort. The objectives were to evaluate the impact on clinical outcomes, including HF and cardiovascular (CV) hospitalizations and mortality, with MOC compared to usual care.
2. Methods
The details of our MOC have been previously described [11]. In summary, the MOC was initiated in July 2020 for patients with HFrEF (EF ≤ 40 %) who are not receiving optimal GDMT. Patients were referred by their primary cardiologist, each of whom was a part of our academic medical center's Heart and Vascular Institute (HVI). Decisions about referral were at the discretion of the provider. However, cardiologists may have been more likely to refer as the MOC program grew in size and impact over time. Therefore, it is possible that the referral process to MOC became more integrated into the cardiologist's workflow for HFrEF over time. Nonetheless, the uptake of referring patients to MOC was heterogenous among cardiologists thus enabling us to evaluate a sizeable control group for comparison.
Patients in MOC were managed by an advanced practice provider (APP) or clinical pharmacist within an average of about two weeks from referral, through a series of weekly telemedicine visits until the optimal, or maximally tolerated, medical regimen is achieved. MOC visits by the APP were billed and reimbursed because of the COVID-19 waiver for telemedicine. In the instance that the APP was not recognized as a provider through insurance, the billing occurred under the supervising HF cardiologist. The treatment algorithm was adapted from contemporary practice guidelines and modified to meet the needs of the MOC [1,11]. The treatment interventions aim to achieve quadruple GDMT (ARNi/ACEi/ARB, BB, MRA, and SGLT2i) within at least four weeks (with titration to target, or maximally tolerated doses, by 12 weeks) [12]. Once on optimized medications, or on maximally tolerated doses, the patient is discharged back to the referring provider.
2.1. Study design and population
Upon institutional review board approval at the University of Pittsburgh, we retrospectively evaluated the impact of MOC versus control (usual care) among patients with HFrEF. Patients with an encounter for MOC were identified from our health system between July 16, 2020, and September 21, 2023. The control group included patients with HFrEF from January 1, 2020, through September 21, 2023. The index visit for the MOC group was the date of the first MOC encounter. For the control group, it was the first visit that met the following criteria: cardiology visit with a diagnosis of HF and left ventricular ejection fraction ≤40 % within 90 days of this visit. Additional criteria for control patients included ongoing treatment with a beta-blocker and an ARNi, ACEi, or ARB, no prior inotrope usage, and no prior MOC encounter. Patients with <3 months of follow-up from the index visit and no encounters after the initial visit were excluded.
Our Heart and Vascular Clinical Analytics dashboard was used to capture medication and clinical outcome data in aggregate based on patient visits recorded in our outpatient electronic health record (EpicCare®, Verona, WI) with the provider template for our MOC. A similar cohort of patients with HFrEF was analyzed in aggregate for the control group. Baseline characteristics that were collected included: demographics, CV co-morbidities, and the number of HF admissions within 2 months of the index visit. We also reported GDMT medication class and dose (ARNi, ACEi, ARB, BB, MRA, and SGLT2i) at baseline for each group and at discharge or last follow-up MOC visit, or last visit within the study period for the control group. Target dose achievement was defined as an increase to a dose at or above daily guideline recommendations, among patients taking less than the target dose [2]. Patients are titrated to either target dose, or the maximally tolerated dose for each patient. Doses achieved are reported by class as <50 % target dose, 50 to <100 % target dose, and target dose. A between-group comparison of GDMT use by class and dose were also provided.
2.2. Outcomes and follow-up
The primary outcome was HF hospitalization. Additional clinical outcomes assessed were CV hospitalizations and all-cause mortality. Each outcome was evaluated at 3 months, 6 months, and 12 months after the index visit. Kaplan−Meier cumulative event curves and Cox proportional hazards regression models with adjustment were conducted. Competing risk analysis was also performed for HF and CV hospitalizations between groups at 3, 6, and 12 months.
2.3. Statistical analyses
Baseline clinical characteristics between study participants were compared using Pearson chi-square test for categorical variables and t-test for continuous variables. Baseline characteristics are shown as frequencies (percentages) for categorical variables and mean (standard deviation [SD]) for continuous variables. Kaplan-Meier cumulative event curves were generated for mortality and differences between groups compared using the log-rank test. A competing risk model, with death as the competing risk was utilized for evaluation of HF admission and CV admission. Death in the readmission analyses was treated as an independent censored event and those patients were no longer at risk for admission. As hospital admission can be a repeating event, cumulative incidence function was presented for HF and CV admissions. Clinical outcomes of the study were analyzed using Cox proportional hazards regression models with adjustment based on the following variables: age, sex, race, area deprivation index, history of atrial fibrillation, chronic kidney disease, hyperlipidemia, ischemic heart disease, stroke, deep vein thrombosis, obstructive sleep apnea, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, end stage renal disease, peripheral arterial disease, Elixhauser van Walraven score, length of stay, and the number of HF admissions within two months of the index visit. The threshold for statistical significance was 2 sided with a type I error rate of 0.05. All analyses were performed using SAS 9.4.
3. Results
3.1. Patient characteristics
A total of 1419 patients from across nine sites within our health system were included in the MOC group. The control group included a total of 5116 patients. Table 1 shows a summary of baseline characteristics between groups. Some differences in demographics between groups were noted (age and race), however, sex was not different. Differences in various CV co-morbidities were also apparent with higher incidences in the control group. All patients in the MOC cohort were referred by a cardiologist. Patients in the MOC group had a mean of 4.14 MOC visits over the study timeframe. The median total follow-up duration for the study was 439 days (IQR 244, 658). The mean number of follow-up visits for the MOC group during the study was: 2.5 (cardiology/office), 1.39 (cardiology/telemedicine), 1.85 (general medicine/office), and 0.23 (general medicine/telemedicine). All patients in the control group were seen by a cardiologist. The control group had a median total follow-up duration of 711 days (IQR 372, 1072.25). The mean number of follow-up visits for the control group during the study was: 3.61 (cardiology/office), 1.86 (cardiology/telemedicine), 3.18 (general medicine/office), and 0.36 (general medicine/telemedicine).
Table 1.
Baseline characteristics.
| MOC (n = 1419) |
Control (n = 5116) |
p-value | |
|---|---|---|---|
| Age | 64.2 (14) | 69.7 (13) | <0.001 |
| Female sex | 449 (31.6 %) | 1616 (31.6 %) | 0.969 |
| Race | 0.002 | ||
| White | 1184 (83.4 %) | 4446 (86.9 %) | |
| Black | 194 (13.7 %) | 572 (11.2 %) | |
| Other | 41 (2.9 %) | 98 (1.9 %) | |
| Hypertension | 946 (66.7 %) | 4001 (78.2 %) | <0.001 |
| Dyslipidemia | 859 (60.5 %) | 3742 (73.1 %) | <0.001 |
| Diabetes mellitus | 432 (30.4 %) | 1872 (36.6 %) | <0.001 |
| Ischemic heart disease | 269 (19 %) | 1274 (24.9 %) | <0.001 |
| Myocardial infarction | 125 (8.8 %) | 710 (13.9 %) | <0.001 |
| Stroke | 178 (12.5 %) | 855 (16.7 %) | <0.001 |
| Transient ischemic attack | 54 (3.8 %) | 230 (4.5 %) | 0.259 |
| Peripheral vascular disease | 137 (9.6 %) | 696 (13.6 %) | <0.001 |
| Deep vein thrombosis | 69 (4.9 %) | 245 (4.8 %) | 0.909 |
| Pulmonary embolism | 55 (3.9 %) | 226 (4.4 %) | 0.374 |
| Major bleeding | 251 (17.7 %) | 1040 (20.3 %) | 0.027 |
| Atrial fibrillation | 412 (29 %) | 1986 (38.8 %) | <0.001 |
| Ventricular tachycardia/ventricular fibrillation | 16 (1.1 %) | 81 (1.6 %) | 0.209 |
| Cardiac arrest | 22 (1.6 %) | 87 (1.7 %) | 0.696 |
| Chronic kidney disease | 164 (11.6 %) | 974 (19 %) | <0.001 |
| End-stage renal disease | 20 (1.4 %) | 118 (2.3 %) | 0.038 |
| Chronic obstructive pulmonary disease | 196 (13.8 %) | 903 (17.6 %) | <0.001 |
| Obstructive sleep apnea | 290 (20.4 %) | 1114 (21.8 %) | 0.278 |
| Left ventricular ejection fraction | 30.4 (11.1) | 26.5 (7.4) | <0.001 |
Continuous data expressed as mean (SD).
Categorical data expressed as n (%).
3.2. GDMT use
The proportion of GDMT use by class between groups is shown in Table 2 (baseline and at discharge or last follow-up visit for MOC and control groups). At MOC discharge or last visit, a significantly higher proportion of patients in MOC versus control received quadruple therapy (49 % vs 19 %), ARNi (62 % vs 45 %), BB (92 % vs. 88 %), MRA (69 % vs 45 %), and SGLT2i (68 % vs 35 %).
Table 2.
Guideline-directed medical therapy use between groups.
| MOC at baseline (n = 1419) |
Control at baseline (n = 5116) |
p-value | MOC at discharge or last visit (n = 1419) |
Control at last visit (n = 5116) |
p-value | |
|---|---|---|---|---|---|---|
| GDMT medications | ||||||
| Quadruple therapy | 243 (17.1 %) | 350 (6.8 %) | <0.0001 | 691 (48.7 %) | 957 (18.7 %) | <0.0001 |
| ARNi | 565 (39.8 %) | 1492 (29.2 %) | <0.0001 | 872 (61.5 %) | 2252 (45.1 %) | <0.0001 |
| <50 % target dose | 457 (80.9 %) | 1141 (76.5 %) | 522 (59.9 %) | 2053 (91.2 %) | ||
| 50 to <100 % target dose | 94 (16.6 %) | 260 (17.4 %) | 324 (37.2 %) | 137 (6.1 %) | ||
| Target dose | 14 (2.5 %) | 91 (6.1 %) | 26 (3 %) | 62 (2.8 %) | ||
| ACEi | 297 (20.9 %) | 1988 (38.9 %) | <0.0001 | 156 (11 %) | 1079 (21.1 %) | <0.0001 |
| <50 % target dose | 163 (54.9 %) | 1043 (52.5 %) | 110 (70.5 %) | 599 (55.5 %) | ||
| 50 to <100 % target dose | 53 (17.8 %) | 396 (19.9 %) | 6 (3.8 %) | 241 (22.3 %) | ||
| Target dose | 81 (27,3 %) | 549 (27.6 %) | 40 (25.6 %) | 239 (22.2 %) | ||
| ARB | 294 (20.7 %) | 1124 (22 %) | 0.3125 | 220 (15.5 %) | 897 (17.5 %) | 0.071 |
| <50 % target dose | 252 (85.7 %) | 865 (77 %) | 190 (86.4 %) | 842 (93.9 %) | ||
| 50 to <100 % target dose | 37 (12.6 %) | 234 (20.8 %) | 28 (12.7 %) | 40 (4.5 %) | ||
| Target dose | 5 (1.7 %) | 25 (2.2 %) | 2 (0.9 %) | 15 (1.7 %) | ||
| BB | 1221 (86 %) | 4257 (83.2 %) | 0.01 | 1313 (92.5 %) | 4517 (88.3 %) | <0.0001 |
| <50 % target dose | 906 (74.2 %) | 3284 (77.1 %) | 810 (61.7 %) | 3303 (73.1 %) | ||
| 50 to <100 % target dose | 253 (20.7 %) | 870 (20.4 %) | 382 (29.1 %) | 1002 (22.2 %) | ||
| Target dose | 62 (5.1 %) | 103 (2.4 %) | 121 (9.2 %) | 212 (4.7 %) | ||
| MRA | 619 (43.6 %) | 1814 (35.5 %) | <0.0001 | 974 (68.6 %) | 2319 (45.3 %) | <0.0001 |
| <50 % target dose | 3 (0.5 %) | 14 (0.8 %) | 1 (0.1 %) | 3 (0.1 %) | ||
| 50 to <100 % target dose | 84 (13.6 %) | 252 (13.9 %) | 59 (6.1 %) | 163 (7 %) | ||
| Target dose | 532 (85.9 %) | 1548 (85.3 %) | 914 (93.8 %) | 2153 (92.8 %) | ||
| SGLT2i | 491 (34.6 %) | 724 (14.2 %) | <0.0001 | 970 (68.4 %) | 1813 (35.4 %) | <0.0001 |
ARNi = angiotensin-receptor neprilysin inhibitor; ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker (e.g., bisoprolol, carvedilol, metoprolol succinate); MRA = mineralocorticoid receptor antagonist; SGLT2i = sodium-glucose cotransporter-2 inhibitor (e.g., dapagliflozin, empagliflozin).
Data expressed as n (%).
Target doses defined as an increase to a dose at or above daily guideline recommendations, among patients taking less than the target dose.
3.3. Clinical outcomes
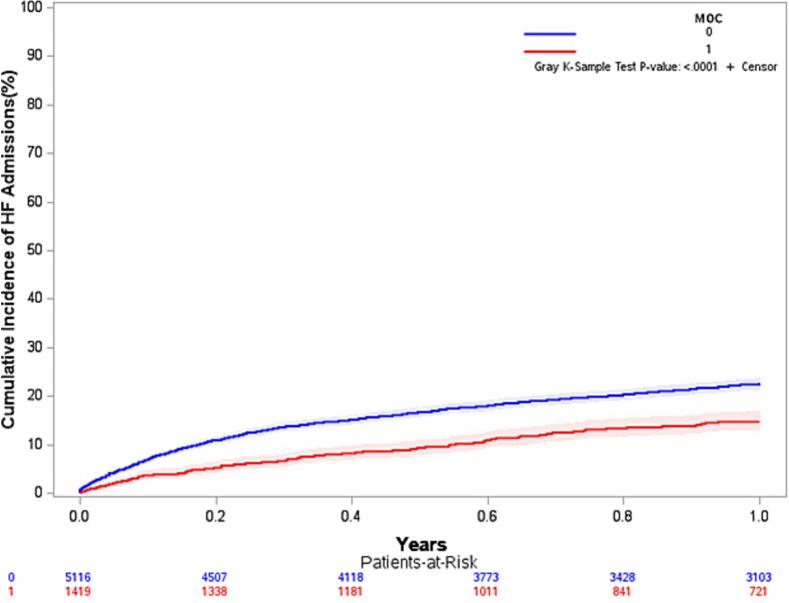
Kaplan-Meier analyses Cumulative Incidence Function for HF hospitalizations at 12 months is shown in Fig. 1. Competing risk analysis for HF hospitalizations at 12 months was significantly lower at each follow-up time point in favor of MOC vs. control (p < 0.001). Cox regression showed that patients in the control group were more likely to be hospitalized because of HF than those in the MOC group at 3 months (HR 1.89, 95 % CI, 1.50–2.38), 6 months (HR 1.68, 95 % CI, 1.39–2.03), and 12 months (HR 1.45, 95 % CI, 1.24–1.70).
Fig. 1.
Kaplan-Meier analysis cumulative incidence function for heart failure hospitalizations at 12 months (MOC vs. control).
CON = control group; MOC = Medication Optimization Clinic group.
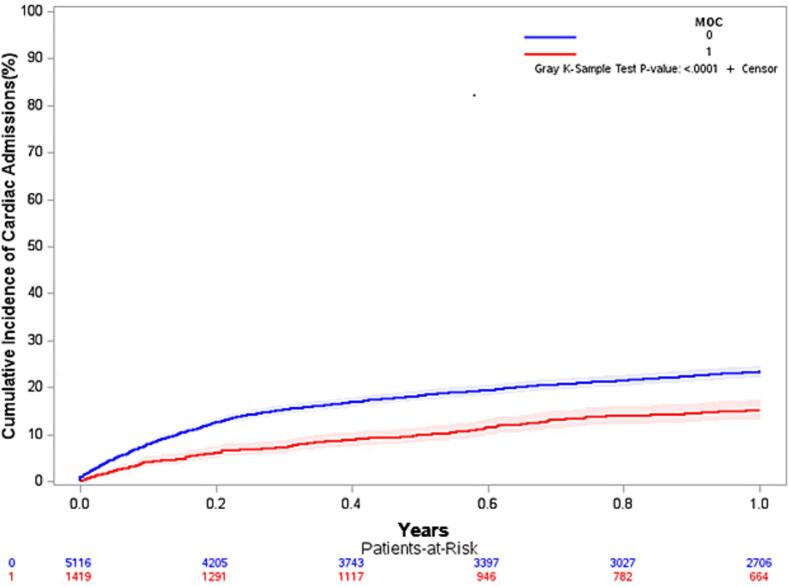
Kaplan-Meier analyses Cumulative Incidence Function for CV hospitalizations at 12 months is shown in Fig. 2. Competing risk analysis for CV hospitalizations was also significantly lower at each follow-up time point in favor of MOC vs. control (p < 0.001). Cox regression showed that patients in the control group were more likely to be hospitalized for any CV cause than those in the MOC group at 3 months (HR 1.92, 95 % CI, 1.60–2.31), 6 months (HR 1.61, 95 % CI, 1.39–1.86), and 12 months (HR 1.42, 95 % CI, 1.25–1.61).
Fig. 2.
Kaplan-Meier analysis cumulative incidence function for cardiovascular hospitalizations at 12 months (MOC vs. control).
CON = control group; MOC = Medication Optimization Clinic group.
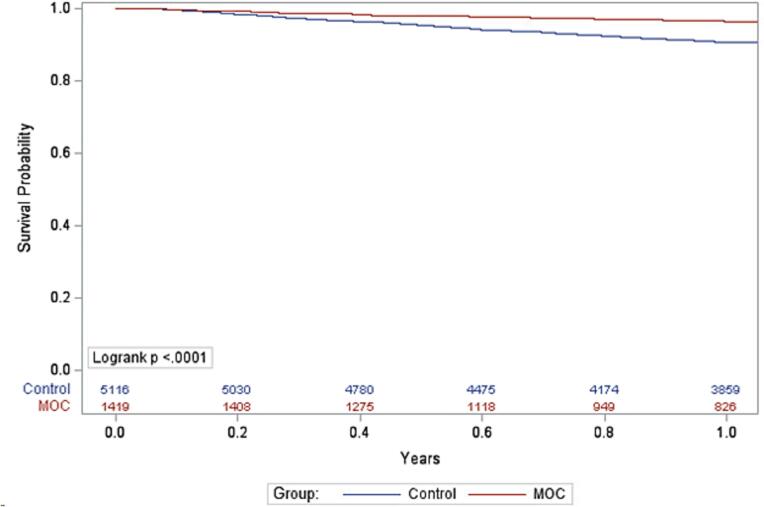
Kaplan-Meier analyses for all-cause mortality are shown in Fig. 3. All-cause mortality was significantly lower at 6 months (p = 0.006) and 12 months (p < 0.001), but did not differ at 3 months (p = 0.35), for MOC vs. control. Cox regression showed that patients in the control group were more like to have all-cause death vs. MOC at 6 months (HR 1.74, 95 % CI, 1.17–2.59) and 12 months (HR 1.93, 95 % CI, 1.41–2.63).
Fig. 3.
Kaplan-Meier analysis for all-cause mortality at 12 months (MOC vs. control).
CON = control group; MOC = Medication Optimization Clinic group.
4. Discussion
4.1. Increase in GDMT use
Results from our study demonstrated that approximately 50 % of patients, compared to about 20 %, received quadruple GDMT through our MOC versus control. Patients in the MOC group were significantly more likely to receive an ARNi, BB, MRA, and an SGLT2i compared to control. The attainment of SGLT2i therapy (68 %) and ARNi (62 %) therapies were particularly notable in the MOC group. Additionally, more patients in MOC achieved moderate to high doses of ARNi (40 % vs. 9 %) and BB (38 % vs. 27 %) than control. Certain GDMT therapies were more prevalent at baseline though in the MOC group. The use of ARNi with dose titration to target dose was one of our main interventions, which explains the lower proportions of ACEi and ARB use in the MOC group. We also prioritized the early initiation of SGLT2i for MOC patients. The emphasis on the use of newer therapies in MOC was enhanced through team efforts to assist with prior authorizations, peer-to-peer reviews with insurers, and directing patients to complete financial assistance applications. The MOC GDMT rates achieved compare favorably to a cohort study that reported delayed initiation of ARNi and SGLT2i after HF hospitalization [13]
Several key studies have evaluated interventions to improve GDMT prescribing. The design, intervention, and setting of each of these studies differ considerably. For example, some focus on intervention in the hospitalized or transitional setting. Others have used electronic health record (EHR) alerting or virtual recommendations as the intervention. An open-label randomized trial (STRONG-HF [Safety, tolerability, and efficacy of up-titration of guideline-directed medical therapies for acute heart failure]) used a structured protocol with in-person visits and biomarker-guided optimization after an acute HF presentation [5]. Increases in GDMT were seen versus usual care, although the study intervention did not include SGLT2i use [5].
A prospective, implementation trial (IMPLEMENT-HF [Implementation of Medical Therapy in Hospitalized Patients With Heart Failure With Reduced Ejection Fraction]) deployed a virtual care team that used algorithm-based recommendations to generate optimization suggestions in the EHR [3]. The authors reported significant increases in use of BB and MRA and a 20 % absolute increase in in-hospital optimization [3]. Less impact on ARNi and SGLT2i use were noted by the investigators, and a majority of patients were not on quadruple therapy. A pragmatic trial (PROMPT-HF [Pragmatic Trial of Messaging to Providers About Treatment of Heart Failure]) used EHR-based clinical alerts to drive modest improvements in GDMT use over a 30 day period in outpatients with HF [4]. More specifically, initiation and titration of BB were significantly increased at 30 days compared to baseline, whereas other medication classes did not differ.
4.2. Improvements in clinical outcomes
The MOC was associated with a significant reduction in the rates of HF and all-cause CV hospitalizations at 3, 6, and 12 months compared to control using competing risk analyses. Cox regression showed that patients in the control group were approximately 1.5 to 2 times more likely to be hospitalized due to HF or any CV cause than those in the MOC group. All-cause mortality in the control group was also about 1.5 to 2 times more likely than those in the MOC group at 6 and 12 months.
The STRONG-HF trial demonstrated a composite improvement in 180-day (amended from 90-day due to low event rates) hospital readmission due to HF or all-cause death with the intervention (initiated before discharge with the goal of up-titration to 100 % of recommended doses within 2 weeks of discharge) versus usual care. At least half of patients attained goal doses by day 90. The clinical improvement was driven by lower hospitalizations since all-cause death was not significantly different. However, more non-serious adverse events were observed in the high-intensity group [5]. In addition, the study intervention appeared to be resource and personnel intensive, and thus may be logistically challenging to implement in certain settings. Other randomized clinical trials to date have not reported improvements in clinical outcomes [6,7].
Despite the multitude of studies which have documented different approaches to improving GDMT, none to our knowledge, have shown a consistent impact on improving clinical outcomes. In contrast, the MOC achieved quadruple GDMT in a high proportion of patients that translated to improvements in both hospitalizations and mortality. These findings align with evidence that demonstrated the combination of ARNi, BB, MRA, and SGLT2i to be the most effective at reducing all-cause death and the composite of CV death or first hospitalization for HF [14].
4.3. MOC structure and process
The design of our MOC has been previously described [11]. In summary, we have deployed a team-based model that is APP-led and clinical pharmacist-supported with HF physician specialist oversight. The MOC has broad institutional and HVI leadership support and clinician acceptance. The MOC is a hospital-based referral clinic with medical-center approved protocol that enables the team to work collaboratively to optimize patient's GDMT through a series of telemedicine visits in a structured timeframe (e.g., weekly phone calls for up to 12 weeks). Medications are proactively initiated and up-titrated in 1 to 2 week intervals, often with simultaneous medication dosing changes made at each visit. This has been productive in addressing the clinical inertia barrier to optimal GDMT dosing [15,16]
The use of telemedicine visits also enables us to provide remote GDMT initiation and titration while promoting patient self-management (with use of at home vital signs monitoring). We were able to secure grant funding to help subsidize blood pressure monitors and scales for patients, as the MOC was expanded to multiple sites. This provides us with multiple data points at each weekly visit to make better-informed medication therapy decisions. Our experience with the MOC adds to the evidence for team-based GDMT optimization efforts in HF [17].
Since the initial deployment, we have scaled the MOC to nine sites within our health system that represents a mix of academic and community-based hospitals. This was accomplished by leveraging shared expertise, processes, workflows, and technologies using a “hub and spoke” model. Therefore, the MOC provides another feasible approach to team-based care to drive improvements in GDMT. This type of intervention is strongly supported given the importance of GDMT to the disease trajectory of HFrEF [1,18]. The MOC also can be paired with hospital-based, or virtual, interventions. At our institution the advanced heart failure service aims for quadruple GDMT prior to discharge (when clinically appropriate), before scheduling the patient to be seen in our discharge clinic (ideally, within 7 days post-discharge). Thereafter, patients may be referred to our MOC for subsequent GDMT initiation (if not previously done) and/or up-titration to target doses.
4.4. Study strengths
The strengths of our study include the large sample size and competing risk analyses for hospitalizations. We were also able to mine a large clinical data repository to evaluate clinical metrics and outcomes data for all patients seen in our health system.
4.5. Study limitations
Limitations of this study include the retrospective design and the potential for residual confounding. Patient referral to MOC was at the discretion of the provider as detailed in the methods However, the MOC has gained broad institutional support among providers as the service has become better established and demonstrated its value [11]. In fact, MOC referral has more recently extended to sicker patients including those who were recently discharged for an acute HF hospitalization. This is also in line with the higher baseline rates of GDMT use in the MOC group, likely a result of earlier initiation of treatment in the hospital setting [1,3,5]. Nonetheless, the control group represents a contemporary cohort of well-treated patients who were managed by a cardiologist.
In our analysis, we do see an imbalance in several baseline characteristics between the MOC and control groups. To minimize bias, we included adjustment for demographics and a multitude of important clinical variables (i.e., atrial fibrillation, chronic kidney disease, ischemic heart disease, stroke, peripheral vascular disease, diabetes, hypertension, and dyslipidemia) in the Cox regression models. We also used a competing risk model to evaluate HF and cardiovascular hospitalizations as a cumulative incidence function.
The generalizability of an MOC-type initiative to augment GDMT is of clear importance to improving outcomes for patients with HFrEF. Locally, we have scaled to a total of nine sites within our health system. In doing so, however, we have extended to sites beyond our academic “hub” that do not provide HF specialty care on-site. Therefore, it was necessary to dedicate clinical expertise and time for education and training to ensure successful implementation of GDMT. Through this process, we acknowledge the possible association between our scaling efforts and lower attainment of target doses of certain medication classes (i.e., ARNi and BB) than anticipated. Overall, we did observe higher rates of SGLT2i, MRA, quadruple therapy, and moderate doses of ARNi versus control which speaks to the success of this initiative.
Finally, we also acknowledge the potential for other reasons for undertreatment beyond scaling, such as affordability barriers during follow-up and medication adjustment by other providers after MOC discharge. There also is the potential for adverse events, such as hypotension or electrolyte abnormalities, leading to undertreatment. These variables were not discretely captured in our study. However, an experienced HF clinician evaluated each of these factors to make appropriate decisions about GDMT.
5. Conclusions
The MOC was associated with improved GDMT that translated to significantly lower risks of HF hospitalizations, CV hospitalizations, and all-cause mortality at 6 and 12 months in patients with HFrEF compared to control. We have demonstrated scalability to multiple academic and community sites within our large health care system. This multidisciplinary approach conducted via telemedicine visits in the outpatient setting provides a scalable model for efficient adoption of GDMT with translation to better clinical outcomes.
Funding
This work was supported by the American Society of Health System Pharmacists Foundation (Bethesda, MD) and the American Nurses Foundation (Silver Spring, MD) [grant number 3.20.205].
Role of the funder/sponsor
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CRediT authorship contribution statement
James C. Coons: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Conceptualization. Jennifer Kliner: Funding acquisition, Conceptualization. Michael A. Mathier: Conceptualization. Suresh Mulukutla: Supervision, Resources. Floyd Thoma: Software, Data curation. Ahmet Sezer: Formal analysis. Mary Keebler: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
James C. Coons, Email: coonsjc@upmc.edu.
Jennifer Kliner, Email: dilascioj@upmc.edu.
Michael A. Mathier, Email: mathierm@upmc.edu.
Suresh Mulukutla, Email: mulukutlasr@upmc.edu.
Floyd Thoma, Email: thomafw3@upmc.edu.
Ahmet Sezer, Email: sezera@upmc.edu.
Mary Keebler, Email: keeblerme@upmc.edu.
References
- 1.Heidenreich P.A., Bozkurt B., Aguilar D., Allen L.A., Byun J.J., Colvin M.M., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2022;79(17):1757–1780. doi: 10.1016/j.jacc.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Maddox T.M., Januzzi J.L., Jr., Allen L.A., Breathett K., Brouse S., Butler J., et al. 2024 ACC expert consensus decision pathway for treatment of heart failure with reduced ejection fraction: a report of the American College of Cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2024;83(15):1444–1488. doi: 10.1016/j.jacc.2023.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt A.S., Varshney A.S., Moscone A., Claggett B.L., Miao Z.M., Chatur S., et al. Virtual care team guided management of patients with heart failure during hospitalization. J. Am. Coll. Cardiol. 2023;81(17):1680–1693. doi: 10.1016/j.jacc.2023.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghazi L., Yamamoto Y., Riello R.J., Coronel-Moreno C., Martin M., O’Connor K.D., et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J. Am. Coll. Cardiol. 2022;79(22):2203–2213. doi: 10.1016/j.jacc.2022.03.338. [DOI] [PubMed] [Google Scholar]
- 5.Mebazaa A., Davison B., Chioncel O., Cohen-Solal A., Diaz R., Filippatos G., et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938–1952. doi: 10.1016/S0140-6736(22)02076-1. [DOI] [PubMed] [Google Scholar]
- 6.DeVore A.D., Granger B.B., Fonarow G.C., Al-Khalidi H.R., Albert N.M., Lewis E.F., et al. Care optimization through patient and hospital engagement clinical trial for heart failure: rationale and design of CONNECT-HF. Am. Heart J. 2020;220:41–50. doi: 10.1016/j.ahj.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Van Spall H.G.C., Lee S.F., Xie F., Oz U.E., Perez R., Mitoff P.R., et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA. 2019;321(8):753–761. doi: 10.1001/jama.2019.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad T., Desai N.R., Yamamoto Y., Biswas A., Ghazi L., Martin M., et al. Alerting clinicians to 1-year mortality risk in patients hospitalized with heart failure: the REVEAL-HF randomized clinical trial. JAMA Cardiol. 2022;7(9):905–912. doi: 10.1001/jamacardio.2022.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow G.C., Van Spall H.G.C., Januzzi J.L., Jr. Team-based virtual nudges to overcome clinician inertia in guideline-directed medical therapy for heart failure. J. Am. Coll. Cardiol. 2023;81(17):1694–1696. doi: 10.1016/j.jacc.2023.02.047. [DOI] [PubMed] [Google Scholar]
- 10.Savarese G., Bodegard J., Norhammar A., Sartipy P., Thuresson M., Cowie M.R., et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden) Eur. J. Heart Fail. 2021;23(9):1499–1511. doi: 10.1002/ejhf.2271. [DOI] [PubMed] [Google Scholar]
- 11.Coons J.C., Kliner J., Mathier M.A., Mulukutla S., Thoma F., Sezer A., et al. Impact of a medication optimization clinic on heart failure hospitalizations. Am. J. Cardiol. 2023;188:102–109. doi: 10.1016/j.amjcard.2022.11.025. [DOI] [PubMed] [Google Scholar]
- 12.McMurray J.J.V., Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: a redefinition of evidence-based medicine. Circulation. 2021;143(9):875–877. doi: 10.1161/CIRCULATIONAHA.120.052926. [DOI] [PubMed] [Google Scholar]
- 13.Savarese G., Kishi T., Vardeny O., Adamsson Eryd S., Bodegard J., Lund L.H., et al. Heart failure drug treatment-inertia, titration, and discontinuation: a multinational observational study (EVOLUTION HF) JACC Heart Fail. 2023;11(1):1–14. doi: 10.1016/j.jchf.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Tromp J., Ouwerkerk W., van Veldhuisen D.J., Hillege H.L., Richards A.M., van der Meer P., et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73–84. doi: 10.1016/j.jchf.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt A.S., DeVore A.D., DeWald T.A., Swedberg K., Mentz R.J. Achieving a maximally tolerated beta-blocker dose in heart failure patients: is there room for improvement? J. Am. Coll. Cardiol. 2017;69(20):2542–2550. doi: 10.1016/j.jacc.2017.03.563. [DOI] [PubMed] [Google Scholar]
- 16.Fiuzat M., Ezekowitz J., Alemayehu W., Westerhout C.M., Sbolli M., Cani D., et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2020;5(7):757–764. doi: 10.1001/jamacardio.2020.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J., Mednick T., Heidenreich P.A., Sandhu A.T. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J. Card. Fail. 2023;29(7):1000–1013. doi: 10.1016/j.cardfail.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava P.K., DeVore A.D., Hellkamp A.S., Thomas L., Albert N.M., Butler J., et al. Heart failure hospitalization and guideline-directed prescribing patterns among heart failure with reduced ejection fraction patients. JACC Heart Fail. 2021;9(1):28–38. doi: 10.1016/j.jchf.2020.08.017. [DOI] [PubMed] [Google Scholar]