Abstract
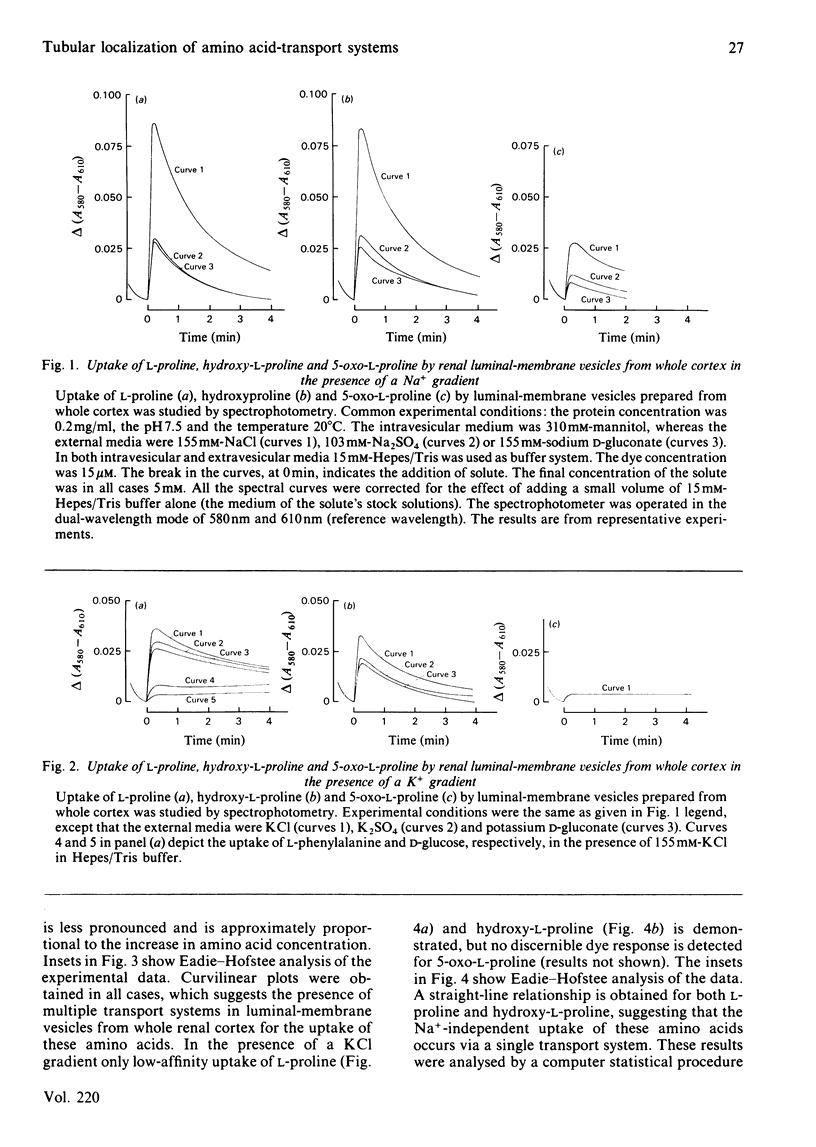
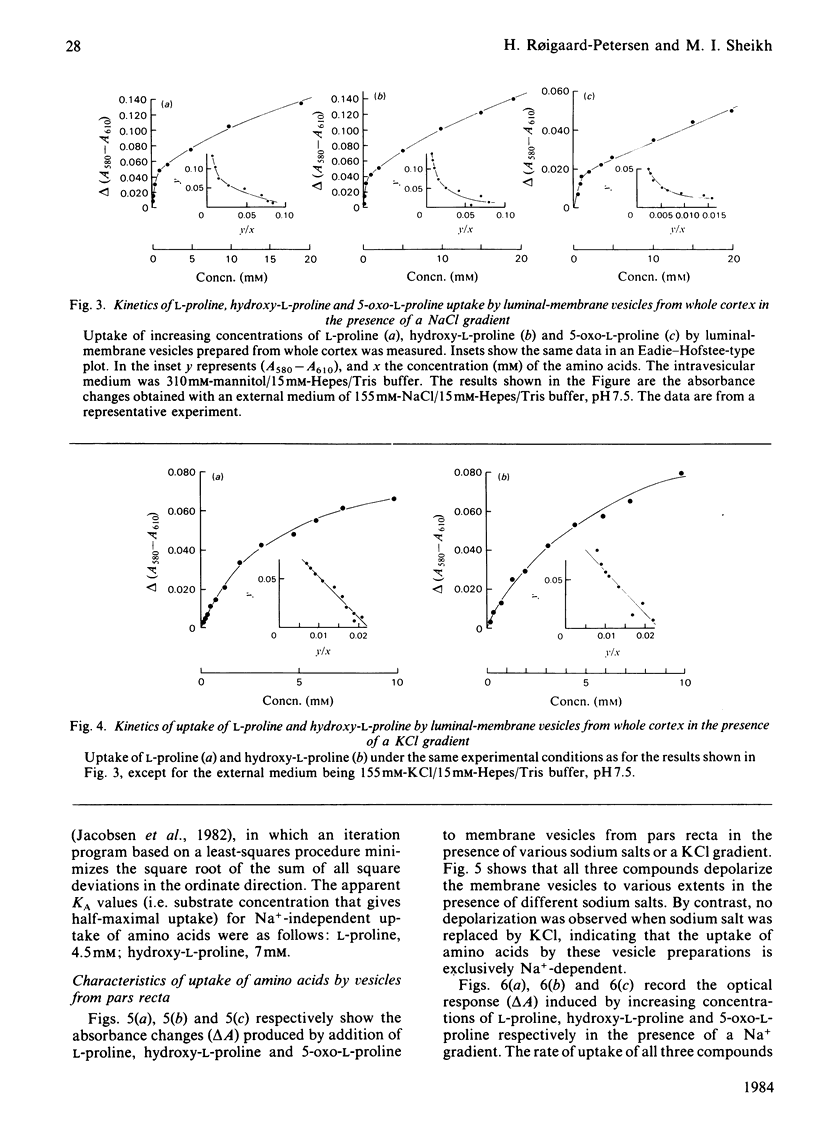
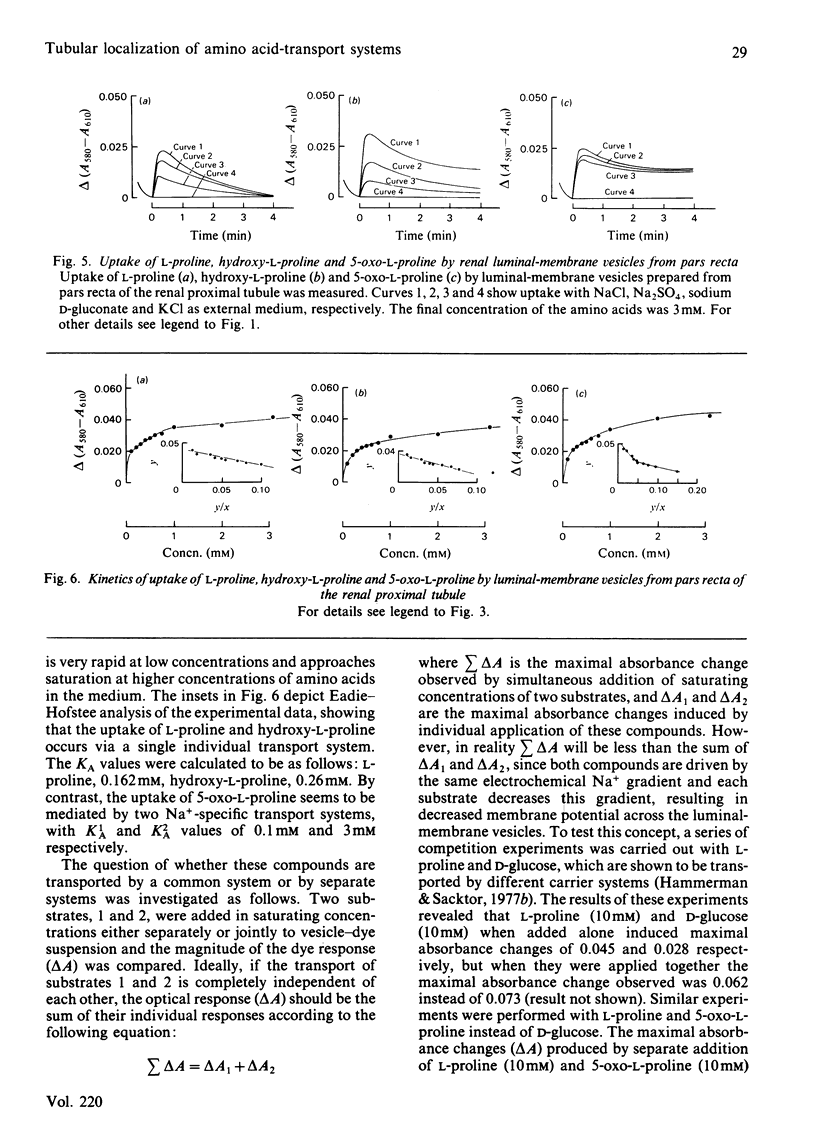
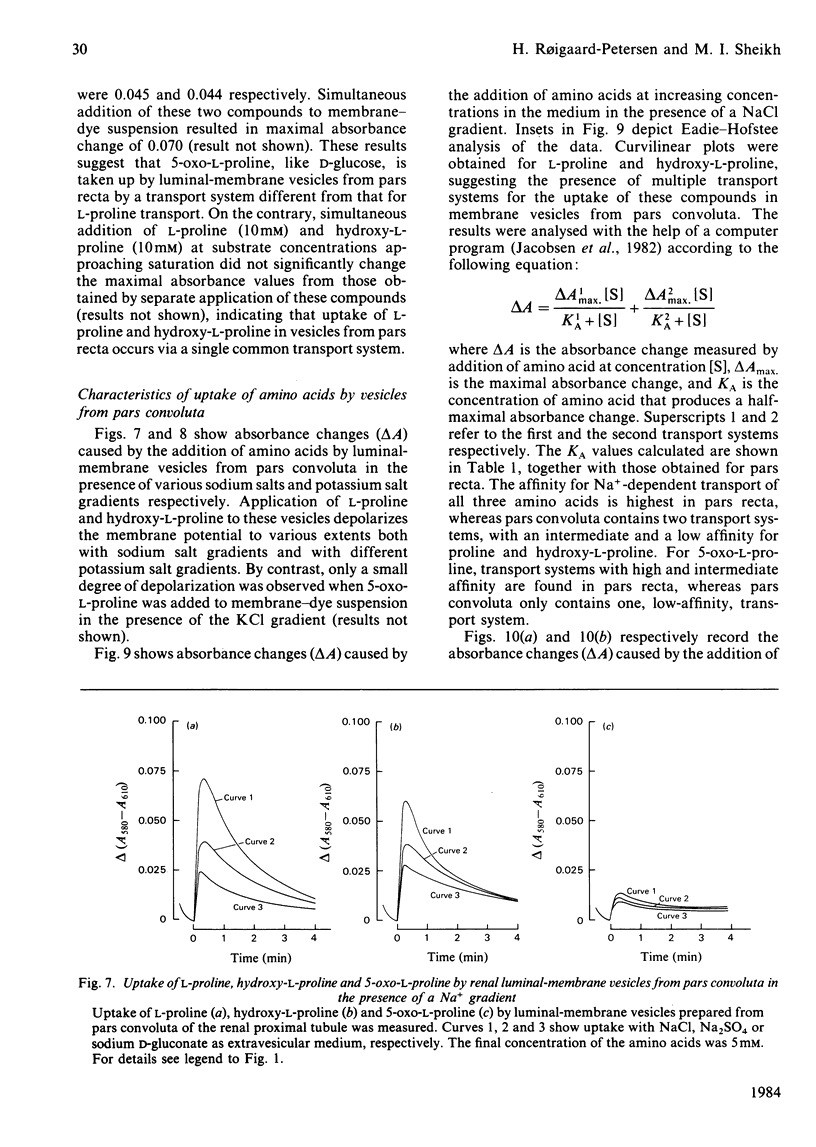
Uptake of L-proline, hydroxy-L-proline and 5-oxo-L-proline by luminal-membrane vesicles isolated either from whole cortex or from pars convoluta or pars recta of proximal tubules was studied by a spectrophotometric method. Uptake of L-proline and hydroxy-L-proline by vesicles from whole cortex was mediated by both Na+-dependent and Na+-independent, but electrogenic, processes, whereas transport of 5-oxo-L-proline in these vesicles was strictly Na+-dependent. Eadie-Hofstee analysis of saturation-kinetic data suggested the presence of multiple transport systems in luminal-membrane vesicles from whole renal cortex for the uptake of all these amino acids. Tubular localization of the transport systems was studied by the use of vesicles derived from pars convoluta and from pars recta. In pars recta transport of all three amino acids was strictly dependent on Na+ and occurred via a high-affinity system (half-saturation: 0.1-0.3 mM). Cation-dependent but Na+-unspecific transport of low affinity for L-proline and hydroxy-L-proline was exclusively localized to the pars convoluta, which also contained a Na+-preferring system of intermediate affinity (half-saturation: L-proline, 0.75 mM; hydroxy-L-proline, 1.3 mM). 5-Oxo-L-proline was transported by low-affinity and Na+-dependent systems in both pars convoluta and pars recta. Competition experiments revealed that transport systems for L-proline and hydroxy-L-proline are common, but indicated separate high-affinity transport systems for 5-oxo-L-proline and L-proline in luminal-membrane vesicles from pars recta. The physiological importance of the presence of various neutral amino acid-transport systems in different segments of the proximal tubule is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen H. N. Some special kinetic problems of transport. Adv Enzymol Relat Areas Mol Biol. 1969;32:1–20. doi: 10.1002/9780470122778.ch1. [DOI] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Fass S. J., Hammerman M. R., Sacktor B. Transport of amino acids in renal brush border membrane vesicles. Uptake of the neutral amino acid L-alanine. J Biol Chem. 1977 Jan 25;252(2):583–590. [PubMed] [Google Scholar]
- Fass S. J., Hammerman M. R., Sacktor B. Transport of amino acids in renal brush border membrane vesicles. Uptake of the neutral amino acid L-alanine. J Biol Chem. 1977 Jan 25;252(2):583–590. [PubMed] [Google Scholar]
- Ganapathy V., Roesel R. A., Howard J. C., Leibach F. H. Interaction of proline, 5-oxoproline, and pipecolic acid for renal transport in the rabbit. J Biol Chem. 1983 Feb 25;258(4):2266–2272. [PubMed] [Google Scholar]
- Ganapathy V., Roesel R. A., Leibach F. H. Transport of 5-oxoproline into rabbit renal brush border membrane vesicles. Biochem Biophys Res Commun. 1982 Mar 15;105(1):28–35. doi: 10.1016/s0006-291x(82)80006-5. [DOI] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B. Na+-dependent transport of glycine in renal brush border membrane vesicles. Evidence for a single specific transport system. Biochim Biophys Acta. 1982 Apr 7;686(2):189–196. doi: 10.1016/0005-2736(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B. Transport of amino acids in renal brush border membrane vesicles. Uptake of L-proline. J Biol Chem. 1977 Jan 25;252(2):591–595. [PubMed] [Google Scholar]
- Hammerman M., Sacktor B. Transport of beta-alanine in renal brush border membrane vesicles. Biochim Biophys Acta. 1978 May 18;509(2):338–347. doi: 10.1016/0005-2736(78)90052-4. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Albrecht I., Rosenberg L. E. Identification and analysis of multiple glycine transport systems in isolated mammalian renal tubules. J Biol Chem. 1968 Nov 10;243(21):5566–5571. [PubMed] [Google Scholar]
- Hillman R. E., Rosenberg L. E. Amino acid transport by isolated mammalian renal tubules. II. Transport systems for L-proline. J Biol Chem. 1969 Aug 25;244(16):4494–4498. [PubMed] [Google Scholar]
- Jacobsen C., Frich J. R., Steensgaard J. Determination of affinity of monoclonal antibodies against human IgG. J Immunol Methods. 1982;50(1):77–88. doi: 10.1016/0022-1759(82)90305-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. E., Kragh-Hansen U., Røigaard-Petersen H., Sheikh M. I. Citrate uptake by basolateral and luminal membrane vesicles from rabbit kidney cortex. Am J Physiol. 1983 Jun;244(6):F686–F695. doi: 10.1152/ajprenal.1983.244.6.F686. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U., Jørgensen K. E., Sheikh M. I. The use of a potential-sensitive cyanine dye for studying ion-dependent electrogenic renal transport of organic solutes. Uptake of L-malate and D-malate by luminal-membrane vesicles. Biochem J. 1982 Nov 15;208(2):369–376. doi: 10.1042/bj2080369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U., Jørgensen K. E., Sheikh M. I. The use of potential-sensitive cyanine dye for studying ion-dependent electrogenic renal transport of organic solutes. Spectrophotometric measurements. Biochem J. 1982 Nov 15;208(2):359–368. doi: 10.1042/bj2080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U., Røigaard-Petersen H., Jacobsen C., Sheikh M. I. Renal transport of neutral amino acids. Tubular localization of Na+-dependent phenylalanine- and glucose-transport systems. Biochem J. 1984 May 15;220(1):15–24. doi: 10.1042/bj2200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNamara P. D., Ozegović B., Pepe L. M., Segal S. Proline and glycine uptake by renal brushborder membrane vesicles. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4521–4525. doi: 10.1073/pnas.73.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sheikh M. I., Kragh-Hansen U., Jørgensen K. E., Røigaard-Petersen H. An efficient method for the isolation and separation of basolateral-membrane and luminal-membrane vesicles from rabbit kidney cortex. Biochem J. 1982 Nov 15;208(2):377–382. doi: 10.1042/bj2080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M. I. Renal handling of phenol red. II. The mechanism of substituted phenolsulphophthalein (PSP) dye transport in rabbit kidney tubules in vitro. J Physiol. 1976 Mar;256(1):175–195. doi: 10.1113/jphysiol.1976.sp011319. [DOI] [PMC free article] [PubMed] [Google Scholar]



