Abstract
Background/Aim
Biomarkers for patients suffering from glioblastoma (GBM) are scarce. Extracellular vesicles (EV) are a promising candidate for a potential biomarker. Therefore, EV concentration could be a potential biomarker of tumor burden, volume, and prognosis.
Patients and Methods
Large EV (lEV) and small EV (sEV) were isolated from 36 GBM patients’ blood plasma by differential centrifugation. Nanoparticle tracking was used to measure EV concentration. Quantitative analysis of tumor volume was performed by evaluating T2/FLAIR relaxation times.
Results
The mean size of lEV was 173.3 nm ± 18.2 nm, while sEV measured 148.3 ± 9.0 nm. Patients with higher lEV concentrations showed a trend towards longer overall survival (36.1 vs. 16.5 months, p=0.08). Regarding inflammatory markers, higher leukocyte count was positively correlated with higher sEV concentration (r2=0.3887, DF 21, p=0.0015). No significant relationship was found between lEV or sEV concentration and tumor volume.
Conclusion
Overall EV concentration in the peripheral blood is not a predictor of tumor volume. sEV concentration is associated with a potential pro-inflammatory metabolism.
Keywords: Extracellular vesicles, biomarker, glioblastoma, liquid biopsy
Glioblastoma (GBM) accounts for 12-15% of all intracranial tumors (1). According to the new WHO classification, they are classified as grade 4 gliomas called “glioblastoma, IDH-wildtype” (90% of cases diagnosed) and “astrocytoma IDH-mutant” [10% of cases diagnosed (2,3)]. GBMs usually reach a median overall survival (OS) of approximately 14 months after diagnosis despite standard therapy based on maximal surgical resection followed by radio-chemotherapy (1,4).
Despite this intensive therapy regimen, the risk of recurrence is high and associated with high mortality. Against this background of therapeutic impasse, new strategies and, in particular, reproducible and easy-to-implement non-invasive measures are needed for a reliable detection of tumor relapse. Possible starting points for this endeavor could be extracellular vesicles (EV), which are easily obtained from the blood, urine, cerebrospinal fluid, ascites, amniotic fluid, and seminal fluid (5). Numerous studies have revealed the importance of EVs as mediators of intercellular communication between gliomas and their microenvironment (6). As reviewed by Young et al., a growing number of studies are investigating the idea of using circulating EV as biomarkers and have already observed a direct correlation between vesiculaemia and tumor burden, as well as OS and recurrence (7).
The aim of this study was to: 1) determine EV concentration at different stages of the disease (pre-operative or post-operative setting, follow-up of patients with stable disease and follow-up of patients in progression), 2) correlate EV concentration with tumor volume as measured using magnetic resonance (MR) scans, 3) evaluate its relationship with OS and 4) assess its association with systemic inflammatory markers (leukocyte count, C-reactive protein) in order to qualify and quantify EV concentration as a possible predictor and biomarker.
Patients and Methods
Patient selection. The project was approved by the local Ethics committee (project number 3/2/14). After providing written consent, GBM patients were included in the study. Overall, 36 patients were recruited between May 2015 and January 2020. Status of disease (pre-operative, stable disease, progressive disease) was assessed at the time of recruitment. Stable and recurrent disease were defined according to the Rano criteria. Maximum time span between MR scans considered for analysis and drawing blood samples was one month. The diagnosis of GBM was verified by the Institute of Pathology at the University Medical Center Goettingen.
Sample preparation and measurement of EV concentration. lEV and sEV were isolated from 10 to 15 ml peripheral blood drawn from GBM patients. After collection, samples were processed immediately (<2 h) following our previously established protocols (8,9). Nano particle tracking analysis (NTA) of lEV and sEV was conducted on a ZetaView PMX-120 device (PMX 120 ZetaView® Mono Laser, Particle Metrix, Inning am Ammersee, Germany) equipped with a 640 nm laser and a CMOS camera (CMOS camera 640×480 pixels, Particle Metrix). Samples were diluted in 1 ml PBS to obtain a concentration of 50-400 particles/frame. For each sample, videos at 11 cell positions were recorded at 25˚C with a camera sensitivity of 80-83 for sEV and 76-79 for lEV, respectively. Data were analyzed with the ZetaView software (v8.02.31).
Assessment of tumor and perifocal edema volume. The MRI examinations were performed on a 3.0 Tesla (Siemens Magnetom Prisma, Siemens Medical Solutions, Erlangen, Germany). A 32-channel head coil was used. A standard protocol for all patients was performed. After native sequences were performed, contrast-enhanced sequences were obtained using a single intravenous dose of gadobutrol (Gd-DO3A-butrol, Gadovist). The following sequences were used: 2D T2w MRI sequence with fluid-attenuated inversion recovery impulse (T2w FLAIR) in axial acquisition (TE=80 ms, TR=8,000 ms, FOV=256×256 mm², FA=120˚, anisotropic voxel size of 1 mm × 1 mm × 3 mm), 3D T1w MPR without contrast enhancement in sagittal acquisition (TE=2.67 ms, TR=1,580 ms, FOV=256×256 mm2, FA=8˚, isotropic voxel size of 1 mm × 1 mm × 1 mm); iv), 3D T1w with gadolinium contrast enhancement in sagittal acquisition (TE=4.57 ms, TR=2,070 ms, FOV=256×256 mm2, FA=15˚, isotropic voxel size of 1 mm × 1 mm × 1 mm).
Quantitative analysis of tumor volume was performed by evaluating four MR sequences (T1w, T1w gadolinium enhanced, T2w, and T2w-FLAIR) simultaneously to annotate a data set. The images were strictly segmented according to their timing, starting with the preoperative image, independent of the other MR acquisitions of the same patient (i.e., in a prospective fashion). Tumors were measured using electronic calipers, in which the largest (a) and smallest (b) tumor extents were determined, and then the volume determination was calculated using the formula V=a*b*b*0.52 (planimetry).
Statistical analysis. GraphPad Prism (Version 9.3.0, GraphPad Software, Boston, MA, USA) was used for statistical analysis. Simple linear regression analysis was used to assess correlations between lEV or sEV concentrations and clinical parameters (OS, inflammatory markers, tumor volume, edema volume). Unpaired student’s t-test was applied comparing subgroups. A p-value <0.05 was considered as significant. To find the cut-off for high and low EV concentration we used program X-tile (10), as previously published by our group (11) before applying Log-rank test and Hazard ratios for survival analysis. Kaplan–Meier curves were drawn using GraphPad Prism (Version 9.3.0).
Results
A cohort of 36 GBM patients was enrolled in the study. Clinical baseline characteristics, disease and mutation status, as well as the number of patients under corticosteroid therapy are listed in Table I. Mean size of lEV was 173.3±18.2 nm, whereas sEV showed a mean size of 148.3±9.0 nm. Regarding inflammatory markers, a higher leukocyte count was positively correlated with higher sEV concentrations in simple linear regression (r2=0.3887; DF=21; p=0.002), whereas leukocyte count was not associated with lEV number (r2=0.013; DF=21; p>0.05). C-reactive protein was neither associated with lEV (r2=0.0024; DF=19; p>0.05) nor sEV (r2=0.0682; DF=19; p>0.05) concentration. Both tumor volume and the edema surrounding the tumor lesion were quantified. Tumor volume was not associated with either lEV (r2=0.03792; DF=31; p>0.05) or sEV concentration (r2=0.04087; DF=30; p>0.05). In line, edema and lEV (r2=0.004; DF=22; p>0.05) and edema and sEV (r2=0.003; DF=22; p>0.05) showed no relevant correlation.
Table I. Clinical characteristics.
Concerning disease status, we were able to show that sEV concentration could be used to predict whether a patient was recruited pre-operatively (87.50% correctly classified patients) or later, during stable disease (66.67% correctly classified patients) in multiple regression analysis. The receiver operating characteristics curve showed an AUC of 0.76 (95%CI=0.56-0.95; p=0.023), and positive predictive power was 80.00%. Pre-operative patient samples showed a significantly lower sEV concentration than samples of patients with stable disease, while no significant differences were observed concerning lEV concentration (Table II).
Table II. Subgroup analysis of large extracellular vesicle (lEV) and small extracellular vesicle (sEV) concentrations in pre-operative samples and samples acquired during stable disease.
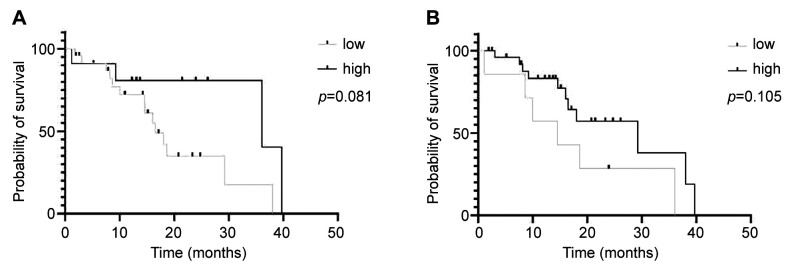
Overall survival is not associated with vesicle concentration. Are lEV or sEV concentrations useful biomarkers for prognosis in GBM patients? Patients were categorized into two groups based on vesicle concentration: high and low. Cut-off for survival analysis was set at a concentration of >1.23×109 lEV particles/ml blood plasma (lEVhigh concentration: 12 patients, lEVlow concentration: 24 patients). The cut-off for high sEV concentration was at >3.61×108 sEV particles/ml blood plasma (sEVhigh concentration: 7 patients, sEVlow concentration: 28 patients). Overall, patients with higher lEV or sEV concentration showed a trend toward a longer OS [36.1 months (high lEV concentration) vs. 16.5 months (low lEV concentration)]; p=0.08; HR=2.45, 95%CI=0.96-6.27; 29.27 months (high sEV concentration) vs. 14.53 months (low sEV concentration); p=0.11; HR=2.19, 95%CI=0.67-7.21 (Figure 1A and B).
Figure 1.
Patients with higher concentrations of large (A) or small extracellular vesicles (B) tend to have a longer overall survival. Kaplan–Meier curve; (A) p=0.08; HR=2.45, 95%CI=0.96-6.27. (B) p=0.11; HR=2.19, 95%CI=0.67-7.21.
Discussion
EV are easily obtained from peripheral blood, making them a promising target of interest in biomarker research. Especially in the case of GBM, there is a need for additional information, as MR diagnostics are not always able to distinguish between pseudo-progression and recurrent disease (12). Furthermore, MR imaging does not adequately correlate the true neoplastic disease burden, as micro-infiltrative processes are beyond the limits of radiological imaging (13). We initially hypothesized that EV concentration might be a marker for cancer volume. Yet, we did not observe a correlation between the volume of the glioma lesion and EV concentration nor between the volume of glioma lesion, surrounding edema, and EV concentration. An explanation might be the immune-privileged environment of the brain, which is less permeable to the transit of cancer-derived EV from the brain to the blood, whereas data on non-brain cancers, such as breast cancer, demonstrated a correlation between EV concentration and tumor size (14).
Only a few studies have evaluated total EV concentration in the peripheral blood of GBM patients. We were able to observe higher sEV concentrations in recurrent disease and could distinguish pre-operative patients from others using ROC analysis. Koch et al. observed a similar phenomenon, with higher IEV concentrations in patients with progressive disease. Compared to our patients, this cohort was much smaller (11 subjects) and underwent radiochemotherapy (15). However, both studies suggest that EV concentration is correlated with disease status. Summing up, EV concentration might be a potential biomarker for detecting early recurrence. Overall, EV concentration could offer a useful addition to monitoring GBM patients. However, the value of monitoring EV concentration as a prognostic marker is still under debate (15-17). Considering the small number of patients included in previous studies (15-17) and our cohort, as well as the heterogeneity of the results, we require larger data sets and longitudinal, prospective studies before establishing EV concentration as a biomarker in GBM patients. Studies investigating other cancer entities already demonstrated the prognostics value of using EV expressing cancer-related proteins in the peripheral blood of patients (18,19). Monitoring e.g. EGFRvIII expression on EV in GBM patients might offer a similar, refined approach.
EV extracted from the peripheral blood are a mixture of cancer-derived EV and EV from various benign cells (20). We observed an association between sEV concentration and leukocyte count A higher GBM burden is likely associated not only with increased secretion of tumor-derived sEVs into the peripheral blood but also with a cancer-induced pro-inflammatory environment. An elevated number of infiltrating and circulating neutrophil granulocytes is known to be associated with the severity of patients’ disease (21). Combining the simplicity of monitoring EV concentration with subtyping the EVs’ cell of origin, e.g., by flow cytometry, may offer additional insights.
This retrospective study is limited due to the small number of patients (n=36) included. No longitudinal samples of patients were available; therefore, we had to rely on sub-group analysis of different patient groups (pre-operative patients, stable disease, and recurrent disease), further restricting patient numbers in sub-analysis.
Still, our data shed light on the application of EV concentration as a clinical biomarker for patients with GBM. In the absence of a reliable biomarker, GBM treatment does not allow early detection of tumor recurrence and timely monitoring of disease progression. Our results suggest that the concentration of EV in the plasma, together with the ability to characterize their specific cargo, may be useful for diagnosing and monitoring treatment in patients with GBM.
Conclusion
The present study is based on a very small cohort. Accordingly, the results have to be carefully analyzed and cautiously interpreted. However, we could demonstrate that sEV concentration is able to differentiate between patients’ surgical status and is associated with a potential pro-inflammatory metabolism. To what extent a higher EV concentration is associated with a better prognosis should be investigated in larger, prospective studies, as we observed a trend towards longer OS in patients with high lEV and sEV concentrations.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Conceptualization J. B., C. Bi. K. D., V. M., M. S.; methodology J. B. C. Bi., K. D.; software J. B., K. D.; formal analysis J. B., investigation J. B., C. Bi. A. B., V. M., V. R., C. Be., K. M. S. M.; resources C. Bi., A. B.; data curation J. B.; writing original draft preparation J. B., K. D.; visualization J. B., K. D.; writing – review and editing all authors, project supervision J. B., K. D., A. B., V. M., C. B.; project administration J. B., C. B.; funding acquisition J. B., C. B. All Authors have read and agreed to the published version of the manuscript.
Funding
This research project was funded by the Else-Kröner-Fresenius Foundation.
References
- 1.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, Sahm F, Koelsche C, Korshunov A, Olar A, Hartmann C, Reijneveld JC, Wesseling P, Unterberg A, Platten M, Wick W, Herold-Mende C, Aldape K, von Deimling A. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, van den Bent MJ, Hegi ME. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5(3):198–206. doi: 10.1007/s11910-005-0047-7. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi K, Ezrin A, Hadjipanayis C. Small extracellular vesicles as tumor biomarkers for glioblastoma. Mol Aspects Med. 2015;45:97–102. doi: 10.1016/j.mam.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Mondal A, Kumari Singh D, Panda S, Shiras A. Extracellular vesicles as modulators of tumor microenvironment and disease progression in glioma. Front Oncol. 2017;7:144. doi: 10.3389/fonc.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soung YH, Ford S, Zhang V, Chung J. Exosomes in cancer diagnostics. Cancers (Basel) 2017;9(1):8. doi: 10.3390/cancers9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menck K, Bleckmann A, Wachter A, Hennies B, Ries L, Schulz M, Balkenhol M, Pukrop T, Schatlo B, Rost U, Wenzel D, Klemm F, Binder C. Characterisation of tumour-derived microvesicles in cancer patients’ blood and correlation with clinical outcome. J Extracell Vesicles. 2017;6(1):1340745. doi: 10.1080/20013078.2017.1340745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menck K, Bleckmann A, Schulz M, Ries L, Binder C. Isolation and characterization of microvesicles from peripheral blood. J Vis Exp. 2017;(119):55057. doi: 10.3791/55057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camp RL, Dolled-Filhart M, Rimm DL. X-Tile. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 11.Buentzel J, Klemp HG, Kraetzner R, Schulz M, Dihazi GH, Streit F, Bleckmann A, Menck K, Wlochowitz D, Binder C. Metabolomic profiling of blood-derived microvesicles in breast cancer patients. Int J Mol Sci. 2021;22(24):13540. doi: 10.3390/ijms222413540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Ma Y, Wu Z, Xie R, Zeng F, Cai H, Lui S, Song B, Chen L, Wu M. Advanced imaging techniques for differentiating pseudoprogression and tumor recurrence after immunotherapy for glioblastoma. Front Immunol. 2021;12:790674. doi: 10.3389/fimmu.2021.790674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia CM, Toms SA. The role of circulating microRNA in glioblastoma liquid biopsy. World Neurosurg. 2020;138:425–435. doi: 10.1016/j.wneu.2020.03.128. [DOI] [PubMed] [Google Scholar]
- 14.Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, González-Vázquez MC, Chavez-Ocaña S, Jimenez-Villanueva X, Sierra-Martinez M, Salazar EP. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44(3):208–214. doi: 10.1016/j.arcmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Koch CJ, Lustig RA, Yang XY, Jenkins WT, Wolf RL, Martinez-Lage M, Desai A, Williams D, Evans SM. Microvesicles as a biomarker for tumor progression versus treatment effect in radiation/temozolomide-treated glioblastoma patients. Transl Oncol. 2014;7(6):752–758. doi: 10.1016/j.tranon.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans SM, Putt M, Yang XY, Lustig RA, Martinez-Lage M, Williams D, Desai A, Wolf R, Brem S, Koch CJ. Initial evidence that blood-borne microvesicles are biomarkers for recurrence and survival in newly diagnosed glioblastoma patients. J Neurooncol. 2016;127(2):391–400. doi: 10.1007/s11060-015-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osti D, Del Bene M, Rappa G, Santos M, Matafora V, Richichi C, Faletti S, Beznoussenko GV, Mironov A, Bachi A, Fornasari L, Bongetta D, Gaetani P, DiMeco F, Lorico A, Pelicci G. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019;25(1):266–276. doi: 10.1158/1078-0432.CCR-18-1941. [DOI] [PubMed] [Google Scholar]
- 18.Brinch CM, Hogdall E, Heer P, Penninga L, Bæk R, Jorgensen MM, Engelmann BE, Rossen PB, Mortensen HJ, Krarup-Hansen A, Aggerholm-Pedersen N. The prognostic value of plasma small extracellular vesicles’ phenotype in patients with gastrointestinal stromal tumor. Anticancer Res. 2022;42(12):5699–5717. doi: 10.21873/anticanres.16078. [DOI] [PubMed] [Google Scholar]
- 19.Asada T, Nakahata S, Fauzi YR, Ichikawa T, Inoue K, Shibata N, Fujii Y, Imamura N, Hiyoshi M, Nanashima A, Morishita K. Integrin α6A (ITGA6A)-type splice variant in extracellular vesicles has a potential as a novel marker of the early recurrence of pancreatic cancer. Anticancer Res. 2022;42(4):1763–1775. doi: 10.21873/anticanres.15653. [DOI] [PubMed] [Google Scholar]
- 20.Menck K, Sivaloganathan S, Bleckmann A, Binder C. Microvesicles in cancer: Small size, large potential. Int J Mol Sci. 2020;21(15) doi: 10.3390/ijms21155373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massara M, Persico P, Bonavita O, Mollica Poeta V, Locati M, Simonelli M, Bonecchi R. Neutrophils in gliomas. Front Immunol. 2017;8:1349. doi: 10.3389/fimmu.2017.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]