Abstract
Background/Aim
Vancomycin-resistant Enterococcus causes significant morbidity, mortality, and excess healthcare costs when compared to vancomycin-susceptible isolates. Patients with hematological malignancies, especially those who undergo hematopoietic stem cell transplantation, are at a particularly high risk for infections with vancomycin-resistant Enterococcus, with mortality ranging from 40-100%. Linezolid and daptomycin are the two most commonly used antibiotics for treatment of vancomycin-resistant enterococcal infections, however, there has been recent emergence of resistance to these drugs as well.
Case Report
We report the case of a 48-year-old male with hematological malignancy and graft failure post hematopoietic stem cell transplantation complicated by dialysis-dependent acute kidney injury and recurrent neutropenic fevers due to vancomycin-resistant Enterococcus faecium (VREf) bacteremia. Despite central line changes, and strict aseptic precautions, the bacteremia returned, showing resistance to daptomycin and linezolid after the second recurrence. As a final effort, using limited clinical data and in vitro studies, we utilized oritavancin off-label as salvage therapy for refractory VREf bacteremia, with subsequent clearance of blood cultures.
Conclusion
This is a rare case of successful off-label use of oritavancin for recurrent multidrug-resistant VREf bacteremia in a patient with hematological malignancy after undergoing hematopoietic stem cell transplantation. It is important to increase awareness of the potential use of this novel antibiotic with increasing resistance of VREf to first-line agents.
Keywords: Enterococcus faecium, VRE, oritavancin, hematopoietic stem cell transplantation, chronic lymphoblastic leukemia
Enterococci are facultative anaerobic Gram-positive cocci that reside in the intestine and female reproductive organs. Depending on the species the bacteria are typically susceptible to ampicillin and vancomycin, amongst other agents (1). Resistance to ampicillin is intrinsic via the penicillin-binding protein 5 (PBP5) gene and development of beta lactamases (2), whereas vancomycin resistance may be intrinsic, as with E. gallinarum and E. casseliflavus, via the VanC gene, or acquired as with E. faecalis and E. faecium via VanA and VanB (1).
According to a comparative study, infections with vancomycin-resistant Enterococcus (VRE) are associated with greater morbidity, mortality, and healthcare costs when compared to those caused by vancomycin-susceptible isolates (3). Specifically, resistance of Enterococcus species to essential antibiotics in the United States is considered a major problem, with 87.1% of Enterococcus faecium isolates being vancomycin resistant (4). Enterococci are most notorious for causing nosocomial infections in those with a weakened immune system (5). Patients with hematological malignancies, especially those who undergo hematopoietic stem cell transplantation (HSCT), are at a particularly high risk for VRE infection, with mortality ranging from 40-100% (6-8). There has not been enough evidence obtained on mortality rates due to VRE during more recent years when effective VRE therapies have been available. However, it has been observed that there are differences in mortality rates between patients with VRE and those with vancomycin-sensitive Enterococcus due to factors such as comorbid conditions and time to receiving effective therapy (9). Despite first- and second-line medications, there is a high mortality rate, as well as a lack of clinical data and treatment options for VRE. Thus, clinicians are forced to rely on information derived from in vitro studies (10).
Source control has always been the primary method to treat VRE, especially when there are no signs of serious infection, such as infective endocarditis and bacteremia. Notable novel therapies have been developed in recent years to treat VRE-associated infections (11). Linezolid and daptomycin are the two most commonly used antibiotics for treatment of VRE infections, however, there has been recent emergence of resistance to these drugs as well (12,13). One of the promising treatments for multiple drug-resistance in VRE is a novel lipoglycopeptide, oritavancin (14). Using the limited clinical data available, supported with in vitro studies, we hereby present a rare case of a patient with hematological malignancy post HSCT on hemodialysis with recurrent multidrug-resistant VRE successfully treated with oritavancin.
Case Report
A 48-year-old male with TP53-mutated chronic lymphoblastic leukemia was admitted for a haploidentical, allogeneic HSCT with donation from his brother. The patient’s conditioning regimen consisted of fludarabine with melphalan and graft versus host disease prophylaxis with post-transplant cyclophosphamide, mycophenolate mofetil and tacrolimus. The patient’s pertinent pre-transplant serology was positive for herpes simplex virus, Epstein-Barr virus and varicella zoster virus. Cytomegalovirus serology was negative for both donor and recipient. Antimicrobial prophylaxis included acyclovir, ciprofloxacin, and micafungin (changed to voriconazole on day +42 from HSCT due to prolonged neutropenia), with sulfamethoxazole-trimethoprim to start on day +28. The patient also had weekly monitoring of plasma cytomegalovirus, Epstein-Barr virus and human herpes virus 6 by polymerized chain reactions, along with rectal VRE swabs as per institutional protocol.
The post transplant hospital course was complicated by hyperbilirubinemia and acute oliguric renal failure (baseline creatinine 1.0 mg/dl that worsened to 5.2 mg/dl) secondary to cytokine-release syndrome refractory to tocilizumab and dexamethasone. On day +6, a non-tunneled hemodialysis (HD) line was placed, and patient was started on continuous renal replacement therapy (CRRT) per nephrology team. He remained on CRRT with stable urine output and serum creatinine. In addition, the patient demonstrated overall improvement in vital signs and liver function. He remained on CRRT until day +30 before transitioning to intermittent hemodialysis on day +31, and for the remainder of his hospital stay.
On day +31, the patient demonstrated signs of engraftment with an absolute neutrophil count of 500×103 cells/μl but failed to achieve complete count recovery and subsequently the count declined to 0. He remained pancytopenic despite receiving granulocyte colony-stimulating factor, as well as blood and platelet transfusions daily. On day +39, he developed gross hematuria that continued for several days. On day +42, human polyomavirus 1 was detected in his urine (284,000 copies/ml) and he was treated for human polyomavirus 1 cystitis with one dose weekly of intravesicular cidofovir for 3 weeks. Urology was consulted on day +47 for intermittent bladder irrigation. On day +49, the patient was found to have VRE on his rectal swab screen.
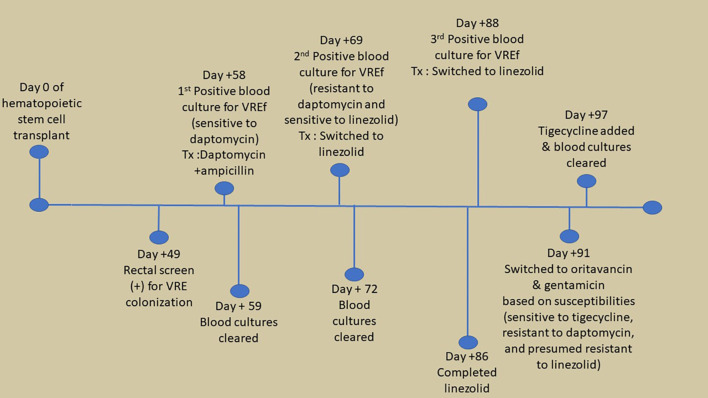
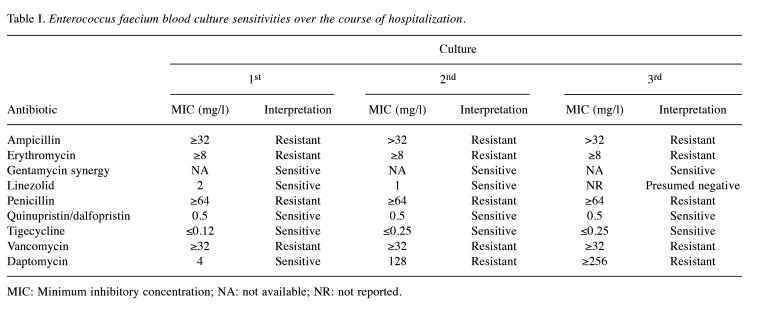
On day +58, the patient developed neutropenic fever and blood cultures [from triple lumen central venous catheter (CVC), non-tunneled HD catheter, and peripheral line] were positive for vancomycin-resistant Enterococcus faecium (VREf) (Figure 1), susceptible to linezolid [minimum inhibitory concentration (MIC)=2 mg/l], tigecycline (MIC ≤0.12 mg/l), gentamicin synergy and dose-dependent susceptible to daptomycin (MIC=4 mg/l) (Table I). The source of his infection was suspected to be translocation from the genitourinary tract in the setting of hematuria and positive urine culture for VRE. The patient was originally treated with high-dose daptomycin (12 mg/kg) every 48 hours plus ampicillin along with removal of the HD catheter and CVC, with a planned duration of 2 weeks of therapy after clearance of bacteremia.
Figure 1.
Timeline of significant events related to recurrent vancomycin-resistant Enteroccous faecium (VREf) bacteremia.
Table I. Enterococcus faecium blood culture sensitivities over the course of hospitalization.
MIC: Minimum inhibitory concentration; NA: not available; NR: not reported.
Bacteremia clearance was documented 24 hours after the initiation of therapy. The tip culture from the HD catheter and CVC were positive for Staphylococcus epidermidis, which was a suspected contaminant. Due to ongoing fever in the setting of profound neutropenia, additional blood cultures were obtained and remained negative, until approximately day 10 of therapy (day +69).
On day +69, the patient again developed neutropenic fever, with a maximum temperature of 38.8˚C and all blood cultures were again positive for VREf (Figure 1), however, the isolate was now also resistant to daptomycin (MIC=128 mg/l), but susceptible to linezolid (MIC=1 mg/l), tigecycline (MIC=0.25 mg/l), quinupristin/dalfopristin (MIC=0.5 mg/l), and gentamycin synergy (Table I). Therapy was switched to 600 mg linezolid every 12 hours on day +70, for a total of 14 days from the first negative blood culture. The source of the recurrent VREf infection was suspected due to be gastrointestinal translocation in the setting of prolonged neutropenia. Computed tomography of the abdomen and pelvis on day +71 was negative for acute pathology, thus all lines including a peripherally inserted central line and HD lines were removed, and the patient was given a 48-hour line holiday. Due to concern for infective endocarditis, a transthoracic echocardiogram was performed on day +71 and was negative for vegetations. Blood cultures cleared on day +72. The patient completed the prespecified linezolid therapy on day +86.
Two days after the completion of therapy, due to new fever, new blood cultures were obtained from his peripherally inserted central line and were found to be positive for VREf (Figure 1). Therapy with linezolid was restarted on day +89-91. This VREf isolate was sensitive to tigecycline (MIC=0.25 mg/l), quinupristin/dalfopristin (MIC=0.5 mg/l), and gentamicin synergy, but resistant to daptomycin and linezolid (Table I). Quinupristin/dalfopristin is not available in the United States. The patient was started on a salvage regimen of daptomycin, ampicillin and tigecycline, pending results of data for susceptibility to oritavancin. Blood culture specimens were sent to an outside laboratory for additional susceptibility testing and revealed that this VREf isolate was sensitive to oritavancin (MIC=0.12 mg/l), and the therapy was changed to oritavancin. The patient was given intravenous oritavancin at 1200 mg every 48 hours (total of three doses), with a single dose of gentamycin at 3 mg/kg. Due to concerns for source control, a transesophageal echocardiogram was recommended to rule out endocarditis but was unable to be done due to pancytopenia. The HD line was again removed on day +93 but blood cultures continued to be positive on days +95-96. Due to persistent bacteremia, oritavancin was changed to 1200 mg weekly, with high-dose tigecycline (100 mg) every 12 hours (days +97-139). In addition, a 2-week course (days +99-113) of oral fosfomycin was started at 3 g every 3 days to attempt decolonization of the gastrointestinal tract. Blood cultures eventually cleared on day +97 and remained negative. Weekly oritavancin was continued at 1,200 mg through day +128. Unfortunately, despite clearance of bacteremia, a repeat bone marrow biopsy on day +125 revealed ongoing primary graft failure. Due to limited options to treat his malignancy, the patient elected to go home with hospice care and further doses of oritavancin were withheld.
Patient consent. The patient voluntarily provided written informed consent to use of their medical information for research purposes and publication.
Discussion
Here we present a patient with multiple risk factors for multidrug-resistant VRE bacteremia. Severe immunosuppression secondary to hematological malignancy, prolonged HSCT-related neutropenia, a prolonged hospitalization stay requiring use of CVCs, and being on multiple prophylactic antimicrobials agents likely contributed to alteration of the gut microbiome towards VRE domination, leading to colonization (15,16). Mucosal disruption related to the immunosuppressive state then allowed bacterial translocation and subsequent recurrent VREf bacteremia.
The primary means of treating VRE bloodstream infections consists of linezolid and daptomycin (15). However, consensus guidelines are lacking, with limited options on how to treat VRE infections resistant to these first-line treatments.
Oritavancin is a long-acting lipoglycopeptide that has proved to act concentration-dependently, with rapid bactericidal activity against Gram-positive bacteria (17). The mechanism of action of this drug involves inhibiting synthesis of the bacterial cell wall and cell membrane disruption, as well as inhibition of bacterial RNA synthesis (18). It is approved by the United States Food and Drug Administration for the treatment of acute bacterial skin and skin structure infections, including methicillin-susceptible and methicillin-resistant Staphylococcus aureus, various streptococci, and vancomycin-susceptible Enterococcus faecalis (5). Fortunately, studies have shown that oritavancin also has potent in-vitro activity against VRE, including VanA-type Enterococcus faecium (19,20). The long half-life of oritavancin (~245 hours) (21) allows for it to be administered as a single intravenous dose based on data comparing its efficacy with 7-10 day courses of intravenous vancomycin in treating Gram-positive bacterial skin and skin structure infections (22,23). However, due to the lack of standardized guidelines on proper dosing in off-label uses, different regimens have been utilized as salvage therapy (24-27).
Our patient was severely immunocompromised with lack of marrow recovery due to graft failure and was developing recurrent VREf bacteremia with increasing resistance to multiple antibiotics, with limited therapeutic options. Although the third VREf strain retained susceptibility to tigecycline, using this drug as a monotherapy is generally not recommended due to its high volume of distribution and low serum concentration (15). Further complicating management was the patient’s ongoing need for renal replacement therapy, requiring multiple removal and re-insertion of catheters, and the inability to obtain a transesophageal echocardiogram to rule out infective endocarditis, thus lack of adequate source control was suspected. As a result, it was decided that a single dose of oritavancin would not be adequate, and an alternative dosing regimen was chosen. We chose to give 1200 mg oritavancin intravenously every 48 hours (total of 3 doses) followed by weekly doses of 1200 mg. This dosing regimen was based on a previously reported case of recurrent daptomycin-resistant VREf bacteremia due to prosthetic valve endocarditis, in which clearance of bacteremia was achieved after receiving 10 weeks of oritavancin, in conjunction with prosthetic valve replacement (14).
Our patient developed acute renal failure requiring hemodialysis throughout their hospital course, making it difficult to determine the optimal dosing of oritavancin. Although oritavancin does not require dose adjustment in patients with mild to moderate renal insufficiency, pharmacokinetic data are lacking in patients with severe renal impairment, including those on hemodialysis (25). In addition, this drug cannot be removed by hemodialysis (18).
Oritavancin has been found to have minimal adverse effects (28) and with our patient’s recurrent bacteremia, and limited treatment options, we believed the benefits of giving it at a continued weekly dose outweighed the risks. Fortunately, our patient tolerated the antibiotic without any adverse effects. To our knowledge, only one other case on the successful use of weekly-dosed oritavancin in the treatment of VREf has been reported for bacteremia related to hepatic abscesses in a patient on chronic hemodialysis (29). We are one of the first to report the safety and tolerability of oritavancin for VREf bacteremia in a patient with severe renal impairment. Nevertheless, randomized control trials are needed to evaluate the optimal dosing schedule of oritavancin in off-label uses and in patients with severe renal disease.
Another highlight of our case was the use of oritavancin specifically in a patient with a hematological malignancy (chronic lymphoblastic leukemia) after undergoing HSCT. There have been very few, but similar cases, which also had a hematological malignancy and were treated with oritavancin for VREf bacteremia. The first case involved a patient with myelodysplastic syndrome, but who had not undergone HSCT, who developed VREf bacteremia likely due to bacterial gut translocation (24). Daptomycin susceptibility testing was not available, so they were initially treated with linezolid, which was later discontinued due to thrombocytopenia. Their antibiotic regimen was then switched to single-dose oritavancin in combination with intravenous fosfomycin, with successful clearing of their bacteremia. Intravenous fosfomycin was added due to its reported in vitro synergism with oritavancin (24). Unfortunately, intravenous fosfomycin was not available at our institution, which is why we used its oral formulation to attempt decolonization of the gastrointestinal tract to prevent recurrent VREf bacteremia. Another case of recurrent VREf bacteremia with meningitis was successfully treated with oritavancin after similarly undergoing HSCT for leukemia (30). They were on multiple concurrent antibiotics (including gentamycin and tigecycline, like our patient) in conjunction with oritavancin due to recurrent bacteremia and concerns for insufficient cerebrospinal fluid penetration. Gastrointestinal decontamination of the VREf was also attempted in that case but oral bacitracin was used instead of oral fosfomycin (30).
With increasing cases of multidrug-resistant VRE worldwide, especially among patients with HSCT, more research looking at further therapeutic options for these complicated infections is needed (15,16).
Conclusion
This is a rare case of successful off-label use of oritavancin for recurrent multidrug-resistant VREf bacteremia in a patient with hematological malignancy after undergoing HSCT. With increasing resistance of VREf to first-line agents, it is important to increase awareness of the potential utility of this novel antibiotic.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
Samuel Jalali: Writing - original draft, review and editing. Hetanshi Bhatt: Writing - original draft. Keval Thakkar: Writing - original draft. Rohan Julka: Writing - original draft, review and editing. Yanina Pasikhova: Patient assessment and treatment; Writing - original draft. Neha Verma: Writing - review and editing.
References
- 1.Gold HS. Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis. 2001;33(2):210–219. doi: 10.1086/321815. [DOI] [PubMed] [Google Scholar]
- 2.Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther. 2014;12(10):1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam S, Singer C, Tucci V, Morthland VH, Pfaller MA, Isenberg HD. The challenge of vancomycin-resistant enterococci: A clinical and epidemiologic study. Am J Infect Control. 1995;23(3):170–180. doi: 10.1016/0196-6553(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 4.Edelsberg J, Weycker D, Barron R, Li X, Wu H, Oster G, Badre S, Langeberg WJ, Weber DJ. Prevalence of antibiotic resistance in US hospitals. Diagn Microbiol Infect Dis. 2014;78(3):255–262. doi: 10.1016/j.diagmicrobio.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Oritavancin [package insert] Lincolnshire, Melinta therapeutics, LLC. 2014 [Google Scholar]
- 6.Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, Young JW, Kiehn TE, Zuccotti G. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13(5):615–621. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 7.Worth LJ, Thursky KA, Seymour JF, Slavin MA. Vancomycin-resistant Enterococcus faecium infection in patients with hematologic malignancy: patients with acute myeloid leukemia are at high-risk. Eur J Haematol. 2007;79(3):226–233. doi: 10.1111/j.1600-0609.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 8.Zirakzadeh A, Gastineau DA, Mandrekar JN, Burke JP, Johnston PB, Patel R. Vancomycin-resistant enterococcal colonization appears associated with increased mortality among allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41(4):385–392. doi: 10.1038/sj.bmt.1705912. [DOI] [PubMed] [Google Scholar]
- 9.Prematunge C, MacDougall C, Johnstone J, Adomako K, Lam F, Robertson J, Garber G. VRE and VSE bacteremia outcomes in the era of effective VRE therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37(1):26–35. doi: 10.1017/ice.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzler E, Santarossa M, Meyer KA, Harrington AT, Reid GE, Clark NM, Albarillo FS, Bulman ZP. In vitro pharmacodynamic analyses help guide the treatment of multidrug-resistant Enterococcus faecium and carbapenem-resistant enterobacter cloacae bacteremia in a liver transplant patient. Open Forum Infect Dis. 2020;7(1):ofz545. doi: 10.1093/ofid/ofz545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217–230. doi: 10.2147/IDR.S54125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamboj M, Cohen N, Gilhuley K, Babady NE, Seo SK, Sepkowitz KA. Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol. 2011;32(4):391–394. doi: 10.1086/659152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olearo F, Both A, Belmar Campos C, Hilgarth H, Klupp EM, Hansen JL, Maurer FP, Christner M, Aepfelbacher M, Rohde H. Emergence of linezolid-resistance in vancomycin-resistant Enterococcus faecium ST117 associated with increased linezolid-consumption. Int J Med Microbiol. 2021;311(2):151477. doi: 10.1016/j.ijmm.2021.151477. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi: 10.1093/ofid/ofv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benamu E, Deresinski S. Vancomycin-resistant enterococcus infection in the hematopoietic stem cell transplant recipient: an overview of epidemiology, management, and prevention. F1000Res. 2018;7:3. doi: 10.12688/f1000research.11831.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krull M, Klare I, Ross B, Trenschel R, Beelen DW, Todt D, Steinmann E, Buer J, Rath PM, Steinmann J. Emergence of linezolid- and vancomycin-resistant Enterococcus faecium in a department for hematologic stem cell transplantation. Antimicrob Resist Infect Control. 2016;5:31. doi: 10.1186/s13756-016-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kmeid J, Kanafani ZA. Oritavancin for the treatment of acute bacterial skin and skin structure infections: an evidence-based review. Core Evid. 2015;10:39–47. doi: 10.2147/CE.S51284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal S, Decano AG, Bandali A, Lai D, Malat GE, Bias TE. Oritavancin (orbactiv): A new-generation lipoglycopeptide for the treatment of acute bacterial skin and skin structure infections. P T. 2018;43(3):143–179. [PMC free article] [PubMed] [Google Scholar]
- 19.Morrissey I, Seifert H, Canton R, Nordmann P, Stefani S, Macgowan A, Janes R, Knight D, Oritavancin Study Group Activity of oritavancin against methicillin-resistant staphylococci, vancomycin-resistant enterococci and -haemolytic streptococci collected from western European countries in 2011. J Antimicrob Chemother. 2013;68(1):164–167. doi: 10.1093/jac/dks344. [DOI] [PubMed] [Google Scholar]
- 20.Sefali S, Nakipoglu Y. Efficacy of oritavancin and nisin alone and their combination against vancomycin resistant enterococci strains in hospitalized patients in Turkiye. Indian J Med Microbiol. 2024;47:100489. doi: 10.1016/j.ijmmb.2023.100489. [DOI] [PubMed] [Google Scholar]
- 21.Moenster RP, Wallace-Lacey A, Western H, Tiefenaur S, Abdulbasir A, Alberts J, Doty J, Abner H, Skouby D, Lorenz M, Fong R, Arora J, Linneman TW. Oritavancin vs. standard of care for treatment of nonendovascular gram-positive bloodstream infections. Open Forum Infect Dis. 2023;10(11):ofad411. doi: 10.1093/ofid/ofad411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corey GR, Good S, Jiang H, Moeck G, Wikler M, Green S, Manos P, Keech R, Singh R, Heller B, Bubnova N, O’Riordan W, SOLO II Investigators Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254–262. doi: 10.1093/cid/ciu778. [DOI] [PubMed] [Google Scholar]
- 23.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O’riordan W. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 24.Di Cecco C, Monticelli J, Di Bella S, Di Maso V, Luzzati R. Vancomycin-resistant enterococcus bloodstream infection successfully managed with oritavancin and fosfomycin as sequential treatment. J Chemother. 2024;36(1):31–34. doi: 10.1080/1120009X.2023.2247205. [DOI] [PubMed] [Google Scholar]
- 25.Lupia T, De Benedetto I, Bosio R, Shbaklo N, De Rosa FG, Corcione S. Role of oritavancin in the treatment of infective endocarditis, catheter- or device-related infections, bloodstream infections, and bone and prosthetic joint infections in humans: narrative review and possible developments. Life (Basel) 2023;13(4):959. doi: 10.3390/life13040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer J, Lata P, Barnett S. Continued dosing of oritavancin for complicated gram-positive infections. Fed Pract. 2020;37(11):502–504. doi: 10.12788/fp.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scoble PJ, Reilly J, Tillotson GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46–54. doi: 10.1007/s40801-020-00194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messina JA, Fowler VG Jr, Corey GR. Oritavancin for acute bacterial skin and skin structure infections. Expert Opin Pharmacother. 2015;16(7):1091–1098. doi: 10.1517/14656566.2015.1026256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzitelli M, Scaglione V, Cattarin L, Franchin E, Stano P, Paci L, Coppi M, Rossolini GM, Mengato D, Calò L, Cattelan AM. Off-label oritavancin treatment outcome and molecular characterization of a vancomycin- and linezolid-resistant Enterococcus faecium causing liver abscesses. J Antimicrob Chemother. 2024;79(3):689–691. doi: 10.1093/jac/dkad410. [DOI] [PubMed] [Google Scholar]
- 30.Wenzler E, Adeel A, Wu T, Jurkovic M, Walder J, Ramasra E, Campion M, Cerny J, Theodoropoulos NM. Inadequate cerebrospinal fluid concentrations of available salvage agents further impedes the optimal treatment of multidrug-resistant Enterococcus faecium meningitis and bacteremia. Infect Dis Rep. 2021;13(3):843–854. doi: 10.3390/idr13030076. [DOI] [PMC free article] [PubMed] [Google Scholar]