Abstract
Background/Aim
To evaluate the difficulty of laparoscopic adrenalectomy by investigating the usefulness of the Mayo Adhesive Probability (MAP) score for assessing adherent perinephric fat and its correlation with histological reality.
Patients and Methods
We retrospectively evaluated 103 patients who underwent laparoscopic adrenalectomies. Based on preoperative computed tomography images, the patients were categorized into two groups: high (3-5 points) and low MAP (0-2 points). Clinical characteristics and perioperative data were compared between the two groups. Additionally, we analyzed the pathological tissue of the tumor and surrounding fat using hematoxylin-eosin-saffron staining.
Results
Compared with the low MAP group, the high MAP group had younger patients (59 vs. 62 years, p=0.097), more male patients (93.3% vs. 44.3%, p<0.001), and higher body mass indices (26.4 vs. 23.8, kg/m2, p=0.029). The MAP group experienced a significantly higher estimated blood loss compared to the low MAP group (10 vs. 52.3, ml, p=0.047). Tumor and adhering perirenal fat tissues of pheochromocytoma, adrenal carcinoma, and metastatic adrenal tumors exhibited significantly higher expression of vascular endothelial growth factor and cluster of differentiation 204 compared to the low MAP group (p<0.001). Additionally, both proteins were highly expressed in the adhering perirenal fat in the high MAP group (p=0.020, p=0.015).
Conclusion
Patients with a preoperative MAP score ≥3, pheochromocytoma, or malignant tumor had a high risk of increased intraoperative blood loss. Strict perioperative management should be performed in such cases.
Keywords: Adrenal tumor, adhering perirenal fat, Mayo adhesive probability score, laparoscopic adrenalectomy, estimated blood loss
Owing to its notable benefits in terms of reduced invasiveness compared with open surgery, laparoscopic adrenalectomy (LA) (1) is recognized as the gold standard surgical treatment for the management of benign adrenal tumors and pheochromocytomas requiring surgical resection. Although there is no definitive upper limit of tumor size for LA, it is recommended to consider tumors measuring ≤12 cm owing to technical challenges and the possibility of malignancy (2).
During LA, there are risks of perioperative complications of Clavien-Dindo grades ≥2, with incidence ranging from 4.4% to 8.4%. The most common complication is bleeding, which requires blood transfusion in approximately 0.8%-1.2% of cases (3,4). To reduce such perioperative complications, it is essential to predict perioperative complications based on preoperative conditions. Previous studies have reported that factors predicting perioperative complications are related to obesity, tumor size, tumor type, and perinephric fat content (4-7). A previous study reported that a scoring system called the Mayo Adhesive Probability (MAP) score focusing on the adhering perirenal fat (APF) was associated with perioperative results in robot-assisted partial nephrectomy (8). In LA, the MAP score may be useful as it is necessary to approach the perinephric fat to remove adrenal tumors surrounded by the perinephric fat. Since adrenal tumors are located within the perirenal fat, it would be beneficial if the MAP score was a predictor of perioperative complications in LA. In recent years, the usefulness of the MAP score in predicting surgical outcomes in partial and donor nephrectomies has been reported (8-10); however, few reports on its correlation with LA have yet been published (11,12). In addition, APF, which is caused by inflammation (13), may be histologically associated with adrenal tumors.
Therefore, this study aimed to retrospectively evaluate MAP score and other predictive factors for APF along with their association with complications. Additionally, we sought to establish a correlation between surgical assessment and histological confirmation of APF.
Patients and Methods
Study cohort and patient selection. The Ethics Committee of Nara Medical University approved this retrospective study (reference ID: 3273). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki (2013). Our team at Nara Medical University pathologically examined 121 patients with functional or nonfunctional adrenal adenoma, pheochromocytoma, adrenocortical cancer, or adrenal metastasis who underwent adrenalectomy between February 2010 and November 2021. Before surgery, all patients underwent computed tomography (CT) and endocrine laboratory examinations at our hospital. We excluded 10 patients because of missing data, six because they underwent open surgery instead of LA, and two because they had bilateral adrenal tumors. A total of 103 patients (85%) who underwent LA were included in the analysis.
Surgeries were performed by experienced urologists in our department, all using the same technique. We collected various clinical variables including patient age, sex, BMI, approach method, history of hypertension, diabetes, abdominal surgery, size and side of the tumor, and diagnostic classifications, such as primary aldosteronism and pheochromocytoma. Surgical outcomes including operative time, estimated blood loss, duration of postoperative hospital stay, complications, and pathological results, were collected from the medical records.
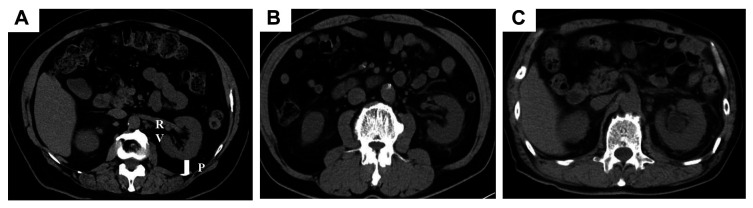
Definition of MAP score. Before surgery, the MAP score was determined through CT and a urologist evaluated this score independently. The MAP score was calculated using two parameters: thickness of the posterior perinephric fat and perinephric stranding type on the ipsilateral side (8). We measured the thickness of the posterior perinephric fat at the renal vein level and assigned a score of 0 for <1.0 cm, 1 for 1.0-2.0 cm, and 2 for ≥2.0 cm. Perinephric fat stranding was scored as 0, 2, or 3 for none, mild, or severe, respectively (Figure 1). The scores for both variables were added to obtain the MAP score. The resulting scores fell within the range of 0-5.
Figure 1.
Representative computed tomography images used to evaluate the Mayo Adhesive Probability score. The method for measuring the amount of fat around the kidney at the location of the renal vein is as follows: (A) None: 0 points. The tissue surrounding the kidney appears completely black on this computed tomography image, with no visible fat stranding. (B) Mild/moderate (type 1): 2 points. The image shows dense areas around the kidneys, but there are no signs of inflammation. (C) Severe stranding (type 2): 3 points. The image depicts a significant accumulation of tissue around the kidney, with thick, dense bars indicating inflammation. P: Posterior perinephric fat thickness (cm); RV: renal vein.
Pathological tissue analysis. Tissues of adrenal tumors and APF that were operated on were fixed, embedded in paraffin, and sliced into 5 mm sections. The sections were then stained with hematoxylin-eosin-saffron (HES) for analysis. Antibodies against vascular endothelial growth factor (VEGF) and cluster of differentiation 204 (CD204) were used to analyze macrophage infiltration due to angiogenesis and inflammation. Slides were incubated overnight at 4˚C with antibodies against VEGF (cat. no. sc-152; 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and CD204 (cat. no. KT022; 1:2,000; Trans Genic, Kobe, Japan). HES-stained sections were examined, and at least three views for each slide were captured at ×100 and ×400 magnification using a light microscope (EVOS® FL Auto Cell Imaging System, Thermo Fisher Scientific). Cytoplasmic and membranous staining of tumor cells and adipocytes were considered positive. The Histochemical scoring (H-score) system, which involves multiplying the intensity score (0-3) by the percentage of stained cells (0-100), was used to classify the specimens into four categories as follows: negative (0; H-score 0-14), weakly positive (1+; H-score 15-99), moderately positive (2+; H-score 100-199), and strongly positive (3+; H-score 200-300) (14).
Statistical analysis. Continuous variables are presented as medians and interquartile ranges. Categorical variables are presented as numbers and percentages. Comparisons between groups were made using the Mann-Whitney U-test, Fisher’s exact test, or the chi-square test, as appropriate. Logistic regression analysis was performed to evaluate these factors. Statistical analysis was performed using EZR statistical software, which is based on the open-source R statistical software (v. 3.0.2) and Prism software 7.00 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was set at p<0.05.
Results
Baseline characteristics. In total, 103 patients who underwent LA were included in the analysis. The clinical characteristics of the patients are summarized in Table I. The 103 patients were categorized into two groups based on their MAP score: low MAP (score <3) and high MAP (score ≥3). There were 88 (85.4%) and 15 (15.6%) patients in the low MAP and high MAP groups, respectively.
Table I. Patients and tumor characteristics.
IQR: Interquartile range. at-test; bFisher’s exact test.
Compared with the low MAP group, significantly fewer abdominal surgeries (34.1% vs. 0%, p=0.005) and females (55.7% vs. 6.7%, p<0.001) were observed in the high MAP group. The high MAP group had higher BMI values (23.8 vs. 26.4, kg/m2, p=0.029) and a higher incidence of hypertension (56.8% vs. 86.7%, p=0.043).
MAP scores and perioperative results. The association between the MAP score and perioperative outcomes of LA is shown in Table II. The high MAP group had a significantly greater estimated blood loss (EBL) than the low MAP group (10 vs. 52.3 ml, p=0.047). The operation time in the high MAP group tended to be longer than that in the low MAP group (173.3 vs. 200.7 min, p=0.081). There was no difference in Clavien-Dindo grade <3 between the two groups, and no Clavien-Dindo grade ≥3 was observed in either group.
Table II. Perioperative outcomes according to the two risk groups based on Mayo Adhesive Probability (MAP) score.
at-test; bFisher’s exact test.
Factors affecting intraoperative EBL. Based on EBL, all patients were divided into two groups: low EBL (<100 ml) and high EBL (≥100 ml). The results of univariate and multivariate logistic analyses are presented in Table III. In univariate analysis, tumor diameter ≥3 cm (OR=2.55, p=0.060), tumor type (OR=2.89, p=0.032), and MAP score ≥3 (OR=3.00, p=0.065) tended to be correlated with bleeding amount ≥100 ml. In multivariate analysis, there were significant differences in tumor type (OR=3.16, p=0.046) and MAP score ≥3 (OR=4.43, p=0.026) in terms of bleeding amount ≥100 ml.
Table III. Univariable and multivariable analysis evaluating the correlation of clinical parameters with estimated blood loss.
MAP: Mayo Adhesive Probability.
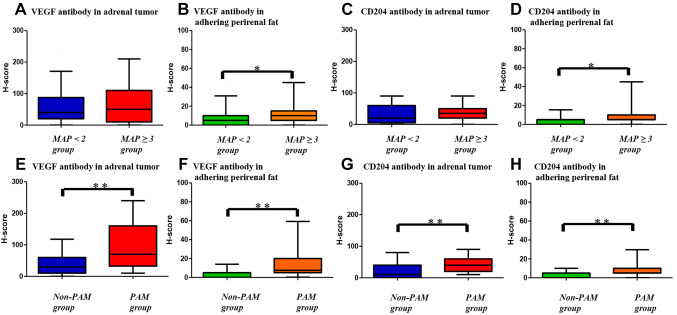
Histological analysis. Tumors pathologically diagnosed as pheochromocytoma, adrenal carcinoma, or metastatic adrenal tumors were classified as the PAM group, whereas the others were classified as the non-PAM group. Based on the tissue analysis of the tumors and APF of the 103 enrolled patients, 40 and 63 patients were categorized into the PAM and non-PAM groups, respectively. In the HES staining, VEGF and CD204 expression in the tumor and APF tissues were compared between the PAM and non-PAM groups or the low- and high-MAP groups using the H-score (Figure 2). The tumor and APF tissues of the PAM group showed significantly higher expression of VEGF and CD204 than the non-PAM group (p<0.001). Furthermore, the high MAP group showed significantly higher expression of VEGF and CD204 in APF tissues than the low MAP group (p=0.020, p=0.015, Figure 3).
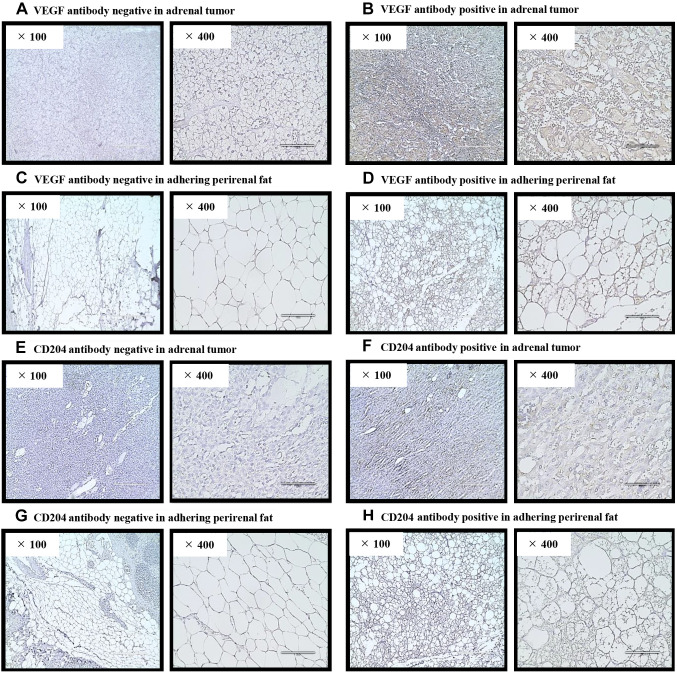
Figure 2.
Representative images of immunohistochemistry. Pathological images (×100 and ×400) of VEGF or CD204 antibodies in adrenal tumors and adhering perirenal fat were obtained using a light microscope. (A) VEGF antibody negativity in the adrenal tumors. (B) VEGF antibody positivity in adrenal tumors. (C) VEGF antibody-negativity in adhering perirenal fat. (D) VEGF antibody positivity in adhering perirenal fat. (E) CD204 antibody negativity in the adrenal tumors. (F) CD204 antibody positivity in adrenal tumors. (G) CD204 antibody-negativity in adhering perirenal fat. (H) CD204 antibody positivity in adhering perirenal fat.
Figure 3.
A, E) VEGF antibody expression in adrenal tumor tissues. B, F) VEGF antibody expression in adhering perirenal fat tissues. C, G) CD204 antibody expression in adrenal tumor tissues. D, H) Comparison of CD204 antibody expression in adhering perirenal fat between MAP <2 and MAP ≥3 groups or PAM and non-PAM groups using Mann-Whitney U-test. *p<0.05, **p<0.001 vs. two groups.
Discussion
As LA for adrenal tumors becomes more common, the predictive factors influencing surgical outcomes play an important role. In this study, we showed that MAP score was related to intraoperative bleeding in LA. Additionally, we identified pathological differences within the APF, which are risk factors for intraoperative bleeding, depending on the tumor type and MAP score. These results may serve as predictive tools for intraoperative bleeding in LA.
Generally, the adrenal gland is surrounded by perinephric fat on the medial side. Consequently, in LA, it is necessary to approach the perinephric fat to remove adrenal tumors. Therefore, it is crucial to preoperatively assess surgical difficulty and risk and systematically evaluate surgical complexity. Abundant visceral fat and obesity are recognized as adverse conditions for LA and have been reported to lead to increased surgical time and complications (15,16). However, the APF reflects the complexity of surgery more accurately than measures of overall body fat, such as obesity or abdominal fat (11,12). APF may make it difficult to remove the perinephric fat from the kidneys, resulting in poor surgical field exposure; increased surgical difficulty, potentially increased operative time; and complications.
The biological role of VEGF has been implicated in neovascularization and angiogenesis. Many studies have suggested that VEGF is a central mediator of wound repair associated with tissue damage, inflammation, and immune responses (17). Additionally, CD204-expressing tumor-associated macrophages may promote angiogenesis, contributing to the composition of a specific microenvironment for tumor progression by interacting with cancer cells and other stromal cells (18). CD204 and VEGF are factors that contribute to angiogenesis and may be involved in bleeding in LA. In this study, we performed immunostaining for VEGF and CD204 expressed in tumor and APF tissues and found that both were expressed at significantly higher levels in the PAM group than in the non-PAM groups (p<0.001). Additionally, APF tissues of the high MAP group showed significantly higher expression of VEGF and CD204 than those of the low MAP group (p=0.020, p=0.015, Figure 3). Changes in the tumor microenvironment and APF may cause intraoperative bleeding depending on the tumor type and MAP score.
The results of this study showed that intraoperative EBL was higher in patients with a MAP score ≥3, pheochromocytoma, adrenal carcinoma, or metastatic adrenal tumor. In their study, Wei et al. reported that the higher the MAP score, the greater the amount of intraoperative EBL and the greater the difference in hemoglobin levels before and after surgery (11). Based on the findings of these studies, patients with high MAP scores might exhibit a correlation with increased EBL. In Japan, robot-assisted adrenalectomy (RAA) has been covered by insurance since 2022. RAA offers several advantages including improved vision and mobility and has been linked to reduced blood loss and shorter surgical times (19). However, the higher cost of RAA is a disadvantage; Bruneau pointed out that the cost of RAA was 2.3 times higher than that of LA at their center and that it was difficult to perform RAA in all patients (20,21). Provided that LA is a relatively safe procedure with few complications, its lower cost compared to RAA makes it a more practical choice for patients, despite the advantages of RAA. The selection of patients eligible for RAA should be compared with those eligible for LA. However, based on the results of our study, patients with high MAP scores and pheochromocytoma with high intraoperative blood loss may be candidates for RAA.
Our study had several limitations including its retrospective nature, relatively small cohort size, and single-institutional design. For example, more patients had a MAP score of 0 and only a few had a score of 5, possibly due to limited sample volume or individual interpretation errors in the imaging data. Most of the patients in our study underwent the intraperitoneal approach; therefore, we were unable to discuss differences between surgical approaches. Immunostaining was performed to assess the risk of complications. However, due to the small number of pathological types, we can only present results for each group individually. Therefore, further verification using expanded data and more patients is necessary to validate the results of this study.
Conclusion
We demonstrated that patients with a preoperative MAP score ≥3, pheochromocytoma, adrenal carcinoma, or metastatic adrenal tumor had a high risk of increased intraoperative blood loss. Additionally, we pathologically demonstrated that VEGF and CD204 were highly expressed in the APF tissues of these patients. Strict perioperative management should be performed in such cases.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Tatsuki Miyamoto: Project development, Data collection, Data analysis, Manuscript writing. Shunta Hori: Project development, Data collection, Manuscript editing. Sayuri Onishi: Data collection. Mitsuru Tomizawa: Data collection. Takuto Shimizu: Data collection. Kenta Onishi: Data collection. Yosuke Morizawa: Data collection. Daisuke Gotoh: Data collection. Yasushi Nakai: Data collection. Makito Miyake: Data collection. Kazumasa Trimoto: Data collection. Nobumichi Tanaka: Data collection. Kiyohide Fujimoto: Data collection, Manuscript editing.
Acknowledgements
The Authors express their gratitude to all the patients who participated in this study for their valuable contributions. The Authors would also like to thank the numerous urologists who provided invaluable assistance in obtaining and summarizing the data utilized in this study.
References
- 1.Ball MW, Hemal AK, Allaf ME. International Consultation on Urological Diseases and European Association of Urology International Consultation on Minimally Invasive Surgery in Urology: laparoscopic and robotic adrenalectomy. BJU Int. 2017;119(1):13–21. doi: 10.1111/bju.13592. [DOI] [PubMed] [Google Scholar]
- 2.Gumbs AA, Gagner M. Laparoscopic adrenalectomy. Best Pract Res Clin Endocrinol Metab. 2006;20(3):483–499. doi: 10.1016/j.beem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Shariq OA, Bews KA, McKenna NP, Dy BM, Lyden ML, Farley DR, Thompson GB, McKenzie TJ, Habermann EB. Is same-day discharge associated with increased 30-day postoperative complications and readmissions in patients undergoing laparoscopic adrenalectomy. Surgery. 2021;169(2):289–297. doi: 10.1016/j.surg.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Scholten A, Chomsky-Higgins K, Nwaogu I, Gosnell JE, Seib C, Shen WT, Suh I, Duh QY. Risk factors associated with perioperative complications and prolonged length of stay after laparoscopic adrenalectomy. JAMA Surg. 2018;153(11):1036–1041. doi: 10.1001/jamasurg.2018.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zonča P, Bužga M, Ihnát P, Martínek L. Retroperitoneoscopic adrenalectomy in obese patients: is it suitable. Obes Surg. 2015;25(7):1203–1208. doi: 10.1007/s11695-014-1475-8. [DOI] [PubMed] [Google Scholar]
- 6.Christakis I, Ng CS, Chen C, Yiin YH, Grubbs EG, Perrier ND, Lee JE, Graham PH. Operation duration and adrenal gland size, but not BMI, are correlated with complication rate for posterior retroperitoneoscopic adrenalectomy for benign diseases. Surgery. 2019;165(3):637–643. doi: 10.1016/j.surg.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ersöz F, Erbil Y, Sarı S, Salmaslıoğlu A, Işsever H, Aral F, Özarmağan S. Predictive value of retroperitoneal fat area measurement for detecting metabolic syndrome in patients undergoing adrenalectomy. World J Surg. 2011;35(5):986–994. doi: 10.1007/s00268-011-1012-z. [DOI] [PubMed] [Google Scholar]
- 8.Davidiuk AJ, Parker AS, Thomas CS, Leibovich BC, Castle EP, Heckman MG, Custer K, Thiel DD. Mayo adhesive probability score: an accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur Urol. 2014;66(6):1165–1171. doi: 10.1016/j.eururo.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 9.Cockerill KJ, Kahn AE, Young SM, Ball CT, Mai ML, Taner CB, Perry DK, Thiel DD. Mayo Adhesive Probability (MAP) score of non-donated kidney aids in predicting post-operative renal function following donor nephrectomy. BMC Urol. 2020;20(1):124. doi: 10.1186/s12894-020-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Y, Xu Y, Gu L, Liu K, Li P, Xuan Y, Gao Y, Zhang X. The Mayo adhesive probability score predicts longer dissection time during laparoscopic partial nephrectomy. J Endourol. 2020;34(5):594–599. doi: 10.1089/end.2019.0687. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Fang Q, Ding S, Wu X, Zhang P, Cao J, Wu D. The adhesive perinephric fat score is correlated with outcomes of retroperitoneal laparoscopic adrenalectomy for benign diseases. World J Surg. 2022;46(11):2687–2694. doi: 10.1007/s00268-022-06671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y, Feng H, Kang Z, Xie Y, Zhang X, Zhang Y. Mayo adhesive probability score is associated with perioperative outcomes in retroperitoneal laparoscopic adrenalectomy. ANZ J Surg. 2022;92(12):3273–3277. doi: 10.1111/ans.17983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khene ZE, Peyronnet B, Mathieu R, Fardoun T, Verhoest G, Bensalah K. Analysis of the impact of adherent perirenal fat on peri-operative outcomes of robotic partial nephrectomy. World J Urol. 2015;33(11):1801–1806. doi: 10.1007/s00345-015-1500-0. [DOI] [PubMed] [Google Scholar]
- 14.McClelland RA, Finlay P, Walker KJ, Nicholson D, Robertson JF, Blamey RW, Nicholson RI. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990;50(12):3545–3550. [PubMed] [Google Scholar]
- 15.Dancea HC, Obradovic V, Sartorius J, Woll N, Blansfield JA. Increased complication rate in obese patients undergoing laparoscopic adrenalectomy. JSLS. 2012;16(1):45–49. doi: 10.4293/108680812X13291597715862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazaure HS, Roman SA, Sosa JA. Obesity is a predictor of morbidity in 1,629 patients who underwent adrenalectomy. World J Surg. 2011;35(6):1287–1295. doi: 10.1007/s00268-011-1070-2. [DOI] [PubMed] [Google Scholar]
- 17.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105(1):1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandao LF, Autorino R, Laydner H, Haber GP, Ouzaid I, De Sio M, Perdonà S, Stein RJ, Porpiglia F, Kaouk JH. Robotic versus laparoscopic adrenalectomy: a systematic review and meta-analysis. Eur Urol. 2014;65(6):1154–1161. doi: 10.1016/j.eururo.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Probst KA, Ohlmann CH, Saar M, Siemer S, Stöeckle M, Janssen M. Robot-assisted vs. open adrenalectomy: evaluation of cost-effectiveness and peri-operative outcome. BJU Int. 2016;118(6):952–957. doi: 10.1111/bju.13529. [DOI] [PubMed] [Google Scholar]
- 21.Brunaud L, Bresler L, Ayav A, Zarnegar R, Raphoz A, Levan T, Weryha G, Boissel P. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy. Am J Surg. 2008;195(4):433–438. doi: 10.1016/j.amjsurg.2007.04.016. [DOI] [PubMed] [Google Scholar]