Abstract
Background/Aim
Non-small-cell lung cancer (NSCLC) is the most frequently diagnosed malignancy and the first cause of cancer-related death. Thus, finding alternative therapeutic options is crucial. Drug repurposing offers therapeutic options in a simplified and affordable manner, especially to cancer patients in developing countries. Several drugs including antihistamines and beta-adrenergic receptor blockers (beta-blockers) display antiproliferative properties on cancer cells. Interestingly, NSCLC patients who had used either antihistamines or beta-blockers showed improved response to chemotherapy or reduced mortality in comparison to non-users of any of these drugs. However, combination therapy is gaining substantial interest in many cancers including non-EGFR mutated NSCLC. Here, we investigated the antineoplastic effect of the combination of the antihistamine loratadine, the beta-blocker nebivolol, and the tyrosine-kinase inhibitor gefitinib on NSCLC cell lines.
Materials and Methods
A-549 and NCI-H1975 cell lines were used. The effect of nebivolol, gefitinib, and loratadine on the metabolic activity was studied using the MTT assay. The inhibitory concentrations (IC20 and IC50) were calculated and used in the drug-combination experiments. Apoptosis was investigated using flow cytometry; and cell survival using the colony formation assay.
Results
The combination nebivolol-loratadine-gefitinib produced a significant synergistic effect on inhibiting the metabolic activity and colony formation, as well as on promoting apoptosis in both cell lines. Noteworthy, the effect on the cell line carrying the EGFR mutation (NCI-H1975) was very similar to the cell line that does not exhibit such mutation (A-549 cells).
Conclusion
The nebivolol-gefitinib-loratadine combination may be a promising alternative for lung cancer treatment.
Keywords: Nebivolol, loratadine, non-small-cell lung cancer, drug combination, drug repurposing
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC), accounts for approximately 80% of all lung cancer cases and is the subtype in which oncogenic mutations are most relevant (2,3). The clinical treatment for NSCLC patients includes monoclonal antibodies, chemotherapeutic agents, and molecular targeted therapy (3-10). However, these therapies usually become ineffective after a relatively short period of time, in most cases due to the development of resistance to treatment (11,12). Therefore, finding better alternative therapeutic options is imperative. To this end, drug combination and repurposing strategies have been used. These therapeutic options possess several advantages including reduced toxicity, low probability of resistance, alternative therapeutic targets, relatively short approval time, and in some cases lower costs (13-18). Antihistamines and beta-adrenergic receptor blockers (beta-blockers) have gained interest in the repurposing of drugs for cancer treatment, including lung cancer. Recent works have demonstrated that the use of antihistamines like loratadine in patients with NSCLC was associated with a significant reduction in mortality (19-21). In addition, nebivolol is a beta-blocker that has demonstrated antiproliferative effects on NSCLC cells (22). Moreover, some studies found that the beta-blocker use was related to longer overall survival and better progression-free survival in patients with NSCLC (23-26). Actually, our group has demonstrated that the combination of antihistamines with other drugs is a potential therapeutic alternative for liver and lung cancer (27,28). In this work, we evaluated the potential antineoplastic effect of the loratadine, nebivolol and gefitinib combination on NSCLC human cell lines.
Materials and Methods
Cell lines and reagents. The human lung cancer cell lines A549 and NCI-H1975 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), and cultured according to the suppliers instructions. The A549 cell line does not have EGFR mutations but has a RAS mutation. The NCI-H1975 cell line exhibits the EGFR T790M mutation that confers resistance to first-generation tyrosine kinase inhibitors, such as erlotinib and gefitinib. The cells were seeded and cultured for 24 h and then exposed to the different drugs. Gefitinib, loratadine and nebivolol, as well as DMSO were purchased from Sigma Aldrich Co. (St Louis, MO, USA).
Metabolic activity. Metabolic activity was assayed using a colorimetric method with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as previously described (27). Briefly, 3,000 A549 and 3,500 NCI-H1975 cells per well were seeded in 96-well plates and incubated for 72 h in culture medium alone or in the presence of either gefitinib, loratadine, nebivolol or their combinations or DMSO as vehicle. MTT (0.5 mg/ml) was added 4 h before completion of the incubation time. Absorbance data were obtained with a Thermo Fisher MultiskanSkyHigh microplate spectrophotometer (Waltham, MA, USA) using two filters (595 nm and 690 nm). The inhibitory concentrations (IC20 and IC50) were obtained by analyzing the concentration-response curves of each drug from the MTT assays.
Apoptosis. Apoptosis was studied using flow cytometry as previously described (27). Briefly, cells (80,000 A549 and 120,000 NCI-H1975) were seeded in 60 mm Petri dishes and incubated for 72 h in culture medium alone or in the presence of the individual drugs or selected combinations, or DMSO. Camptothecin (apoptosis inducer) and methanol (necrosis inducer) were used as controls. Apoptosis was determined with the Annexin V-FITC kit (Thermo Fisher Scientific, Waltham, MA, USA) binding to phosphatidylserine and DNA staining with propidium iodide (PI). Experiments were performed using a flow cytometer (CYAN ADP; Dako, Glostrup, Denmark). Percentages of viable (FITC-negative and PI-negative), apoptotic (FITC-positive and PI-negative) and late apoptotic (FITC-positive and PI-positive) cells were obtained by quadrant analysis using the Kaluza 2.1 software (Beckman Coulter, Inc. Brea, CA, USA).
Colony formation assay. Cell survival was studied using the colony formation assay. Briefly, 250 A549 and 2,500 NCI-H1975 cells were cultured in 60 mm Petri dishes to allow the growth of colonies from single separated cells. 24 h after plating, the cells were incubated for 72 h in culture medium alone or in the presence of the individual drugs or selected combinations, or DMSO. Afterwards, cells were left to grow for 10 and 14 days more, respectively, in the absence of the drugs. Then, cells were fixed in ethanol (absolute grade) for 15 min, stained with crystal violet (1%) for 15 min, rinsed four times with water, observed and counted.
Statistical analysis. Data statistical analysis was performed using the GraphPad Prism software 8.1 (La Jolla, CA, USA). One-way ANOVA analysis, followed by Tukey-Kramer test was performed. p-Values <0.05 were considered as statistically significant.
Results
Calculation of the ICs of gefitinib, loratadine, and nebivolol on the NSCLC cell lines. Concentration-response curves of the drugs were obtained using the MTT assay. We used different concentrations of each drug and compared their effect against that of DMSO (data not shown). From these curves, the IC20 and IC50 were calculated for both cell lines and used in the next experiments; in the case of the A549 cells the IC10 was also studied (Table I).
Table I. Inhibitory concentrations [IC (μM)] obtained for each drug and cell line.
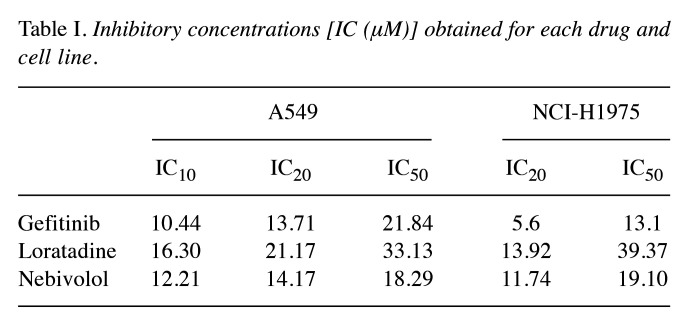
Enhanced effect of the combinations on the metabolic activity in both cell lines. First, we determined the effect of the drugs alone on the metabolic activity of A549 and NCI-H1975 cells at the corresponding ICs (Figure 1A). Then, the metabolic activity experiments with drug combinations were performed at different ICs. We observed a strong metabolic activity inhibition with the loratadine-nebivolol combination in A549 cells, thus we decided to test combinations with lower concentrations (IC10) of these drugs in this cell line. Even at low concentrations (IC10) some of the two-drug combinations resulted in significantly higher inhibition of the metabolic activity (Figure 1B). Interestingly, the loratadine-nebivolol combination was more effective than the combination of any of these drugs with gefitinib. The strong effect of the two-drug combinations was also observed at the other ICs tested in both cell lines (Figure 1C). In accordance, all the three-drug combinations inhibited almost completely the metabolic activity in both cell lines (Figure 1B and D).
Figure 1.
The combinations of gefitinib (G), loratadine (L) and nebivolol (N) abolish the metabolic activity in A549 and NCI-H1975 cell lines. A) Drug-alone effect; B) L-IC10 and N-IC10 and their two- and three-drug combination effects on the A549 cell line; C) two-drug combinations effects at different ICs on both cell lines; D) three-drug combination effects. The combination of two or three drugs significantly inhibited metabolic activity in both cell lines compared to the effect of individual drugs. Cells were cultured in the presence of the drug combinations for 72 h and results are shown as the mean±S.D. of eight replicates for each group and from three different experiments. Statistically significant differences vs. the DMSO group (*), vs. L-IC20 (▽) (or L-IC10 in panel B), vs. L-IC50 (∂) (or L-IC20 in panel B), vs. N-IC20 (φ) (or N-IC10 in panel B), vs. N-IC50 (●) (or N-IC20 in panel B), vs. G-IC20 (∍), vs. G-IC50 (γ), vs. groups using G-IC20 (♦) in the two-drug combination assay and vs. groups using G-IC50 (φ) also in the two-drug combination assay. p<0.05 in all cases.
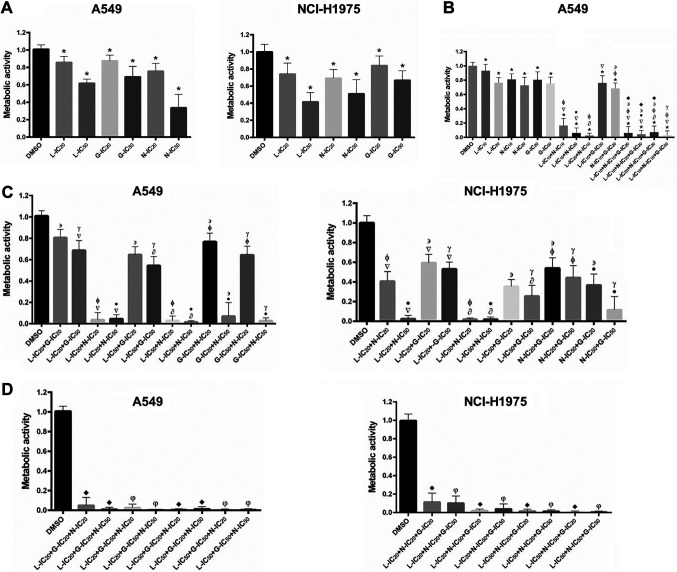
Apoptosis was increased in A549 and NCI-H1975 cells especially by the drug combinations. With the results obtained in the metabolic activity experiments, the effect of the drug-alone and selected drug combinations on apoptosis was studied. In A549 cells, loratadine and gefitinib alone showed no effect on apoptosis when compared to the control. However, nebivolol alone and its combinations increased apoptosis (Figure 2A). Interestingly, the drugs alone did not increase apoptosis in NCI-H1975 cells, but the combinations did (Figure 2B).
Figure 2.
Apoptosis induction by gefitinib (G), loratadine (L), nebivolol (N), and their combinations in lung cancer cell lines. A) Nebivolol and drug combination increased apoptosis in comparison with the DMSO group in A549 cells. B) Drug combination increased apoptosis vs DMSO and drug-alone in NCI-H1975 cells. Cells were cultured in the presence of the combinations or individual drugs for 72 h. Results are shown as the mean±S.D. of three different experiments. C) Representative plots from the flow-cytometry data indicating the different quadrants separating the cell populations: viable cells (left panel), necrosis cells (center panel), apoptosis and late apoptosis cells (right panel). Statistically significant difference vs. the DMSO group (*), vs. L-IC10 (▽) (or L-IC20 in panel B), vs. N-IC20 (∂), vs. G-IC20 (φ) (or G-IC50 in panel B). p<0.05 in all cases.
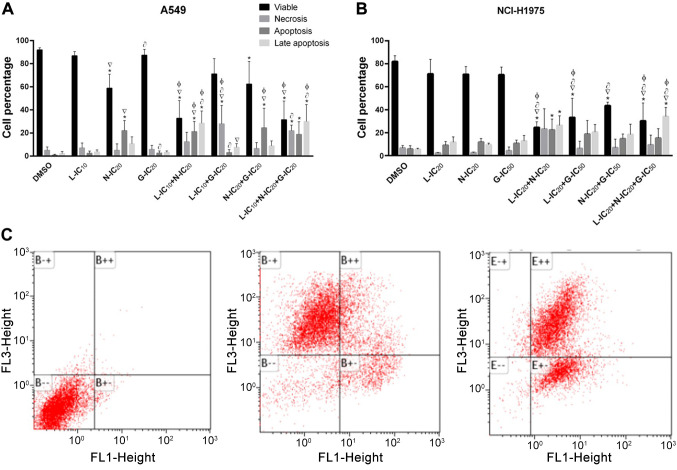
Drug combination effect on cell survival. Cell survival (assessed by the colony formation assay) was abolished completely in most cases by the drug combinations in comparison with the drugs alone in A549 cells. Surprisingly, the particular triple combination L-IC10+N-IC10+G-IC50 showed no effect on the cell survival of these cells (Figure 3A). Interestingly, single-drug treatment significantly decreased cell survival in some cases in NCI-H1975 cells, while all the drug combinations tested completely abolished cell survival in these cells (Figure 3B).
Figure 3.
Drug combinations of gefitinib (G), loratadine (L), and nebivolol (N) suppress cell survival in A549 and NCI-H1975 cells. The combinations tested were able to completely inhibit the formation of colonies. Percentage of colonies formed under each condition in A) A549 and B) NCI-H1975 cell lines. Cells were cultured in the presence of the individual drugs or their combinations or DMSO for 72 h. Results are shown as the mean±S.D. from three different assays. C) Representative examples of colony formation in Petri dishes, cells were stained with crystal violet (1%). Statistically significant differences vs. the DMSO group (*), vs. L-IC10 (▽), vs. N-IC10 (φ), vs. N-IC20 (●), vs. G-IC20 (∍), vs. G-IC50 (γ). p<0.05 in all cases.
Discussion
The current panorama in the management of lung cancer worldwide is almost hopeless. To date, lung cancer continues to be the deadliest neoplasm and represents the sixth cause of death worldwide (1,29). Unfortunately, despite the existence of several therapeutic strategies offered to these patients, they either do not respond to the treatment or develop resistance in a short period of time, as in the case of gefitinib-treated patients (3,4,5-9,10,11,12). Gefitinib is a tyrosine kinase inhibitor (TKI) of the epidermal growth factor receptor (EGFR), which is over-expressed in 15 to 50% of the patients with NSCLC (30-32). In many countries, gefitinib is the first-choice treatment for patients with some common EGFR mutations; however, due to multiple resistance mechanisms, its effectiveness is limited to a few months (33,34). For this reason, new TKIs such as afatinib and osimertinib have been developed; however, these have shown only a modest improvement compared to gefitinib (35,36).
In this work, we demonstrate that the combinations of two or three drugs (gefitinib, loratadine and nebivolol) have a greater effect on the lung cancer cell lines A549 and NCI-H1975 compared to the effect of each drug alone. In addition, we clearly show that both cell lines are sensitive to the three drugs studied, but with different sensitivity. The inhibitory concentrations found were very similar to those reported (where available) in several studies including our own work (19,20,32,37). In the drug combination experiments, we observed a synergistic effect in both cell lines when combining nebivolol with loratadine. Interestingly, we observed a greater sensitivity in the A549 cell line, therefore we also decided to use the IC10 of both drugs in these cells. Noteworthy, even at these low ICs used, we still observed a significant synergistic effect. In a few cases, antagonistic effects were observed when combining gefitinib with any of the other drugs; however, this was avoided when using higher ICs of the drugs.
Remarkably, all the three-drug combinations studied abolished almost completely the metabolic activity and colony formation even when using the IC10 of loratadine and nebivolol. The two-drug combination of loratadine and nebivolol displayed notable effects on apoptosis, as well as in combination with gefitinib.
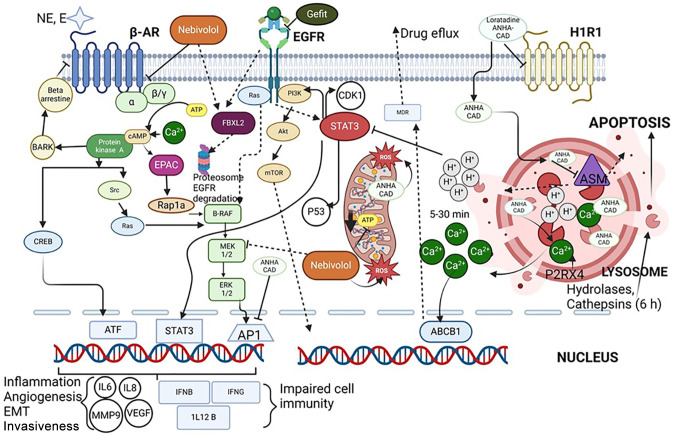
The potential molecular mechanisms explaining the observed anticancer effects are depicted in Figure 4. Loratadine accumulation in the lysosomes causes the leak of proteolytic enzymes and H+ and Ca++ ions into the cytoplasm inducing the apoptotic process (20). Nebivolol could trigger several processes such as cell arrest, mitochondrial respiration inhibition (38), and proteasome-mediated EGFR degradation (22). In addition, the activation of the beta-adrenergic receptor leads to increased levels of PKA, which in turn enhances cell proliferation via the RAS-RAF-MEK- ERK signaling pathways. As a consequence, beta-blockers like nebivolol decrease cell proliferation. Besides, nebivolol is also capable of inhibiting MEK kinase, which has been associated with resistance to treatments with TKIs. Furthermore, nebivolol has recently been shown to enhance the sensitivity of breast cancer cells to the fibroblast growth factor receptor (FGFR) inhibitor erdafitinib by reduction in AKT activation (39). However, nebivolol can bind to the FBXL2 chaperone, which becomes more efficient in degrading the EGFR receptor regardless of the resistance mutations, which could be favoring the disappearance of cellular resistance to gefitinib (22,40). Therefore, the signaling pathways targeted by loratadine and/or nebivolol may create a suitable environment leading to tumor cell eradication.
Figure 4.
Potential molecular mechanisms involved in the anticancer effects of the combination gefitinib-loratadine-nebivolol on lung cancer cells. The β-AR and EGFR signaling pathways are proliferative in nature; their overstimulation (β-AR) or oncogenic mutations (EGFR) lead to uncontrolled proliferation of neoplastic cells. However, antagonism of these receptors by nebivolol and gefitinib, respectively, inhibits these pathways. Additionally, nebivolol promotes apoptosis by producing oxidative stress at the mitochondrial level while simultaneously inhibiting mitochondrial respiration and facilitating the degradation of the EGFR receptor in proteasomes through the activation of FBXL2. It is also theorized that it can directly inhibit MEK, in the MAPK proliferative pathway. Furthermore, the lysosomal accumulation of loratadine, an amphiphilic cationic antihistamine (ANHA-CAD) (19) stimulates the release of Ca2+ and H+, producing inhibition of multidrug-resistant (MDR) proteins by Ca2+ and acidification of the cytosol by H+, which dephosphorylates and inactivates STAT3, thus inhibiting the STAT3-PI3K-Akt-mTOR signaling. The accumulation of loratadine inside lysosomes stimulates P2RX4, which is responsible for expelling Ca2+ into the cytosol, and inhibits ASM (acid sphingomyelinase), which causes an accumulation of ceramides and destabilization of the lysosomal membrane, producing its fragmentation and consequently the release of hydrolases and cathepsins into the cytosol, which favors the initiation of apoptosis and pyroptosis.
We demonstrate that combining two or three-drugs- gefitinib, loratadine, and nebivolol- is a potential approach for lung cancer treatment. Although several studies explaining the precise mechanism of the combination effect are needed, these results suggest that the triple combination gefitinib-loratadine-nebivolol could serve as a novel therapeutic strategy for lung cancer. The potential anticancer effect of this combination on lung cancer cells displaying other mutations deserves further investigation. We propose this combination as a worthy translational approach (for instance in Phase II clinical trials), that may help to decrease the mortality from this disease.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
JLM-L and EH-G performed the experiments; JLM-L, EH-G, MdGC-L and JC analyzed the data; JLM-L, MdGC-L and JC wrote the paper; JLM-L, MDGC-L, RV-V and JC made substantial contributions to the manuscript; all Authors contributed to the design of the experiments, reviewed, and approved the manuscript.
Acknowledgements
JLM-L received the CONAHCYT fellowship number 817175 supporting his M.Sc. studies.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am. 2016;25(3):447–468. doi: 10.1016/j.soc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Chevallier M, Borgeaud M, Addeo A, Friedlaender A. Oncogenic driver mutations in non-small cell lung cancer: Past, present and future. World J Clin Oncol. 2021;12(4):217–237. doi: 10.5306/wjco.v12.i4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S. Metastatic non-small cell lung cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 5.Hellmann MD, Li BT, Chaft JE, Kris MG. Chemotherapy remains an essential element of personalized care for persons with lung cancers. Ann Oncol. 2016;27(10):1829–1835. doi: 10.1093/annonc/mdw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planchard D, Jänne PA, Cheng Y, Yang JC, Yanagitani N, Kim SW, Sugawara S, Yu Y, Fan Y, Geater SL, Laktionov K, Lee CK, Valdiviezo N, Ahmed S, Maurel JM, Andrasina I, Goldman J, Ghiorghiu D, Rukazenkov Y, Todd A, Kobayashi K, FLAURA2 Investigators Osimertinib with or without chemotherapy in EGFR -mutated advanced NSCLC. N Engl J Med. 2023;389(21):1935–1948. doi: 10.1056/NEJMoa2306434. [DOI] [PubMed] [Google Scholar]
- 7.Chapman AM, Sun KY, Ruestow P, Cowan DM, Madl AK. Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–134. doi: 10.1016/j.lungcan.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC, FLAURA Investigators Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 9.Xia L, Liu Y, Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist. 2019;24(Suppl 1):S31–S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N, CheckMate 816 Investigators Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stock-Martineau S, Laurie K, McKinnon M, Zhang T, Wheatley-Price P. Evolution of systemic treatment uptake and survival in advanced non-small cell lung cancer. Curr Oncol. 2020;28(1):60–68. doi: 10.3390/curroncol28010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jourdan JP, Bureau R, Rochais C, Dallemagne P. Drug repositioning: a brief overview. J Pharm Pharmacol. 2020;72(9):1145–1151. doi: 10.1111/jphp.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RR, Stonier PD. Repurposing old drugs in oncology: Opportunities with clinical and regulatory challenges ahead. J Clin Pharm Ther. 2019;44(1):6–22. doi: 10.1111/jcpt.12759. [DOI] [PubMed] [Google Scholar]
- 15.MotieGhader H, Tabrizi-Nezhadi P, Deldar Abad Paskeh M, Baradaran B, Mokhtarzadeh A, Hashemi M, Lanjanian H, Jazayeri SM, Maleki M, Khodadadi E, Nematzadeh S, Kiani F, Maghsoudloo M, Masoudi-Nejad A. Drug repositioning in non-small cell lung cancer (NSCLC) using gene co-expression and drug-gene interaction networks analysis. Sci Rep. 2022;12(1):9417. doi: 10.1038/s41598-022-13719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh AB, Kozuch P, Rohs N, Becker DJ, Levy BP. Metformin as a repurposed therapy in advanced non-small cell lung cancer (NSCLC): results of a phase II trial. Invest New Drugs. 2017;35(6):813–819. doi: 10.1007/s10637-017-0511-7. [DOI] [PubMed] [Google Scholar]
- 17.Marrone KA, Zhou X, Forde PM, Purtell M, Brahmer JR, Hann CL, Kelly RJ, Coleman B, Gabrielson E, Rosner GL, Ettinger DS. A randomized phase II study of metformin plus paclitaxel/carboplatin/bevacizumab in patients with chemotherapy-naïve advanced or metastatic nonsquamous non-small cell lung cancer. Oncologist. 2018;23(7):859–865. doi: 10.1634/theoncologist.2017-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrieta Rodriguez OG, Barron FB, Salinas Padilla MÁ, Ramirez-Tirado LA, Flores-Estrada D, Cruz-Rico G, Arguelles Jiménez MJ, Cardona Zorrilla AF. Combination of metformin plus TKI vs. TKI alone in EGFR(+) LUNG adenocarcinoma: A randomized phase II study. J Clin Oncol. 2018;36(15_suppl):9013–9013. doi: 10.1200/JCO.2018.36.15_suppl.9013. [DOI] [Google Scholar]
- 19.Ellegaard AM, Dehlendorff C, Vind AC, Anand A, Cederkvist L, Petersen NHT, Nylandsted J, Stenvang J, Mellemgaard A, Østerlind K, Friis S, Jäättelä M. Repurposing cationic amphiphilic antihistamines for cancer treatment. EBioMedicine. 2016;9:130–139. doi: 10.1016/j.ebiom.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Zhong R, Huang J, Chen Z, Xu H, Lin L, Cai Q, He M, Lao S, Deng H, Li C, Li J, Zheng Y, Liu X, Zeng R, He J, Liang W. Loratidine is associated with improved prognosis and exerts antineoplastic effects via apoptotic and pyroptotic crosstalk in lung cancer. J Exp Clin Cancer Res. 2024;43(1):5. doi: 10.1186/s13046-023-02914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Zhong R, Huang J, Chen Z, Xu H, Lin L, Cai Q, He M, Lao S, Deng H, Li CC, Li J, Zeng R, Zheng Y, Liu X, Liang W, He J. 1310P Loratadine: A potential game-changer in lung cancer treatment with improved survival outcomes. Ann Oncol. 2023;34:S752–3. doi: 10.1016/j.annonc.2023.09.784. [DOI] [Google Scholar]
- 22.Niu M, Xu J, Liu Y, Li Y, He T, Ding L, He Y, Yi Y, Li F, Guo R, Gao Y, Li R, Li L, Fu M, Hu Q, Luo Y, Zhang C, Qin K, Yi J, Yu S, Yang J, Chen H, Wang L, Li Z, Dong B, Qi S, Ouyang L, Zhang Y, Cao Y, Xiao ZJ. FBXL2 counteracts Grp94 to destabilize EGFR and inhibit EGFR-driven NSCLC growth. Nat Commun. 2021;12(1):5919. doi: 10.1038/s41467-021-26222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CH, Lee CH, Ko JC, Chang LY, Lee MC, Zhang JF, Wang JY, Shih JY, Yu CJ. Effect of β-blocker in treatment-naïve patients with advanced lung adenocarcinoma receiving first-generation EGFR-TKIs. Front Oncol. 2020;10:583529. doi: 10.3389/fonc.2020.583529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson MB, Le X, Heymach JV. β-adrenergic signaling in lung cancer: a potential role for beta-blockers. J Neuroimmune Pharmacol. 2020;15(1):27–36. doi: 10.1007/s11481-019-09891-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhary KR, Yan SX, Heilbroner SP, Sonett JR, Stoopler MB, Shu C, Halmos B, Wang TJC, Hei TK, Cheng SK. Effects of β-adrenergic antagonists on chemoradiation therapy for locally advanced non-small cell lung cancer. J Clin Med. 2019;8(5):575. doi: 10.3390/jcm8050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh MS, Guzner A, Wainwright DA, Mohindra NA, Chae YK, Behdad A, Villaflor VM. The impact of beta blockers on survival outcomes in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer. 2021;22(1):e57–e62. doi: 10.1016/j.cllc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chávez-López MG, Zúñiga-García V, Hernández-Gallegos E, Vera E, Chasiquiza-Anchatuña CA, Viteri-Yánez M, Sanchez-Ramos J, Garrido E, Camacho J. The combination astemizole-gefitinib as a potential therapy for human lung cancer. Onco Targets Ther. 2017;10:5795–5803. doi: 10.2147/OTT.S144506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villarruel-Melquiades F, Hernandez-Gallegos E, Solano-Agama C, Mendoza-Garrido ME, Camacho J. The combination sorafenib-raloxifene-loratadine as a novel potential therapeutic approach against human liver cancer. In Vivo. 2023;37(3):1156–1163. doi: 10.21873/invivo.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The top 10 causes of death. Geneva, Switzerland, World Health Organization, 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. [Last accessed on March 30, 2024]
- 30.Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ríos CPS, Alexander JA, Flores Soto MDR. P2.10 Mutational EGFR Profile in Mexican patients with pulmonary adenocarcinoma measured by new generation sequencing (NGS) J Thorac Oncol. 2019;14(11):S1188. [Google Scholar]
- 32.Rodríguez-Lara V, Ramírez-Tirado LA, Barrón F, Zatarain-Barrón ZL, Flores-Estrada D, Arrieta O. Characteristics of non-small cell lung cancer: Differences by sex and hormonal status in a Mexican population. Salud Publica Mex. 2019;61(3):265. doi: 10.21149/10094. [DOI] [PubMed] [Google Scholar]
- 33.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 34.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/s1470-2045(11)70393-x. [DOI] [PubMed] [Google Scholar]
- 35.Svaton M, Bratova M, Fischer O, Krejci J, Koubkova L, Cernovska M, Hrnciarik M, Zemanova M, Coupkova H, Porzer B, Dolezal D, Tuzova T, Hurdalkova K, Barinova M, Skrickova J. Real-life effectiveness of afatinib versus gefitinib in patients with non-small-cell lung cancer: a Czech multicentre study. Anticancer Res. 2021;41(4):2059–2065. doi: 10.21873/anticanres.14975. [DOI] [PubMed] [Google Scholar]
- 36.Tamiya M, Tamiya A, Suzuki H, Moriizumi K, Nakahama K, Taniguchi Y, Kunimasa K, Kimura M, Inoue T, Kuhara H, Nishino K, Hirashima T, Atagi S, Imamura F, Kumagai T. Which is better EGFR-TKI followed by Osimertinib: Afatinib or Gefitinib/Erlotinib. Anticancer Res. 2019;39(7):3923–3929. doi: 10.21873/anticanres.13544. [DOI] [PubMed] [Google Scholar]
- 37.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuevo-Tapioles C, Santacatterina F, Stamatakis K, Núñez de Arenas C, Gómez de Cedrón M, Formentini L, Cuezva JM. Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat Commun. 2020;11(1):3606. doi: 10.1038/s41467-020-17384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YJ, Jang SK, Kim G, Hong SE, Park CS, Seong MK, Kim HA, Kim KS, Kim CH, Park KS, Hong J, Jin HO, Park IC. Nebivolol sensitizes BT-474 breast cancer cells to FGFR inhibitors. Anticancer Res. 2023;43(5):1973–1980. doi: 10.21873/anticanres.16357. [DOI] [PubMed] [Google Scholar]
- 40.Qu GP, Shi M, Wang D, Wu JH, Wang P, Gong ML, Zhang ZJ. Dual targeting of MEK and PI3K effectively controls the proliferation of human EGFR-TKI resistant non-small cell lung carcinoma cell lines with different genetic backgrounds. BMC Pulm Med. 2021;21(1):208. doi: 10.1186/s12890-021-01571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]