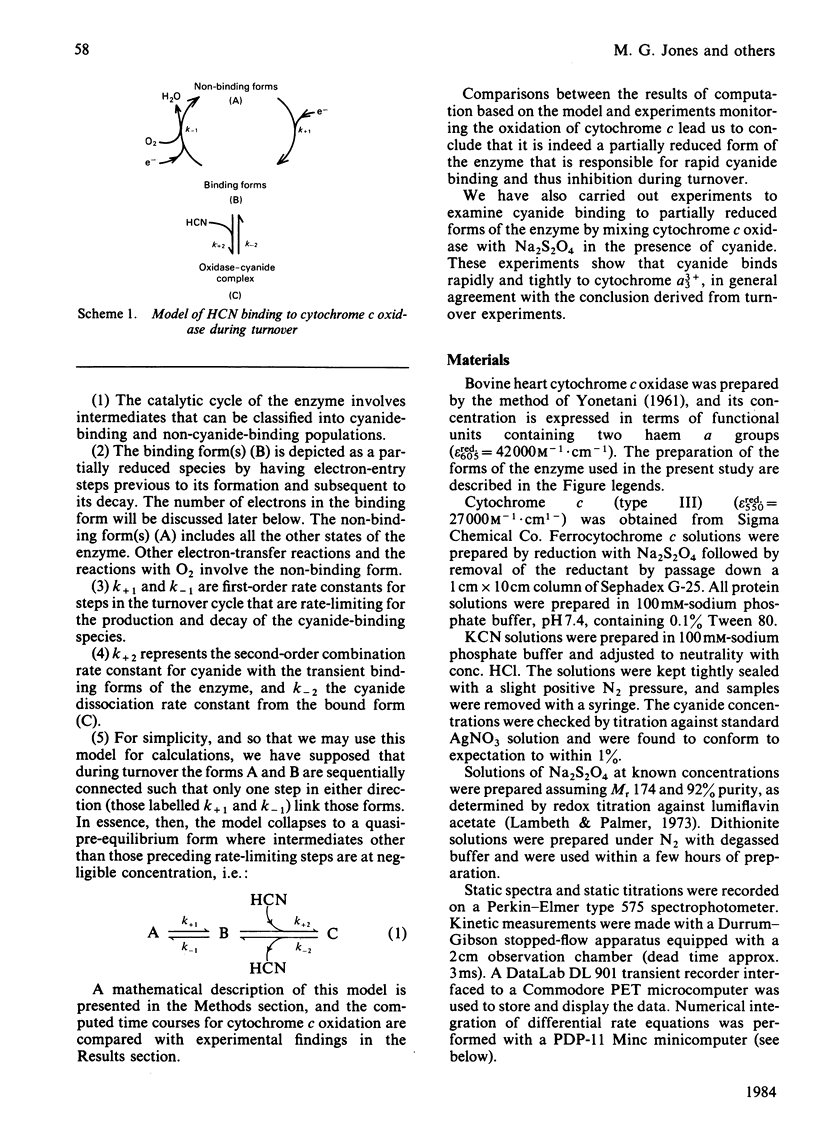
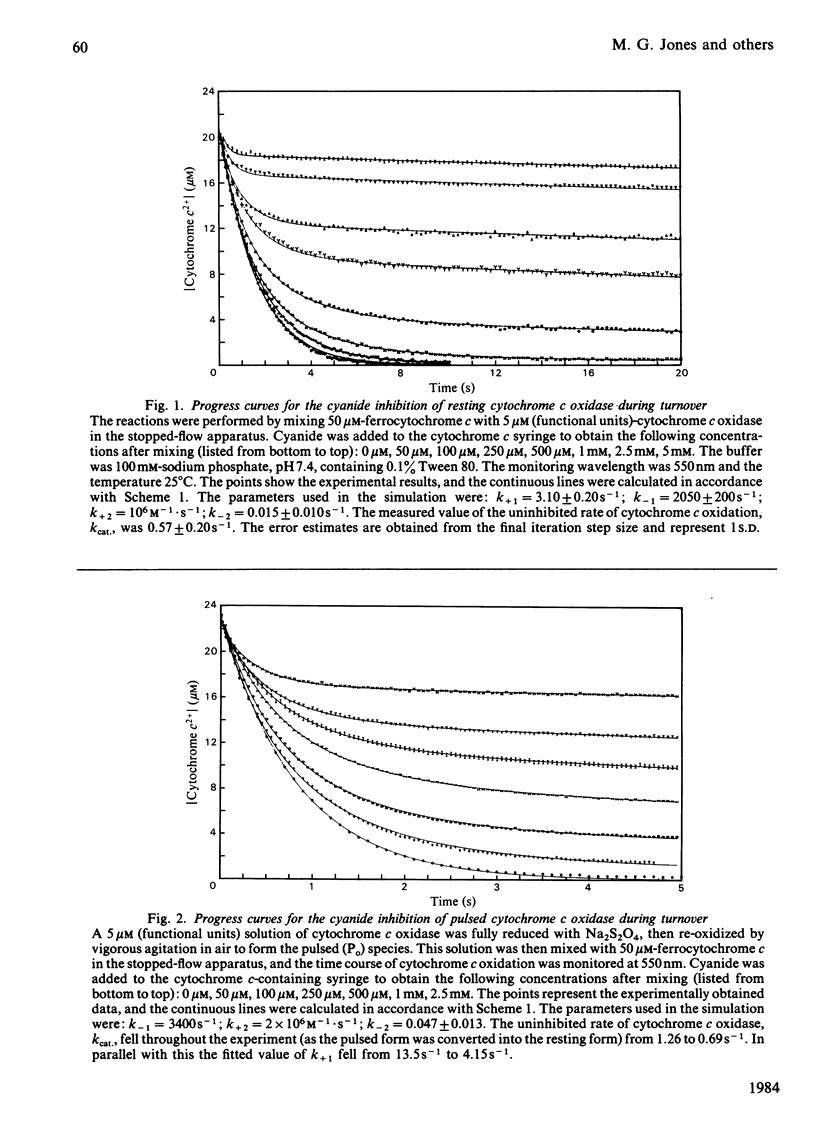
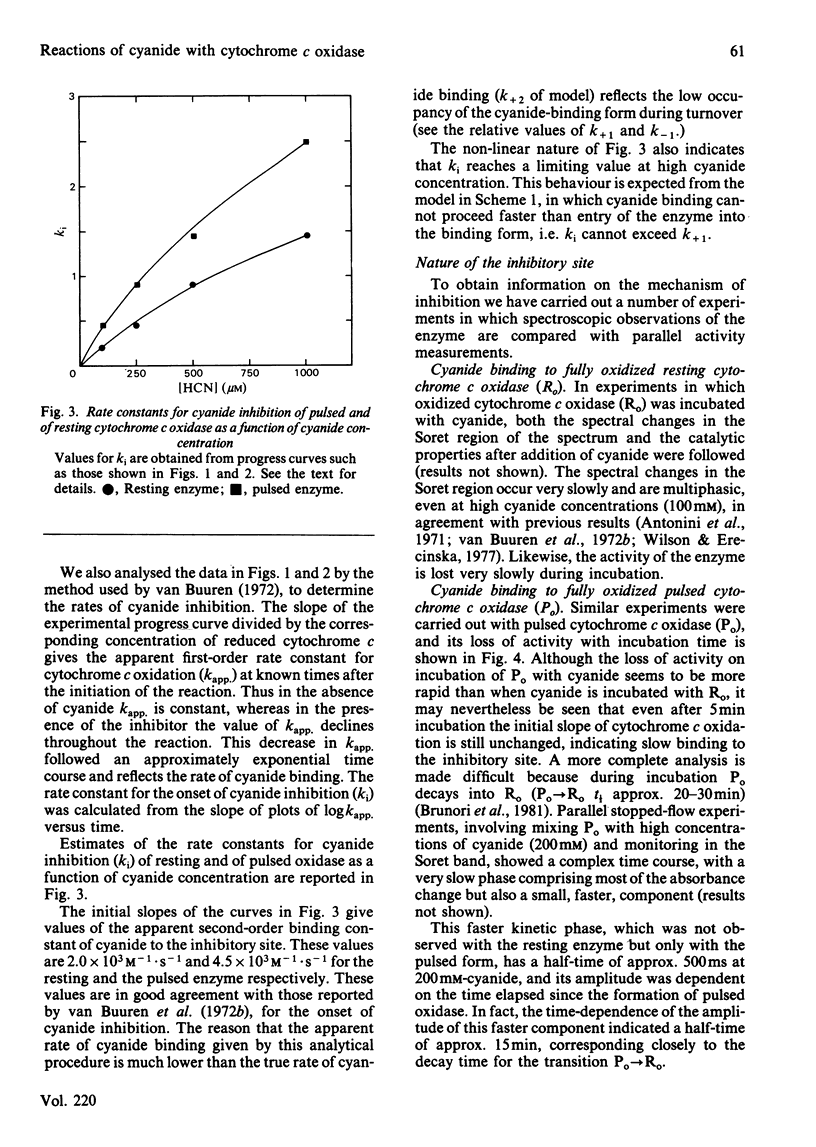
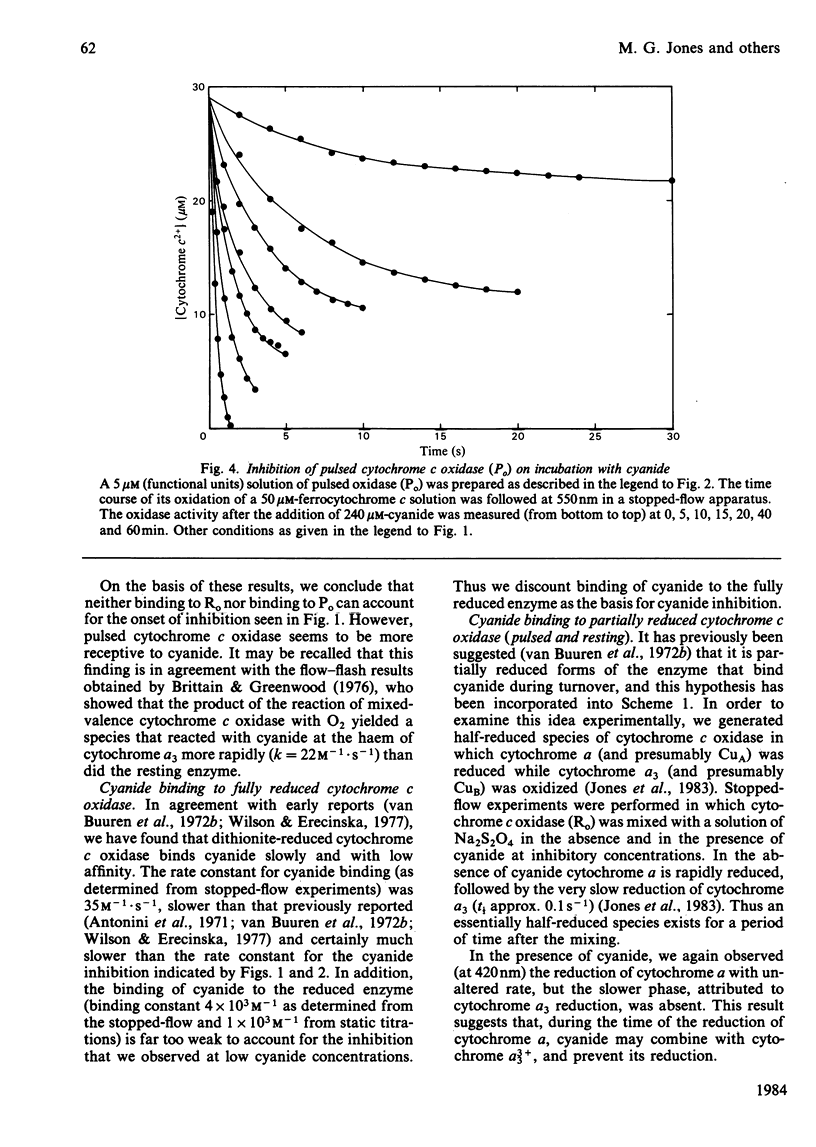
Abstract
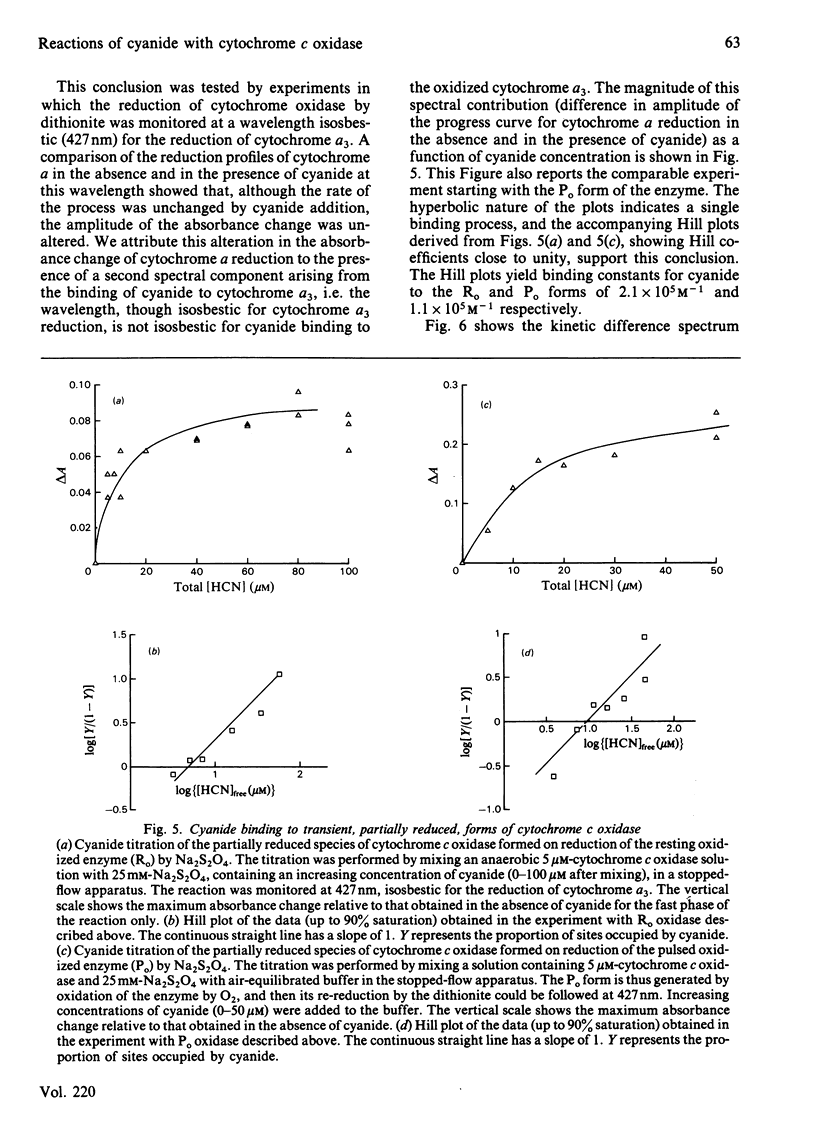
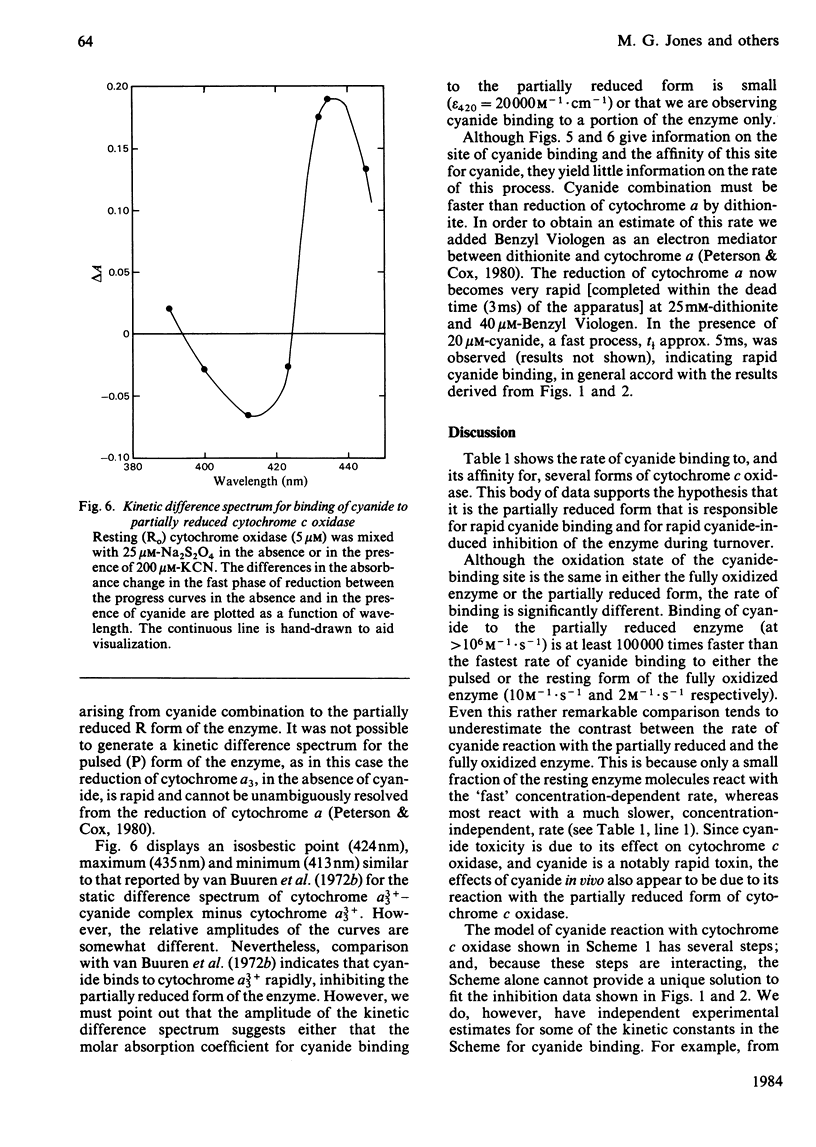
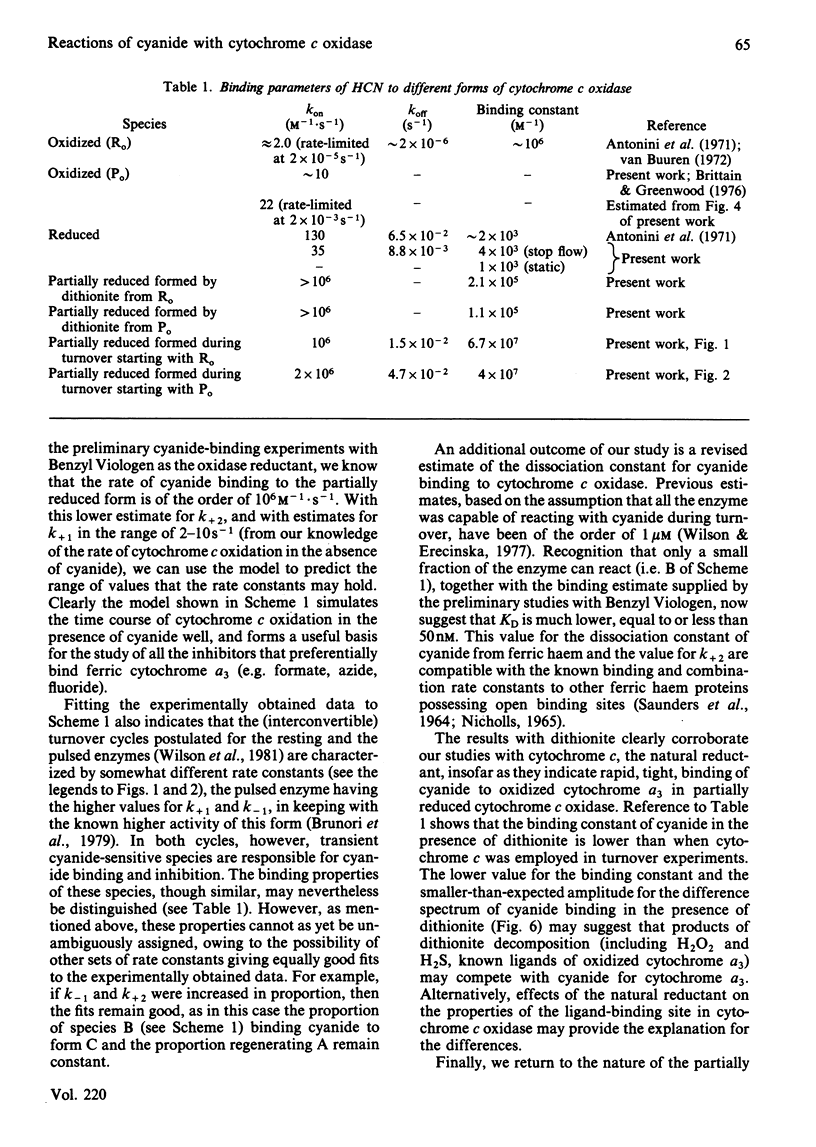
Experiments were performed to examine the cyanide-binding properties of resting and pulsed cytochrome c oxidase in both their stable and transient turnover states. Inhibition of the oxidation of ferrocytochrome c was monitored as a function of cyanide concentration. Cyanide binding to partially reduced forms produced by mixing cytochrome c oxidase with sodium dithionite was also examined. A model is presented that accounts fully for cyanide inhibition of the enzyme, the essential feature of which is the rapid, tight, binding of cyanide to transient, partially reduced, forms of the enzyme populated during turnover. Computer fitting of the experimentally obtained data to the kinetic predictions given by this model indicate that the cyanide-sensitive form of the enzyme binds the ligand with combination constants in excess of 10(6) M-1 X s-1 and with KD values of 50 nM or less. Kinetic difference spectra indicate that cyanide binds to oxidized cytochrome a33+ and that this occurs rapidly only when cytochrome a and CuA are reduced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Brunori M., Colosimo A., Greenwood C., Wilson M. T. Oxygen "pulsed" cytochrome c oxidase: functional properties and catalytic relevance. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3128–3132. doi: 10.1073/pnas.74.8.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini E., Brunori M., Greenwood C., Malmström B. G., Rotilio G. C. The interaction of cyanide with cytochrome oxidase. Eur J Biochem. 1971 Nov 11;23(2):396–400. doi: 10.1111/j.1432-1033.1971.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Brittain T., Greenwood C. Kinetic studies on the binding of cyanide to oxygenated cytochrome c oxidase. Biochem J. 1976 May 1;155(2):453–455. doi: 10.1042/bj1550453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Colosimo A., Rainoni G., Wilson M. T., Antonini E. Functional intermediates of cytochrome oxidase. Role of "pulsed" oxidase in the pre-steady state and steady state reactions of the beef enzyme. J Biol Chem. 1979 Nov 10;254(21):10769–10775. [PubMed] [Google Scholar]
- Brunori M., Colosimo A., Sarti P., Antonini E., Wilson M. T. 'Pulsed' cytochrome oxidase may be produced without the advent of dioxygen. FEBS Lett. 1981 Apr 20;126(2):195–198. doi: 10.1016/0014-5793(81)80240-2. [DOI] [PubMed] [Google Scholar]
- Errede B., Kamen M. D. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978 Mar 21;17(6):1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Eglinton D. G., Gooding P. E., Greenwood C., Thomson A. J. Characterization of the partially reduced cyanide-inhibited derivative of cytochrome c oxidase by optical, electron-paramagnetic-resonance and magnetic-circular-dichroism spectroscopy. Biochem J. 1981 Mar 1;193(3):699–708. doi: 10.1042/bj1930699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. D., Jones M. G., Wilson M. T., Brunori M., Colosimo A., Sarti P. Reactions of cytochrome c oxidase with sodium dithionite. Biochem J. 1983 Jan 1;209(1):175–182. doi: 10.1042/bj2090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth D. O., Palmer G. The kinetics and mechanism of reduction of electron transfer proteins and other compounds of biological interest by dithionite. J Biol Chem. 1973 Sep 10;248(17):6095–6103. [PubMed] [Google Scholar]
- Nicholls P. Oxidation and peroxidation. J Gen Physiol. 1965 Sep;49(1 Suppl):131–147. [PubMed] [Google Scholar]
- Petersen L. C., Cox R. P. Reduction of oxygen-pulsed cytochrome c oxidase by cytochrome c and other electron donors. Biochim Biophys Acta. 1980 Mar 7;590(1):128–137. doi: 10.1016/0005-2728(80)90152-8. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Peterson J., Antonini E., Brunori M., Colosimo A., Wyman J. A plausible two-state model for cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7115–7118. doi: 10.1073/pnas.78.11.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. III. Improved preparation and some properties. J Biol Chem. 1961 Jun;236:1680–1688. [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]
- van Buuren K. J., Zuurendonk P. F., van Gelder B. F., Muijsers A. O. Biochemical and biophysical studies on cytochrome aa 3 . V. Binding of cyanide to cytochrome aa 3 . Biochim Biophys Acta. 1972 Feb 28;256(2):243–257. doi: 10.1016/0005-2728(72)90056-4. [DOI] [PubMed] [Google Scholar]


