Abstract
Background/Aim
Tremelimumab plus durvalumab is an approved first-line therapy for advanced hepatocellular carcinoma (HCC). Previous studies identified WNT/β-catenin mutations or CD8+ tumor-infiltrating lymphocytes (TILs) as biomarkers that can predict responsiveness to immune checkpoint inhibitor therapy in HCC. However, biomarkers for effectiveness of tremelimumab plus durvalumab in HCC have not been reported. This study investigated whether evaluation of WNT/β-catenin signaling and CD8+ TILs by immunohistochemical staining of tumor biopsy tissues can predict the response to tremelimumab plus durvalumab in patients with HCC.
Patients and Methods
Fifteen HCC patients who underwent tumor biopsies were classified into three groups based on WNT/β-catenin signal activation and CD8+ TIL infiltration. The clinical responses to treatment in the groups were evaluated.
Results
Four patients had HCC with WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration, four patients had HCC with WNT/β-catenin signal activation and low-level CD8+ TIL infiltration, and seven patients had WNT/β-catenin signal activation and high-level CD8+ TIL infiltration or WNT/β-catenin signal inactivation and low-level CD8+ TIL infiltration. A better response rate was observed in the WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration group, and a worse response rate was observed in the WNT/β-catenin signal activation and low-level CD8+ TIL infiltration group.
Conclusion
Although the present study involved a small number of patients, the findings suggest that the efficacy of tremelimumab plus durvalumab may be affected by WNT/β-catenin signaling and CD8+ TIL infiltration.
Keywords: Hepatocellular carcinoma, tremelimumab plus durvalumab, WNT/β-catenin signal, CD8+ tumor-infiltrating lymphocytes, objective response
In 2020, hepatocellular carcinoma (HCC) accounted for 900,000 new cancer cases and 830,000 deaths worldwide, making it the sixth most common neoplasm and the third leading cause of cancer-related deaths worldwide (1,2). Patients with advanced HCC have benefited from recent advances in systemic chemotherapy, including therapy with immune checkpoint inhibitors (ICIs) and molecular targeted agents (3-9).
Tremelimumab is a human monoclonal antibody that targets cytotoxic T-lymphocyte-associated protein-4 (CTLA-4). Tremelimumab inhibits CTLA-4 activity, thereby contributing to T-cell activation, priming the immune response to cancer, and fostering the death of cancer cells. As a monoclonal antibody, durvalumab binds to programmed death ligand (PD-L1) and blocks PD-L1 interactions with PD-1 and CD80, thus countering the tumor immune-evading tactics and releasing the inhibition of immune responses. Combination therapy with tremelimumab plus durvalumab, which targets CTLA-4 and PD-L1, showed superior outcomes in the HIMALAYA trial compared with sorafenib, displaying a median survival time of 16.4 months (8). Therefore, atezolizumab plus bevacizumab or tremelimumab plus durvalumab may be offered as first-line therapy for patients with advanced HCC, Child–Pugh class A liver disease, and Eastern Cooperative Oncology Group performance status 0-1 (10).
The prognosis of patients with advanced HCC may be improved by selecting the appropriate chemotherapy. Thus, it is critical to choose agents that are suitable for personalized HCC treatment. Consequently, it is essential to identify potential predictive biomarkers and understand the mechanisms of resistance or response to systemic chemotherapy regimens. Tremelimumab plus durvalumab has not been associated with any established biomarkers that can predict responsiveness in HCC patients.
HCC progression depends on the tumor immune microenvironment, which facilitates interactions between tumor cells and immune cells. Treatment for HCC has focused on the classification of tumors according to WNT/β-catenin mutations. Approximately 40% of HCC patients harbor WNT/β-catenin mutations that result in the immune microenvironment lacking immune cell infiltration, so-called ‘immune-exclusion HCC’ or ‘non-inflamed cold HCC’ (11,12). Immune-exclusion HCC associated with WNT/β-catenin signal activation is resistant to ICI therapy (13-15). As well as reflecting the local immune response, CD8+ tumor-infiltrating lymphocytes (TILs) may also play an important role in controlling tumor progression (16-18). In the tumor immune microenvironment, CD8+ T cells can promote the accumulation of distinct endogenous CD8+ and CD4+ T cells that support antitumor activity (19-21). Positive correlations between CD8+ TIL levels in the tumor immune microenvironment and responses to ICI therapy have been noted in various types of cancer (22,23). Patients with immune-exclusion HCC, who have decreased CD8+ TIL infiltration, are resistant to ICI therapy, based on a recent study (11). CD8+ TILs may also be correlated with the clinical response to atezolizumab plus bevacizumab (24,25).
While WNT/β-catenin signal activation is known to cause immune exclusion in other cancer types, HCC with WNT/β-catenin signal activation can be classified into two distinct types: inflamed tumors with CD8+ T cell infiltration and non-inflamed tumors without CD8+ T cell infiltration (26-28).
In this study, we investigated whether WNT/β-catenin signal activation and CD8+ TIL infiltration on HCC tissues could be useful biomarkers for predicting response to tremelimumab plus durvalumab.
Patients and Methods
Patients. This single-center prospective study analyzed the efficacy of tremelimumab plus durvalumab in HCC patients based on WNT/β-catenin signaling and CD8+ TIL infiltration. These parameters were evaluated by immunohistochemical staining of tumor tissues obtained by liver biopsy or lymph node metastasis biopsy using endoscopic ultrasound-guided fine needle aspiration at Aso Iizuka Hospital between April 2023 and May 2024. Thirty-two patients received tremelimumab plus durvalumab for advanced HCC. There were 10 patients who did not undergo tumor biopsy prior to chemotherapy and seven patients who were followed up within four weeks before evaluation of treatment response. We evaluated the remaining 15 patients. According to the Declaration of Helsinki, the study was approved by the Ethics Committee of Aso Iizuka Hospital (approval no. 23056). Consent for the study was obtained using the opt-out method.
Treatment protocol. This regimen consisted of tremelimumab 300 mg and durvalumab 1,500 mg every four weeks (termed the STRIDE regimen) administered intravenously according to the HIMALAYA study conducted by AstraZeneca Co., Ltd. (8). The treatment was continued until the disease progressed or the side effects became intolerable.
Evaluation of efficacy. Every 4-12 weeks following treatment initiation, computed tomography or magnetic resonance imaging was performed to determine the treatment’s effectiveness. Response Evaluation Criteria in Solid Tumors (RECIST) criteria were used by the treating physician to evaluate the antitumor response (29). A complete response (CR), partial response (PR), or stable disease (SD) lasting at least four months was defined as the disease control rate (DCR). The objective response rate (ORR) was defined as PR+CR. Patients were followed up every two weeks.
Immunohistochemistry (IHC). We conducted immunostaining in accordance with our previous reports (25,30). The presence of β-catenin, glutamine synthetase (GS), and CD8 was evaluated using the following primary antibodies: monoclonal mouse anti-human β-catenin (#610153; 1:300; BD Biosciences, Franklin Lakes, NJ, USA), monoclonal mouse anti-human GS (#GS-6; 1:500; Millipore, Burlington, MA, USA), and mouse anti-human monoclonal CD8 (clone C8/144B; 1:50; DAKO, Agilent, Santa Clara, CA, USA). The activation of WNT/β-catenin signaling was defined by β-catenin nuclear staining in ≥5% of cancer cells or diffuse GS staining in ≥50% of cancer cells, as previously reported (31-35). The high and low level of CD8+ cell infiltration in the tumors was defined using a cut off value of 15.9 cells/high-power field according to our previous report (25).
Statistical analysis. All statistical analyses were conducted using JMP Pro version 11 software (SAS Institute Inc., Cary, NC, USA). Data are presented as medians (interquartile ranges), and Fisher’s exact test and Mann-Whitney U-tests are used to compare groups. Statistical significance was accepted at p<0.05.
Results
Patient characteristics. The characteristics of the 15 patients who received tremelimumab plus durvalumab are shown in Table I. We divided the patients into three groups based on WNT/β-catenin signaling and CD8+ T cell infiltration: Group A, WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration; Group B, WNT/β-catenin signal activation and high-level CD8+ TIL infiltration or WNT/β-catenin signal inactivation and low-level CD8+ TIL infiltration; Group C, WNT/β-catenin signal inactivation and low-level CD8+ TIL infiltration.
Table I. Characteristics of the study patients.
Data are expressed as median (interquartile range) unless otherwise indicated. Group A: WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration. Group B: WNT/β-catenin signal activation and high-level CD8+ TIL infiltration or WNT/β-catenin signal inactivation and low-level CD8+ TIL infiltration. Group C: WNT/β-catenin signal activation and low-level CD8+ TIL infiltration. TIL: Tumor-infiltrating lymphocyte; HCV: hepatitis C; HBV: hepatitis B; NBNC: non-hepatitis B non-hepatitis C; MVI: macroscopic portal vein invasion; EHS: extrahepatic spread; BCLC: Barcelona Clinic Liver Cancer; AFP: α-fetoprotein; PIVKA-II: protein induced by vitamin K absence or antagonist-II.
There were four patients in Group A, seven patients in Group B, and four patients in Group C. There were more female patients and more patients with Barcelona Clinic Liver Cancer stage C in Group A than in the other groups. The level of protein induced by vitamin K absence or antagonist-II was lower in Group C than in the other groups. Age, etiology, Child–Pugh grade, tumor size, number of intrahepatic lesions, microvascular invasion, extrahepatic spread, and serum α-fetoprotein (AFP) levels were similar among the groups.
Effect of tremelimumab plus durvalumab. The ORR (CR+PR) was 4/4 (100%) in Group A, 2/7 (33.3%) in Group B, and 0/0 (0%) in Group C (p=0.0027). The DCR (CR+PR+SD) was 4/4 (100%) in Group A, 3/7 (42.9%) in Group B, and 2/4 (50%) in Group C (p=0.0787) (Table II). Therefore, WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration affected the efficacy of tremelimumab plus durvalumab.
Table II. Comparisons of responses between the groups.
Data are shown as n (%). Group A: WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration. Group B: WNT/β-catenin signal activation and high-level CD8+ TIL infiltration or WNT/β-catenin signal inactivation and low-level CD8+ TIL infiltration. Group C: WNT/β-catenin signal activation and low-level CD8+ TIL infiltration. CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: objective response rate; DCR: disease control rate; TIL: tumor-infiltrating lymphocyte.
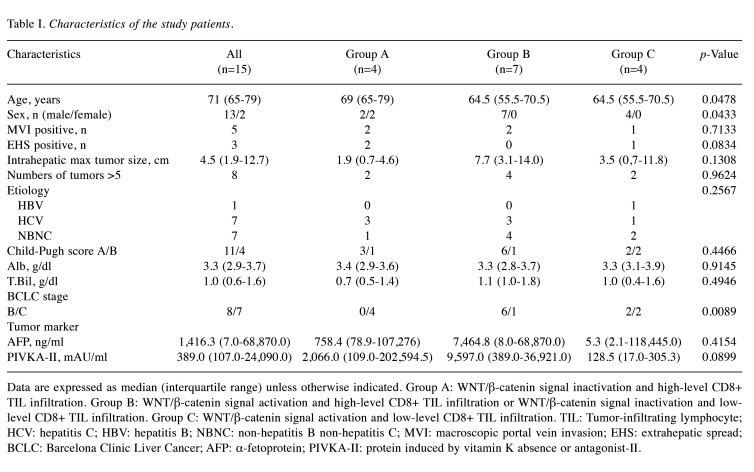
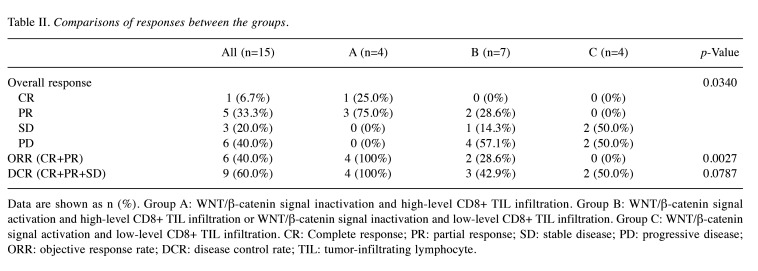
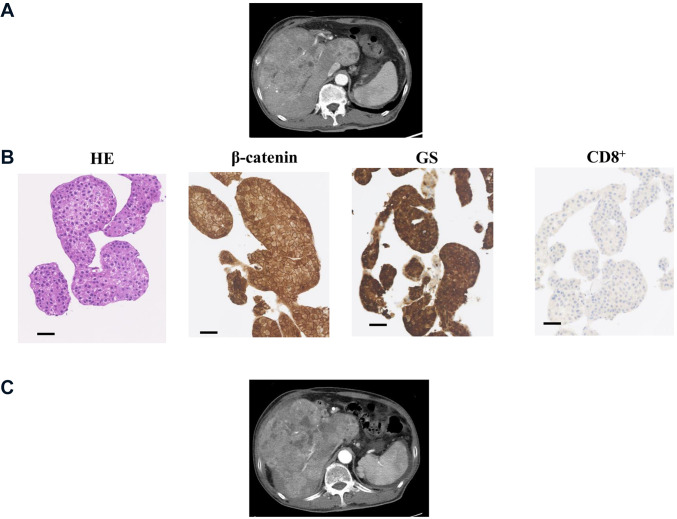
IHC for β-catenin, glutamine synthetase and CD8 in HCC tissues. The expression levels of β-catenin, GS, and CD8 were assessed by IHC prior to tremelimumab plus durvalumab therapy (Table III). Representative cases are shown in Figure 1 and Figure 2. Case 2 was a 79-year-old man with hepatitis C virus-related multiple HCC (Figure 1). The immunostaining in this moderately differentiated HCC revealed WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration. After administration of tremelimumab plus durvalumab, CT imaging of the liver showed decreased tumor size and enhancement in the arterial phase, indicating PR. The serum AFP level was 142,562.5 ng/ml before treatment and decreased to 8,544.8 ng/ml after three cycles. Case 9 was a 53-year-old man with hepatitis B virus-related HCC (Figure 2). The biopsy specimen was diagnosed as moderately differentiated HCC, and immunostaining revealed WNT/β-catenin signal activation with low-level CD8+ TIL infiltration. CT imaging demonstrated increased tumor size and enhancement in the arterial phase, indicating PD. The serum AFP level was 1,141,188.0 ng/ml before treatment and increased to 2,206,150.0 ng/ml after three cycles.
Table III. Wnt/β-catenin signal activation and CD8+ TIL infiltration in the 15 patients.
Group A: WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration. Group B: WNT/β-catenin signal activation and high-level CD8+ TIL infiltration or WNT/β-catenin signal inactivation and low-level CD8+ TIL infiltration. Group C: WNT/β-catenin signal activation and low-level CD8+ TIL infiltration. TIL: Tumor-infiltrating lymphocyte; HCV: hepatitis C; HBV: hepatitis B; NBNC: non-hepatitis B non-hepatitis C; GC: glutamine synthetase; PD: progressive disease; PR: partial response.
Figure 1.
Representative Case 2: WNT/β-catenin signal inactivation and high-level CD8+ tumor-infiltrating lymphocyte infiltration. (A) Computed tomography image of the early arterial phase prior to treatment. (B) Images of liver specimens subjected to hematoxylin-eosin (HE) staining (magnification: 100×; scale bar: 100 μm) and immunohistochemical staining for β-catenin, glutamine synthetase (GS), and CD8 (magnification: 100×, scale bar: 100 μm). (C) CT image of the early arterial phase to evaluate treatment efficacy.
Figure 2.
Representative Case 9: WNT/β-catenin signal activation and low-level CD8+ tumor-infiltrating lymphocyte infiltration. (A) Computed tomography image of the early arterial phase prior to treatment. (B) Images of liver specimens subjected to hematoxylin-eosin (HE) staining (magnification: 100×; scale bar: 100 μm) and immunohistochemical staining for β-catenin, glutamine synthetase (GS), and CD8 (magnification: 100×; scale bar: 100 μm). (C) CT image of the arterial phase to evaluate treatment efficacy.
Discussion
The immune response can play an important role in cancer progression. Based on the cellular and molecular characteristics, Llovet et al. (36) classified HCC into inflamed and non-inflamed classes. The inflamed class includes the immune-active, exhausted, and immune-like subtypes, while the non-inflamed class includes the intermediate and excluded subtypes.
The WNT/β-catenin cascade is a major signaling pathway for the regulation of liver carcinogenesis. Approximately 30%-40% of HCC cases were reported to show WNT/β-catenin signaling activation induced by gene mutations (37,38). WNT/β-catenin activation was shown to inhibit cytotoxic TIL infiltration in the immune microenvironment of melanoma, resulting in resistance to anti-PD-L1/anti-CTLA-4 monoclonal antibody therapy (39). WNT/β-catenin activation had negative effects on the DCR and progression-free survival (PFS) in patients with HCC who received anti-PD-L1 antibody therapy (11-13). Taken together, these studies indicate that WNT/β-catenin activation may be a biomarker for the response to ICI therapy in patients with HCC. We previously reported that CD8+ TIL infiltration could be a biomarker for predicting the response to atezolizumab plus bevacizumab and that the efficacy of atezolizumab plus bevacizumab may be unaffected by WNT/β-catenin signal activation (25,30). WNT/β-catenin signal activation causes immune exclusion, and HCC with WNT/β-catenin signal activation can be classified into two distinct types. Specifically, HCC with mutations causing WNT/β-catenin signal activation can be either inflamed with CD8+ T cell infiltration or non-inflamed without CD8+ T cell infiltration (26,27). We evaluated whether IHC staining of tumor tissues to assess WNT/β-catenin activation and CD8+ TIL infiltration classified into three groups could predict response to tremelimumab plus durvalumab in patients with HCC. We found that tremelimumab plus durvalumab was remarkably effective in HCC patients with WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration.
In this study, PFS and overall survival (OS) were not evaluated because of the small number of cases. ORR has been shown to predict OS in individuals with unresectable HCC (40,41). Regarding tremelimumab plus durvalumab therapy, the good response in HCC with WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration may result in long OS.
A limitation of the present study was the inclusion of only a small number of HCC patients who underwent tumor biopsies because of its nature as a single-center study. Furthermore, the study included advanced HCC cases of different stages. Finally, when analyzing a small number of cases, it is difficult to match groups according to liver function and tumor stage.
Conclusion
The present findings suggest that WNT/β-catenin signaling and CD8+ TIL infiltration, as evaluated by IHC staining of tumor biopsy tissues, may be useful biomarkers for predicting response to tremelimumab plus durvalumab therapy in patients with HCC. The findings suggest that tremelimumab plus durvalumab can be recommended for HCC with WNT/β-catenin signal inactivation and high-level CD8+ TIL infiltration based on IHC staining of tumor biopsy tissues. To improve the prognosis of patients with advanced HCC, further studies regarding chemotherapy selection are needed.
Funding
This study was conducted with the assistance of an Aso Iizuka Hospital Clinical Research Grant (grant no. AIH-CRG2024-4).
Conflicts of Interest
The Authors declare that they have no competing interests in relation to this study.
Authors’ Contributions
A.K., K.T., and K.M. designed the study. A.K., K.T., J.T., and H.S. assisted with data analyses. Y.O. performed pathological examinations, including immunostaining. A.K. wrote the initial draft of the manuscript. K.T. contributed to the analysis and interpretation of the data. K.T. and K.M. assisted in the preparation and critical review of the manuscript. All Authors approved the final version of the manuscript and have agreed to be accountable for all aspects of the work.
Acknowledgements
The Authors thank Y. Ishibashi for assistance with manuscript preparation. The Authors also thank Alison Sherwin, Ph.D., from Edanz (https://jp.edanz/com/ac) for editing a draft of this manuscript.
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44(S19):96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, IMbrave150 Investigators Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M, KEYNOTE-224 investigators Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 7.Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol Res. 2018;48(8):597–607. doi: 10.1111/hepr.13181. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 9.Kuwano A, Yada M, Tanaka K, Koga Y, Nagasawa S, Masumoto A, Motomura K. Systemic chemotherapy for advanced hepatocellular carcinoma in patients with Child-Pugh class B. Cancer Diagn Progn. 2024;4(2):111–116. doi: 10.21873/cdp.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordan JD, Kennedy EB, Abou-Alfa GK, Beal E, Finn RS, Gade TP, Goff L, Gupta S, Guy J, Hoang HT, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Kortmansky J, Leaf A, Remak WM, Sohal DPS, Taddei TH, Wilson Woods A, Yarchoan M, Rose MG. Systemic therapy for advanced hepatocellular carcinoma: ASCO Guideline update. J Clin Oncol. 2024;42(15):1830–1850. doi: 10.1200/JCO.23.02745. [DOI] [PubMed] [Google Scholar]
- 11.Pinyol R, Sia D, Llovet JM. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res. 2019;25(7):2021–2023. doi: 10.1158/1078-0432.CCR-18-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika I, Do RK, Sun Y, Kingham TP, D’Angelica MI, Berger MF, Hyman DM, Jarnagin W, Klimstra DS, Janjigian YY, Solit DB, Schultz N, Abou-Alfa GK. Prospective genotyping of hepatocellular carcinoma: Clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25(7):2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Zhou Q, Liu J, Zhang W. CTNNB1 alternation is a potential biomarker for immunotherapy prognosis in patients with hepatocellular carcinoma. Front Immunol. 2021;12:759565. doi: 10.3389/fimmu.2021.759565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwano A, Tanaka K, Yada M, Nagasawa S, Morita Y, Masumoto A, Motomura K. Therapeutic efficacy of lenvatinib for hepatocellular carcinoma with iso-high intensity in the hepatobiliary phase of Gd-EOB-DTPA-MRI. Mol Clin Oncol. 2022;16(2):53. doi: 10.3892/mco.2021.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuta K, Ishii G, Kim E, Shiono S, Nishiwaki Y, Endoh Y, Kodama T, Nagai K, Nagai K. Primary lung adenocarcinoma with massive lymphocyte infiltration. Am J Clin Pathol. 2005;123(4):547–552. doi: 10.1309/APKQ-4Q9D-52GN-LR8W. [DOI] [PubMed] [Google Scholar]
- 17.Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92(4):651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Matsutani S, Hirakawa K, Ohira M. The prognostic significance of the tumor-infiltrating programmed cell death-1+ to CD8+ lymphocyte ratio in patients with colorectal cancer. Anticancer Res. 2017;37(8):4165–4172. doi: 10.21873/anticanres.11804. [DOI] [PubMed] [Google Scholar]
- 19.Schillaci R, Salatino M, Cassataro J, Proietti CJ, Giambartolomei GH, Rivas MA, Carnevale RP, Charreau EH, Elizalde PV. Immunization with murine breast cancer cells treated with antisense oligodeoxynucleotides to type I insulin-like growth factor receptor induced an antitumoral effect mediated by a CD8+ response involving Fas/Fas ligand cytotoxic pathway. J Immunol. 2006;176(6):3426–3437. doi: 10.4049/jimmunol.176.6.3426. [DOI] [PubMed] [Google Scholar]
- 20.Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA. CD8-mediated type 1 antitumor responses selectively modulate endogenous differentiated and nondifferentiated T cell localization, activation, and function in progressive breast cancer. J Immunol. 2006;177(11):8191–8201. doi: 10.4049/jimmunol.177.11.8191. [DOI] [PubMed] [Google Scholar]
- 21.Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147(4):366. doi: 10.1001/archsurg.2012.35. [DOI] [PubMed] [Google Scholar]
- 22.Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, Kinoshita T, Mizukami N, Ono H, Kage M, Hoshino T. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi: 10.1016/j.ejca.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, Yau T, Kaseb AO, Burgoyne AM, Dayyani F, Spahn J, Verret W, Finn RS, Toh HC, Lujambio A, Wang Y. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28(8):1599–1611. doi: 10.1038/s41591-022-01868-2. [DOI] [PubMed] [Google Scholar]
- 25.Kuwano A, Yada M, Miyazaki Y, Tanaka K, Kurosaka K, Ohishi Y, Masumoto A, Motomura K. Tumor-infiltrating CD8(+) T cells as a biomarker for chemotherapy efficacy in unresectable hepatocellular carcinoma. Oncol Lett. 2023;25(6):259. doi: 10.3892/ol.2023.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montironi C, Castet F, Haber PK, Pinyol R, Torres-Martin M, Torrens L, Mesropian A, Wang H, Puigvehi M, Maeda M, Leow WQ, Harrod E, Taik P, Chinburen J, Taivanbaatar E, Chinbold E, Solé Arqués M, Donovan M, Thung S, Neely J, Mazzaferro V, Anderson J, Roayaie S, Schwartz M, Villanueva A, Friedman SL, Uzilov A, Sia D, Llovet JM. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut. 2023;72(1):129–140. doi: 10.1136/gutjnl-2021-325918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M. Combination immunotherapy with anti-PD-1/PD-L1 antibody plus anti-VEGF antibody may promote cytotoxic T lymphocyte infiltration in hepatocellular carcinoma, including in the noninflamed subclass. Liver Cancer. 2022;11(3):185–191. doi: 10.1159/000524977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019;25(10):3074–3083. doi: 10.1158/1078-0432.CCR-18-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Kuwano A, Yada M, Narutomi F, Nagasawa S, Tanaka K, Kurosaka K, Ohishi Y, Masumoto A, Motomura K. Therapeutic efficacy of atezolizumab plus bevacizumab for hepatocellular carcinoma with WNT/β-catenin signal activation. Oncol Lett. 2022;24(1):216. doi: 10.3892/ol.2022.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157(3):763–770. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P, Perret C. Differential effects of inactivated Axin1 and activated β-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26(5):774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 33.Tsujikawa H, Masugi Y, Yamazaki K, Itano O, Kitagawa Y, Sakamoto M. Immunohistochemical molecular analysis indicates hepatocellular carcinoma subgroups that reflect tumor aggressiveness. Hum Pathol. 2016;50:24–33. doi: 10.1016/j.humpath.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Ueno A, Masugi Y, Yamazaki K, Komuta M, Effendi K, Tanami Y, Tsujikawa H, Tanimoto A, Okuda S, Itano O, Kitagawa Y, Kuribayashi S, Sakamoto M. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014;61(5):1080–1087. doi: 10.1016/j.jhep.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TB, Roncalli M, Di Tommaso L, Kakar S. Combined use of heat-shock protein 70 and glutamine synthetase is useful in the distinction of typical hepatocellular adenoma from atypical hepatocellular neoplasms and well-differentiated hepatocellular carcinoma. Mod Pathol. 2016;29(3):283–292. doi: 10.1038/modpathol.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 37.Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N, Losic B, Waxman S, Thung SN, Mazzaferro V, Esteller M, Friedman SL, Schwartz M, Villanueva A, Llovet JM. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153(3):812–826. doi: 10.1053/j.gastro.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 40.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Cheng AL, Vogel A, Tovoli F, Ueshima K, Aikata H, López CL, Pracht M, Meng Z, Daniele B, Park JW, Palmer D, Tamai T, Saito K, Dutcus CE, Lencioni R. Overall survival and objective response in advanced unresectable hepatocellular carcinoma: A subanalysis of the REFLECT study. J Hepatol. 2023;78(1):133–141. doi: 10.1016/j.jhep.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Kudo M, Montal R, Finn RS, Castet F, Ueshima K, Nishida N, Haber PK, Hu Y, Chiba Y, Schwartz M, Meyer T, Lencioni R, Llovet JM. Objective response predicts survival in advanced hepatocellular carcinoma treated with systemic therapies. Clin Cancer Res. 2022;28(16):3443–3451. doi: 10.1158/1078-0432.CCR-21-3135. [DOI] [PubMed] [Google Scholar]