Abstract
Background/Aim
Despite the well-publicized clinical outcomes after unplanned excision (UE) and re-excision (re-excision) in patients with soft-tissue sarcoma (STS), there is little information about the real-life referral patterns for UE, such as patient profile, details of procedures, and subsequent management after UE. We aimed to investigate the characteristics of patients with UE who were referred to sarcoma-specific centers.
Patients and Methods
Between May 2022 and June 2023, we registered 97 patients who underwent UE and were referred to sarcoma-specific centers in Japan. We excluded those with well-differentiated liposarcomas and dermatofibrosarcoma protuberances. We investigated the details of UE and additional treatment after UE.
Results
There were 49 men and 48 women, with a mean age of 62 years. A broad range of surgeons performed UE; 36 plastic surgeons, 22 orthopedic surgeons, 17 general surgeons, 17 dermatologists, and 5 others. The mean tumor size was 4.1 cm. Local anesthesia was administered to 58 patients. Forty-five patients underwent UE without prior magnetic resonance imaging. Inappropriate transverse skin incisions were performed in 42 patients. Of the 97 patients, 82 underwent re-excision after UE. The mean time between UE and date of initial presentation at the referral hospital was 46 days. The mean interval between UE and re-excision was 96 days. Of the 82 patients, 59 underwent soft-tissue reconstruction after re-excision.
Conclusion
A broad range of surgeons performed UE. Continuous education about STS should be considered for all surgeons. UE should be avoided because residual tumors are common, and reconstructive surgery may be necessary.
Keywords: Soft-tissue sarcoma, unplanned excision, re-excision
Soft tissue sarcomas (STS) are rare and heterogeneous. The age-adjusted incidence rate of STS was reported to be 2.4 per 100,000 people per year, and STS accounted for 0.7% of all cancers diagnosed in the United States in 2022 (1). This rarity may lead to STS not being considered as a differential diagnosis when physicians find a mass. Unfortunately, unplanned excision (UE) tends to occur in nonspecialized sarcoma centers, where STS is seldom treated. A lack of knowledge regarding the principles of STS treatment results in incomplete preoperative imaging, such as magnetic resonance imaging (MRI), inappropriate surgical procedures, and incomplete tumor resection (R1 or R2 resection). Although complete tumor resection (R0 resection) is necessary to prevent local recurrence, the rate of residual tumors after re-excision following UE varies from 31% to 74% (2). Therefore, re-excision should be considered to achieve complete tumor removal. Patients who undergo UE have been reported to have worse outcomes (3) than those who undergo planned excision, however, some authors have shown that patients who undergo UE have similar or improved outcomes (4-6). Despite these well-publicized clinical outcomes after UE and re-excision in patients with STS, there is little information on the real-life referral patterns of UE, such as patient profiles, details of procedures, and subsequent management after UE (7,8). Therefore, we aimed to investigate the details of patients with UE who were referred to sarcoma-specific centers belonging to the Bone and Soft Tissue Tumor Study Group of the Japan Clinical Oncology Group (JCOG).
Patients and Methods
This study was approved by the Institutional Review Board of the Authors’ affiliated hospitals. The requirement for informed consent was waived due to the nature of the study. Between May 2022 and June 2023, we registered 97 patients who underwent UE and were referred to specialists belonging to the JCOG at 32 institutions in Japan. We excluded patients with well-differentiated liposarcomas and dermatofibrosarcoma protuberances. Excisional biopsy was allowed after preoperative MRI when the tumor size and depth were <2 cm and superficial, respectively (9).
The following data were collected: (i) type of hospital (private clinic, general hospital, or cancer center/university) and department that undertook the UE; (ii) tumor characteristics recorded at the previous hospital (tumor size, depth, and site); (iii) details of the UE (aim of resection, anesthesia, skin incision, preoperative MRI examination); (iv) pathological diagnosis; and (v) information of re-excision (preoperative examination, duration of UE and re-excision, pathological evaluation and surgical procedure).
UE was defined as follows: (a) tumor resection without awareness of STS; (b) surgery without preoperative MRI; (c) surgery under local anesthesia; or (d) an inappropriate skin incision (transverse skin incision).
Statistical analysis. Statistical associations were evaluated between clinical backgrounds using the Mann-Whitney U-test and Kruskal-Wallis test for continuous variables and the chi-squared test for categorical variables. All statistical analyses were performed using the EZR graphical user interface (Saitama Medical Center, Jichi Medical University, Saitama, Japan) in R (The R Foundation for Statistical Computing, Vienna, Austria). This package is a modified version of the R Commander, which is frequently used in biostatistics to add statistical functions. Statistical significance was set at p<0.05.
Results
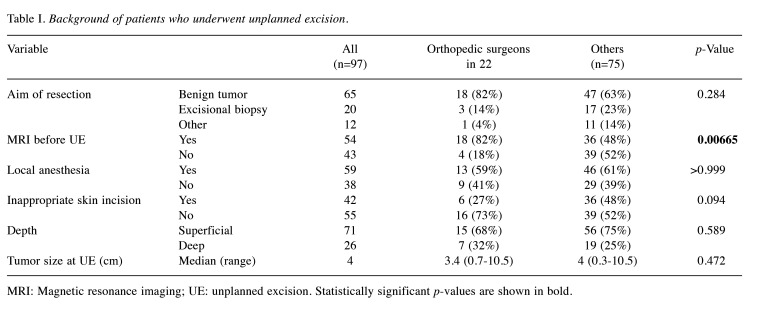
A total of 97 patients were included in the study. There were 49 men and 48 women with a mean age of 62 years (range=9-94 years). UE was performed in private clinics in 23, general hospitals in 66, and university hospitals in 8 cases. A broad range of surgeons performed UE: plastic surgeons in 36 cases, orthopedic surgeons in 22, general surgeons in 17, dermatologists in 17, and other types in 5 cases. The mean and median tumor size was 4.1 cm and 4 cm, respectively, ranging from 0.3 to 10.5 cm. The tumor size was unknown in 11 patients. Tumors were superficial in 75 patients and deep in 22 patients. The tumor sites included the thigh in 16, forearm in 12, leg in 12, upper arm in 9, inguinal region in 9, back in 8, shoulder in 5, and others in 26. The aim at UE was resection of a benign tumor in 65, excisional biopsy in 20, and others, such as a diagnosis of inguinal herniation and hematoma, in 12. Of the 20 patients who underwent excisional biopsy, five had STS <2 cm; however, they did not fulfill the definition for excisional biopsy according to the Japanese Orthopaedic Association (JOA) guidelines (9).
In addition to the aim of UE, we investigated inappropriate surgical procedures. Local anesthesia was administered to 58 patients. Forty-five patients underwent UE without preoperative MRI. Inappropriate transverse skin incisions were made in 42 patients (Table I). Drain insertion was performed in 19 patients. UE cases were classified into two groups, according to the surgeons who performed it: orthopedic surgeons in 22 cases and other surgeons in 75 cases. MRI was more frequently performed by orthopedic surgeons (p=0.0067). The aim of resection of the mass by orthopedic surgeons was benign tumor resection in 18, excisional biopsy in 3, and other in 1, while the aim of mass resection by other surgeons was benign tumor resection in 47, excisional biopsy in 17, and other in 11. Three out of 20 patients who were treated with the aim of excisional biopsy satisfied the definition of excisional biopsy according to guidelines. The reasons for inappropriate excisional biopsy are shown in Table I.
Table I. Background of patients who underwent unplanned excision.
MRI: Magnetic resonance imaging; UE: unplanned excision. Statistically significant p-values are shown in bold.
The histological diagnoses included myxofibrosarcoma in 24, undifferentiated pleomorphic sarcoma in 18, leiomyosarcoma in 11, dedifferentiated liposarcoma in 7, synovial sarcoma in 6, and others in 31. Surgical margins were classified as R0 in 2, R1 in 38, R2 in 29, and unknown in 29.
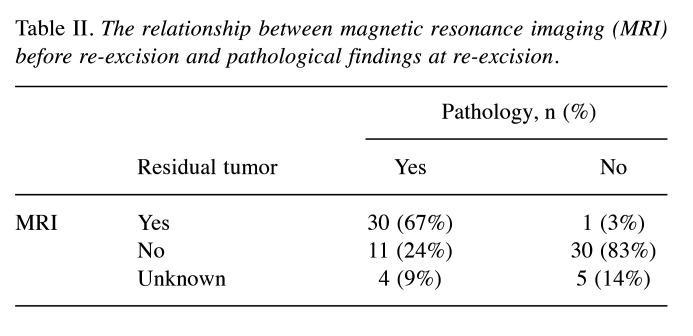
Of the 97 patients, 82 underwent re-excision after UE. The median and mean intervals between UE and the date of initial presentation at the referral hospital were 34 and 46 days (range=8-197 days), respectively. The median and mean intervals between UE and re-excision were 81 and 96 days (range=32-290 days, respectively. Of the 82 patients, 81 underwent MRI before re-excision. Seventy-five patients underwent gadolinium-enhanced MRI. On MRI, residual tumor was observed in 31 patients, whereas no residual tumor was observed in 41 patients. It was not possible to evaluate residual tumor on MRI in nine patients. Thirty out of the 31 patients with possible residual tumor on MRI after UE had pathologically-proven residual tumors at re-excision, while 30 out of the 41 patients with no apparent residual tumor on MRI after UE had no residual tumor at re-excision. The relationship between MRI findings and pathological evaluation of the residual tumor in the re-excision is shown in Table II.
Table II. The relationship between magnetic resonance imaging (MRI) before re-excision and pathological findings at re-excision.
For the 82 patients undergoing re-excision, soft-tissue reconstruction after re-excision was performed in 59 (72%) as follows: pedicle or free soft-tissue flaps in 44, skin grafts in 17, and other in 3. Artificial blood vessels were replaced in two patients. Of the 36 patients without pathological evidence of residual tumor at the re-excision, 18 underwent soft-tissue flap reconstruction.
Re-excision was not performed in 15/97 patients for the following reasons: patient refusal in 10, comorbidities in 41, and chemotherapy and radiotherapy without re-excision in 1.
Discussion
Using the bone and soft-tissue tumor registry in Japan, the mean incidence rate of UE for STS was reported to be 11.3% (10). Over 11 years, out of the 8,761 registered patients with STS, 991 were referred after undergoing UE. Although re-excision after UE for STS may improve local control and survival, other aspects of the effects of UE should be considered. Alamanda et al. reported a detailed analysis of the financial burden of UE and found that the total cost of UE was almost double that of planned excision at a specialized sarcoma center (11). Umer et al. commented that from the patient’s point of view, one operation was better than two, as it meant less time in the hospital and away from work (12). Another problem with UE is that it is often performed using inappropriate approaches (e.g. transverse incisions) and may be accompanied by the insertion of a drain, which further extends the field of contamination, leading to a need for soft-tissue reconstruction (13-15).
In the United Kingdom, the National Institute of Clinical Excellence proposed urgent referral criteria for STS to reduce UE (16). In Japan, clinical practice guidelines for STS published by the JOA propose that STS should be suspected if the tumors are >5 cm or are deep (9). In the present study, the mean tumor size was 4.1 cm and 77% of the tumors were superficial. In addition to assessing tumor size, depth, and rapid progression, MRI should be performed before surgery according to the guidelines (9,17). However, MRI is often not performed in patients with UE. Some authors have reported that only 21% and 23.8% of patients treated with UE underwent preoperative MRI (14,18). In this study, MRI was frequently performed by orthopedic surgeons (82%) (Table I). Orthopedic surgeons may diagnose the tumor as benign, on the basis of MRI reports, and resect the tumor as benign. In contrast, only half of the patients (48%) treated by other surgeons, including plastic, general, and dermatology surgeons, underwent preoperative MRI. This finding may indicate that there is a lack of consideration of the possibility of STS before UE. In addition, for UE, in our study the aim of resection by surgeons, except for orthopedic surgeons, was an excisional biopsy. However, a few patients satisfied the definition for undergoing an excisional biopsy according to the JOA guidelines (9). The surgeons may not have been aware of the existence of guidelines for STS because they do not belong to the JOA. Therefore, continuous education about STS should be considered for all surgeons.
Differentiating between residual tumor and postsurgical changes remains a challenge, irrespective of whether MRI has been performed after previous UE. Examinations are often confounded by the presence of postoperative edema, hematoma, and seroma (19).
Davies et al. reported that the diagnostic performance of MRI for residual tumor in 104 patients with UE had a sensitivity of 64% and a specificity of 93% (20). Gingrich et al. analyzed 76 patients with UE and reported that the sensitivity and specificity of MRI for predicting residual STS were 86.7% and 57.9%, respectively, with an overall accuracy of 78.1% (21). In our study, 30 out of 31 patients (97%) with possible residual tumor on MRI had a pathologically confirmed residual tumor in the re-excision. However, only 30 out of the 41 patients (73%) with no evidence of residual tumor on MRI had no residual tumor in the re-excision. Despite this, MRI remains a valuable tool before revision surgery that is performed after UE. It can aid in preoperative planning by identifying the site and extent of previous surgery.
Although urgent re-excision is logically appropriate after UE, the time window before re-excision to minimize the risk of local recurrence and increase survival has not been established. Funovics et al. reported, for 310 patients undergoing UE, that overall survival was a significantly better for patients with earlier re-excision (within 12 weeks of UE), than when re-excision occurred after a longer time (15). In their study, the duration between the UE and re-excision was approximately 12 weeks. The causes of delays in re-excision are complicated. Firstly, surgeons may refer patients to a specialized sarcoma center after a pathological diagnosis. In our study the median and mean intervals between UE and the date of initial presentation at the referral hospital were 34 and 46 days, respectively. Furthermore, pathological examinations may be performed at a specialized referral sarcoma center because pathologists at nonspecialized centers may be less familiar with STS. In our study, 72% of patients underwent soft-tissue reconstruction after re-excision. Re-excision after UE often requires a larger excision than might have been performed if it was an initially planned, and can sometimes be mutilating, necessitating additional techniques. Combined surgery by orthopedic and plastic surgeons may be necessary, and arrangement of the date for re-excision and reconstruction is necessary.
A literature review revealed a wide range (24-91%) in the incidence of residual tumor cells in re-excision (21). Recently, however, a French research group reported that the ‘watch and wait’ approach for re-excision after UE in R0 or R1 excision of STS was safe and did not affect the metastatic risk (22). They suggested that the advantage of delaying re-excision until the time of local recurrence was that surgery was better guided by the tumor mass than immediate re-excision after UE. However, concerns remain regarding survival due to the timing of the additional excision.
The major limitations of our study are inherent in its observational nature. Therefore, management within the JCOG is heterogeneous, reflecting the absence of a consensus and shared guidelines for the management of patients after UE.
Conclusion
A broad range of surgeons perform UE. Continuous education about STS should be considered for all surgeons. In particular, surgeons should be familiar with the guidelines for STS, including the definition of excisional biopsy. UE should be avoided because residual tumors are common, and reconstructive surgery may be necessary.
Conflicts of Interest
The Authors declare no conflicts of interest for this study.
Authors’ Contributions
Tomoki Nakamura contributed to the study conception and design. Material preparation, data collection and analysis were performed by all Authors. The first draft of the manuscript was written by Tomoki Nakamura and all Authors commented on previous versions of the article. All Authors read and approved the final article.
Acknowledgements
The Authors thank Masanori Okamoto, Tomohiro Fujiwara, Jungo Imanishi, Daisuke Kubota, Toshio Kojima, Hideki Nishimura and Yuki Funauchi for their contributions to the study protocol. We also appreciate the support by the Network of Exploration for Orthopaedic Tumor Surgeons (NEOS) of the Bone and Soft Tissue Tumor Study Group of the JCOG.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Grimer R, Parry M, James S. Inadvertent excision of malignant soft tissue tumours. EFORT Open Rev. 2019;4(6):321–329. doi: 10.1302/2058-5241.4.180060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeed H, King DM, Johnstone CA, Charlson JA, Hackbarth DA, Neilson JC, Bedi M. Preoperative radiation therapy followed by reexcision may improve local control and progression-free survival in unplanned excisions of soft tissue sarcomas of the extremity and chest-wall. Int J Surg Oncol. 2016;2016:5963167. doi: 10.1155/2016/5963167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Kawai A, Hagi T, Asanuma K, Sudo A. A comparison of clinical outcomes between additional excision after unplanned and planned excisions in patients with soft-tissue sarcoma of the limb: A propensity-matching cohort study. Bone Joint J. 2021;103-B(12):1809–1814. doi: 10.1302/0301-620X.103B12.BJJ-2021-0037.R1. [DOI] [PubMed] [Google Scholar]
- 5.Sacchetti F, Alsina AC, Morganti R, Innocenti M, Andreani L, Muratori F, Scoccianti G, Totti F, Campanacci DA, Capanna R. Re-excision after unplanned excision of soft tissue sarcoma: A systematic review and metanalysis. The rationale of systematic re-excision. J Orthop. 2021;25:244–251. doi: 10.1016/j.jor.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scoccianti G, Innocenti M, Frenos F, Muratori F, Sacchetti F, Beltrami G, Capanna R, Campanacci DA. Re-excision after unplanned excision of soft tissue sarcomas: Long-term results. Surg Oncol. 2020;34:212–217. doi: 10.1016/j.suronc.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesan M, Richards CJ, McCulloch TA, Perks AGB, Raurell A, Ashford RU, East Midland Sarcoma Service Inadvertent surgical resection of soft tissue sarcomas. Eur J Surg Oncol. 2012;38(4):346–351. doi: 10.1016/j.ejso.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Belzarena AC, Binitie O, Letson GD, Joyce DM. Unplanned sarcoma excisions: understanding how they happen. J Am Acad Orthop Surg Glob Res Rev. 2024;8(1):e23.00176. doi: 10.5435/JAAOSGlobal-D-23-00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Japanese Orthopaedic Association . Tokyo: Nankodo. 2020. Clinical Practice Guidelines on the Management of Soft Tissue Tumors. First Edition. [Google Scholar]
- 10.Nakamura T, Kawai A, Sudo A. The incidence of unplanned excision in patients with soft tissue sarcoma: Reports from the Bone and Soft Tissue Tumor registry in Japan. J Orthop Sci. 2022;27(2):468–472. doi: 10.1016/j.jos.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Alamanda VK, Delisca GO, Mathis SL, Archer KR, Ehrenfeld JM, Miller MW, Homlar KC, Halpern JL, Schwartz HS, Holt GE. The financial burden of reexcising incompletely excised soft tissue sarcomas: a cost analysis. Ann Surg Oncol. 2013;20(9):2808–2814. doi: 10.1245/s10434-013-2995-5. [DOI] [PubMed] [Google Scholar]
- 12.Umer HM, Umer M, Qadir I, Abbasi N, Masood N. Impact of unplanned excision on prognosis of patients with extremity soft tissue sarcoma. Sarcoma. 2013;2013:498604. doi: 10.1155/2013/498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, Kawai A, Asanuma K, Hagi T, Sudo A. Is no additional excision after unplanned excision with positive margins justified in patients with small (≤5 cm) high-grade soft-tissue sarcoma?: Analysis from the Bone and Soft Tissue Tumor registry in Japan. J Orthop Sci. 2022;27(2):463–467. doi: 10.1016/j.jos.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Morinaga S, Miwa S, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, Tada K, Langit MB, Yonezawa H, Araki Y, Asano Y, Tsuchiya H. Clinical characteristics of patients with undergoing unplanned excisions of malignant soft tissue tumors. J Orthop Surg. 2021;29(3):230949902110575. doi: 10.1177/23094990211057597. [DOI] [PubMed] [Google Scholar]
- 15.Funovics PT, Vaselic S, Panotopoulos J, Kotz RI, Dominkus M. The impact of re-excision of inadequately resected soft tissue sarcomas on surgical therapy, results, and prognosis: A single institution experience with 682 patients. J Surg Oncol. 2010;102(6):626–633. doi: 10.1002/jso.21639. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara T, Evans S, Stevenson J, Tsuda Y, Gregory J, Grimer R, Abudu A. Impact of the national sarcoma guidelines on the prevalence and outcome of inadvertent excisions of soft tissue sarcomas: An observational study from a UK tertiary referral centre. Eur J Surg Oncol. 2022;48(3):533–540. doi: 10.1016/j.ejso.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, De Álava E, Del Muro XG, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hecker-Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss S, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Casali PG, Stacchiotti S, ESMO Guidelines Committee, EURACAN and GENTURIS Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up✩. Ann Oncol. 2021;32(11):1348–1365. doi: 10.1016/j.annonc.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi M, Ieguchi M, Takami M, Aono M, Taguchi S, Kuroda T, Takaoka K. Clinical problems after initial unplanned resection of sarcoma. Jpn J Clin Oncol. 2008;38(10):701–709. doi: 10.1093/jjco/hyn093. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Pretell-Mazzini J, Kerr DA, Chelala L, Yang X, Jose J, Subhawnog TK. MRI findings associated with microscopic residual tumor following unplanned excision of soft-tissue sarcomas in the extremities. Skeletal Radiol. 2018;47(2):181–190. doi: 10.1007/s00256-017-2762-y. [DOI] [PubMed] [Google Scholar]
- 20.Davies AM, Mehr A, Parsonage S, Evans N, Grimer RJ, Pynsent PB. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol. 2004;14(3):506–513. doi: 10.1007/s00330-003-2023-4. [DOI] [PubMed] [Google Scholar]
- 21.Gingrich AA, Elias A, Michael Lee CY, Nakache YN, Li CS, Shah DR, Boutin RD, Canter RJ. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J Surg Res. 2017;208:26–32. doi: 10.1016/j.jss.2016.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decanter G, Stoeckle E, Honore C, Meeus P, Mattei JC, Dubray-Longeras P, Ferron G, Carrere S, Causeret S, Guilloit JM, Fau M, Rosset P, Machiavello JC, Delhorme JB, Regenet N, Gouin F, Blay JY, Coindre JM, Penel N, Bonvalot S. Watch and wait approach for re-excision after unplanned yet macroscopically complete excision of extremity and superficial truncal soft tissue sarcoma is safe and does not affect metastatic risk or amputation rate. Ann Surg Oncol. 2019;26(11):3526–3534. doi: 10.1245/s10434-019-07494-6. [DOI] [PubMed] [Google Scholar]