Abstract
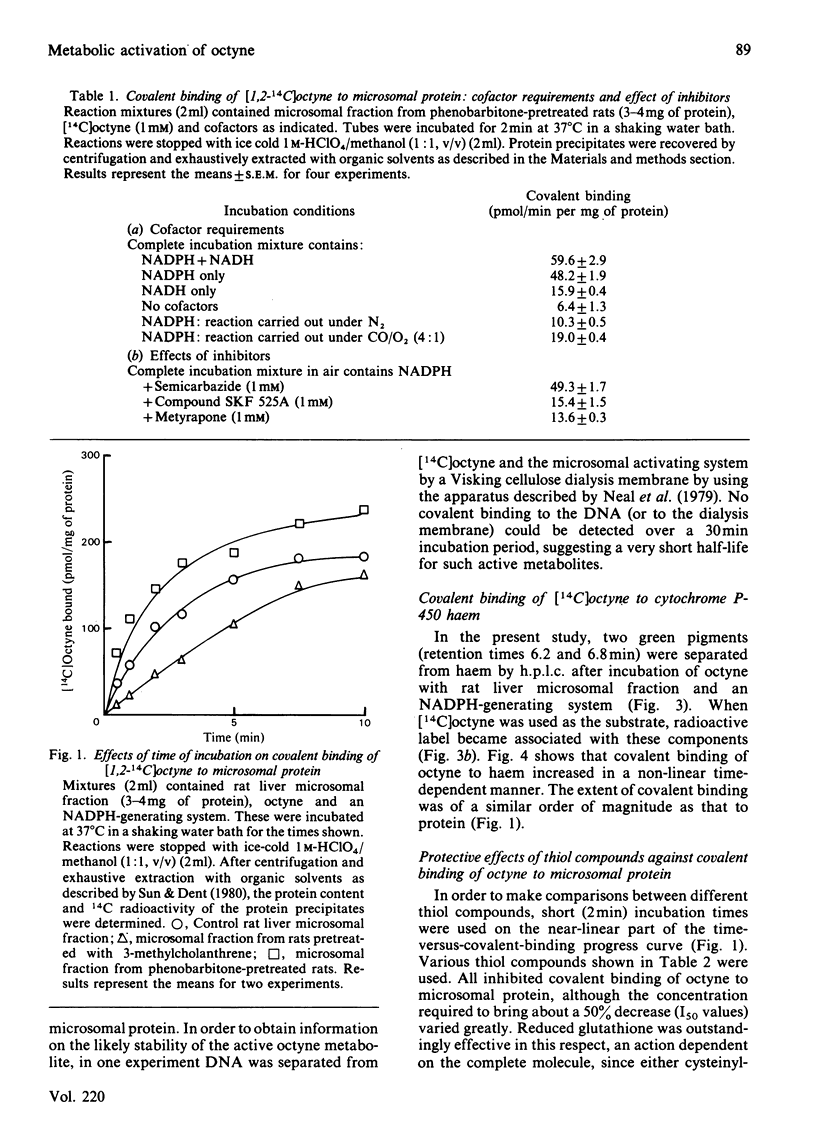
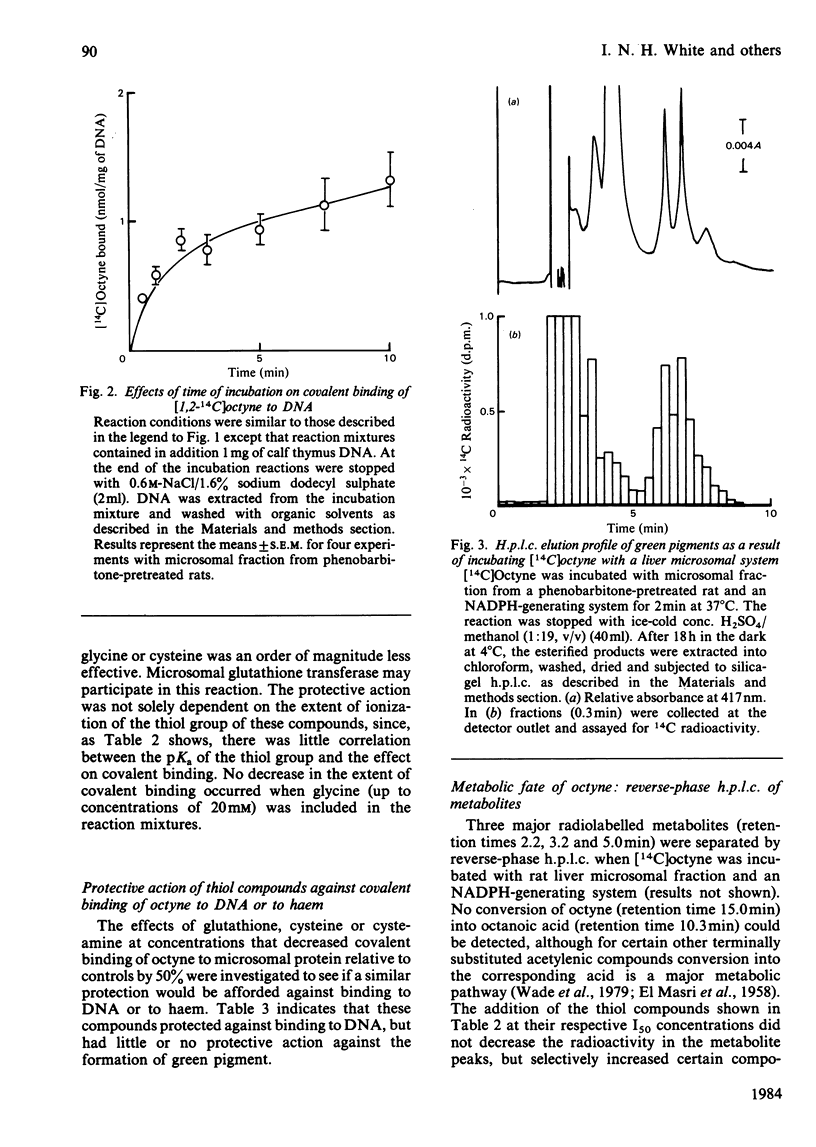
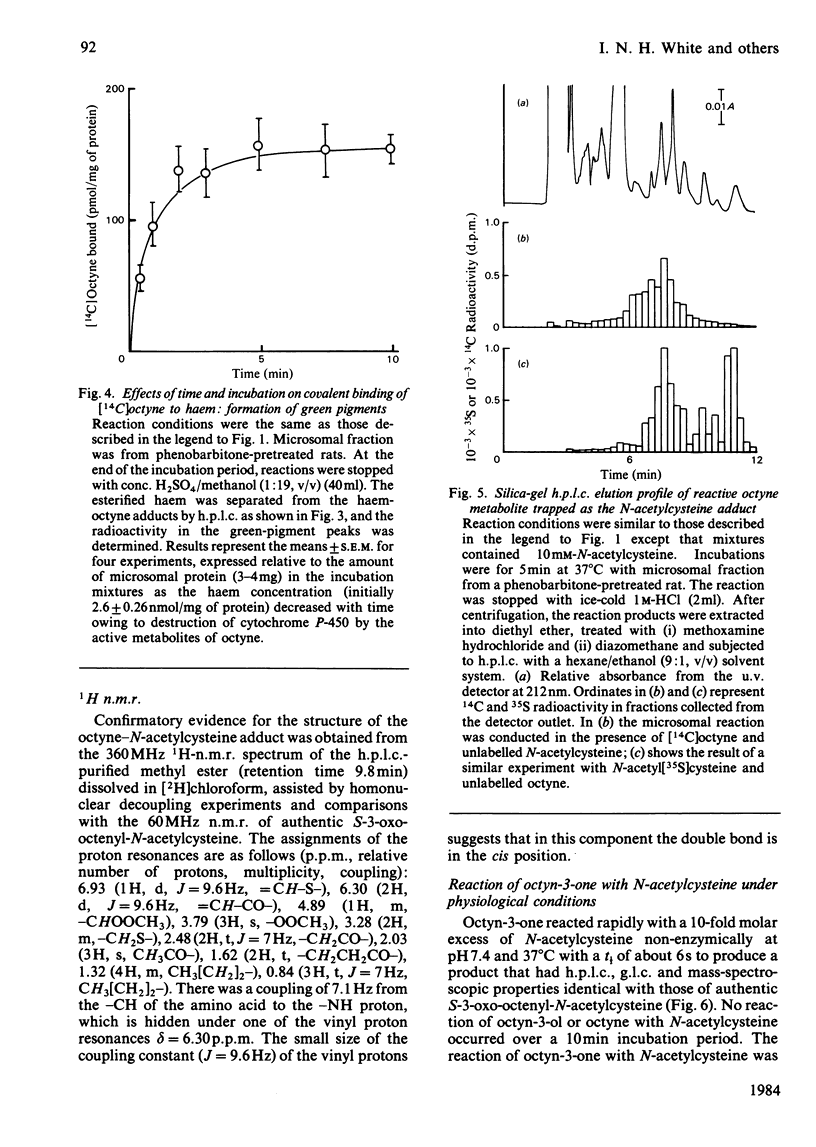
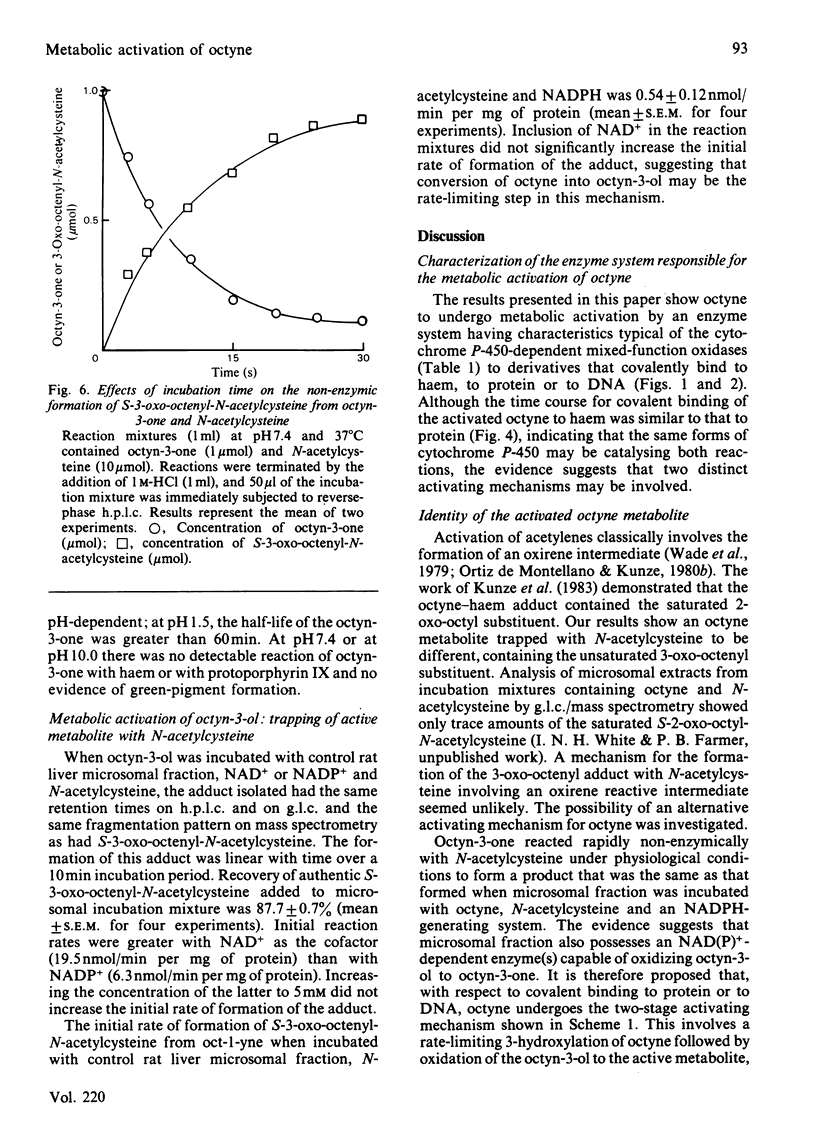
[1,2-14C]Oct-l-yne was used to investigate metabolic activation of the ethynyl substituent in vitro. Activation of octyne by liver microsomal cytochrome P-450-dependent enzymes gave intermediate(s) that bound covalently to protein, DNA and to haem. The time course and extent of covalent binding of octyne to haem and to protein were similar. However, two different activating mechanisms are probably involved. Whereas covalent binding to protein or to DNA was inhibited by nucleophiles such as N-acetylcysteine, that to haem was little affected. When N-acetylcysteine was included in the reaction mixtures, two major octyne-N-acetylcysteine adducts were isolated and purified by high-pressure liquid chromatography. G.l.c.-mass spectrometry and n.m.r. suggest that these are the cis-trans isomers of S-3-oxo-oct-1-enyl-N-acetylcysteine. Oct-1-yn-3-one reacted non-enzymically with N-acetylcysteine at pH 7.4 and 37 degrees C with a t1/2 of about 6 s also to yield S-3-oxo-oct-l-enyl-N-acetylcysteine. The same product was formed when microsomal fractions were incubated with oct-1-yn-3-ol, N-acetylcysteine and NAD(P)+. Octyn-3-one did not appear to react with haem or protoporphyrin IX. 5. A mechanism for the metabolic activation of oct-1-yne is proposed, consisting in (a) microsomal hydroxylation of the carbon atom alpha to the acetylenic bond and (b) oxidation to yield octyn-3-one as the reactive species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolt H. M. Structural modifications in contraceptive steroids altering their metabolism and toxicity. Arch Toxicol. 1977 Dec 30;39(1-2):13–19. doi: 10.1007/BF00343271. [DOI] [PubMed] [Google Scholar]
- EL MASRI A. M., SMITH J. N., WILLIAMS R. T. Studies in detoxication. 73. The metabolism of alkylbenzenes: phenylacetylene and phenylethylene (styrene). Biochem J. 1958 Feb;68(2):199–204. doi: 10.1042/bj0680199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze K. L., Mangold B. L., Wheeler C., Beilan H. S., Ortiz de Montellano P. R. The cytochrome P-450 active site. Regiospecificity of prosthetic heme alkylation by olefins and acetylenes. J Biol Chem. 1983 Apr 10;258(7):4202–4207. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindeke B., Hallström G., Anderson E. Enzymic oxidation alpha to the acetylenic group in the metabolism of N-(5-pyrrolidinopent-3-ynyl)-succinimide (BL 14) in vitro. Xenobiotica. 1978 Jun;8(6):341–348. doi: 10.3109/00498257809070017. [DOI] [PubMed] [Google Scholar]
- Loosemore M. J., Wogan G. N., Walsh C. Determination of partition ratios for allylisopropylacetamide during suicidal processing by a phenobarbital-induced cytochrome P-450 isozyme from rat liver. J Biol Chem. 1981 Aug 25;256(16):8705–8712. [PubMed] [Google Scholar]
- Metcalf J. F., Helmsen R. Immunoelectron microscopic localization of herpes simplex virus antigens in rabbit cornea with antihuman IgG-antiferritin hybrid antibodies. Invest Ophthalmol Vis Sci. 1977 Sep;16(9):779–786. [PubMed] [Google Scholar]
- Neal G. E., Mattocks A. R., Judah D. J. The microsomal activation of aflatoxin B1 and 2-(N-ethylcarbamoyloxymethyl)furan in vitro using a novel diffusion apparatus. Biochim Biophys Acta. 1979 Jun 1;585(1):134–142. doi: 10.1016/0304-4165(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L. Self-catalyzed inactivation of hepatic cytochrome P-450 by ethynyl substrates. J Biol Chem. 1980 Jun 25;255(12):5578–5585. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Yost G. S., Mico B. A. Self-catalyzed destruction of cytochrome P-450: covalent binding of ethynyl sterols to prosthetic heme. Proc Natl Acad Sci U S A. 1979 Feb;76(2):746–749. doi: 10.1073/pnas.76.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. D., Dent J. G. A new method for measuring covalent binding of chemicals to cellular macromolecules. Chem Biol Interact. 1980 Oct;32(1-2):41–61. doi: 10.1016/0009-2797(80)90067-8. [DOI] [PubMed] [Google Scholar]
- Wade A., Symons A. M., Martin L., Parke D. V. Metabolic oxidation of the ethynyl group in 4-ethynylbiphenyl. Biochem J. 1979 Dec 15;184(3):509–517. doi: 10.1042/bj1840509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N. Destruction of liver haem by norethindrone. Conversion into green pigments. Biochem J. 1981 May 15;196(2):575–583. doi: 10.1042/bj1960575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N. Metabolic activation of acetylenic substituents to derivatives in the rat causing the loss of hepatic cytochrome P-450 and haem. Biochem J. 1978 Sep 15;174(3):853–861. doi: 10.1042/bj1740853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N. Metabolic activation of saturated aldehydes to cause destruction of cytochrome P-450 in vitro. Chem Biol Interact. 1982 Mar 15;39(2):231–243. doi: 10.1016/0009-2797(82)90124-7. [DOI] [PubMed] [Google Scholar]
- White I. N., Muller-Eberhard U. Decreased liver cytochrome P-450 in rats caused by norethindrone or ethynyloestradiol. Biochem J. 1977 Jul 15;166(1):57–64. doi: 10.1042/bj1660057. [DOI] [PMC free article] [PubMed] [Google Scholar]



