Abstract
Objective
Although thyroid disease is a common condition, there is limited research examining the prevalence of thyroid disease over a long period of time, including both before and during the COVID-19 pandemic. Therefore, we aimed to investigate sociodemographic aspects that might be associated with thyroid disease and how its prevalence has varied during the pandemic.
Methods
We assessed the prevalence of thyroid disease among Korean adults by using data from the Korea National Health and Nutrition Examination Survey from 1998 to 2021. We evaluated weighted prevalence and β-coefficients with 95 % CI for factors such as age, sex, residential area, education level, household income, perceived stress level, weight change, occupation category, and body mass index groups.
Results
From 1998 to 2021, the prevalence of thyroid disease among 159,896 Korean adults (88,120 females [55.1 %]) aged 20 years and older exhibited an overall increasing trend. The weighted prevalence in the general population rose from 1.52 % (95 % CI, 1.41–1.64) in 1998–2005 to 3.84 % (3.30–4.39) in 2021, with a higher likelihood of thyroid disease exposure as age increased. In addition, females, individuals with lower education levels, those with high levels of perceived stress, those who gained weight, and those classified as overweight or obese emerged as vulnerable groups for thyroid disease. For the majority of subgroups, the change amid the effect of the pandemic on prevalence was minimal. However, the aged ≥60 years group showed a greater increase in prevalence during the pandemic than before the pandemic (βdiff: 0.52 [95 % CI, 0.37–0.68]).
Conclusions
A nationwide representative study in South Korea revealed an increasing trend in the prevalence of thyroid disease over 24 years, particularly among the older population. Despite the minimal variation during the pandemic, our findings emphasize the need for targeted thyroid disease policies and further research, especially for specific subgroups such as the older population.
Keywords: Thyroid disease, Trend, Prevalence, Epidemiology, South Korea
1. Introduction
Thyroid disease is a critical endocrine disorder, affecting approximately 200 million people globally [1]. It includes a variety of abnormal symptoms resulting from either the excessive or insufficient secretion of thyroid hormones [2,3]. Its repercussions extend beyond individual well-being, affecting societal structures by placing a strain on healthcare resources and social infrastructure [4].
Prior research highlights that thyroid disease can lead to various complications, including diabetes, fractures, and arrhythmias, and even serve as a causal factor in thyroid cancer, potentially resulting in severe consequences [5,6]. Despite the severe implications and wide-reaching consequences of thyroid disease, it continues to be remarkably overlooked compared to other medical conditions [7,8]. Therefore, early detection and treatment of thyroid disease are of utmost significance. Over the past several decades, the prevalence of thyroid disease has increased [9], influenced by a multitude of factors. Environmental changes, genetic elements, lifestyle patterns, rapid urbanization, industrialization, and excessive stress, are identified as key factors affecting thyroid function [7,10].
In particular, the COVID-19 pandemic was associated with increased stress levels, reduced physical activity, and a rise in overweight and obesity rates [11,12]. Research has shown that chronic stress and metabolic disruptions can adversely affect thyroid function by influencing hormone levels and immune responses. For example, elevated stress hormones such as cortisol have been linked to alterations in thyroid hormone synthesis and metabolism [13]. Additionally, obesity is known to contribute to thyroid dysfunction through mechanisms such as inflammatory cytokine release and changes in thyroid hormone binding proteins [14]. These interrelated factors may collectively contribute to an increased prevalence of thyroid disorders during and after the pandemic. Accordingly, previous studies have looked at thyroid disease during the COVID-19 pandemic [15], but to the best of the authors’ knowledge, no large-scale comprehensive study has been conducted to specifically address the long-term trends and effects of thyroid disease before and during the COVID-19 pandemic. In this context, our study aimed to identify large-scale and long-term trends in the prevalence of thyroid disease from 1998 to 2021, including both pre and during COVID-19 pandemic periods. Additionally, we aimed to comprehensively understand associations of various factors on thyroid disease by quantitatively analyzing relationships between socio-demographic factors and thyroid disease. Findings from this study, may be used in public health and clinical medicine by providing information for the development of prevention and treatment strategies for thyroid diseases.
2. Methods
2.1. Participant selection and data collection
In this study, we employed data from the Korea National Health and Nutrition Examination Survey (KNHANES) conducted by the Korea Disease Control and Prevention Agency (KDCA) over 24 years between 1998 and 2021 [16,17]. Our study covered the population aged ≥20 years and the dataset included information on age, sex, region of residence, body mass index (BMI), education level, household income, perceived stress level, weight change, occupation category and whether there is a thyroid disease in the population. This is cross-sectional research, incorporating both questionnaire and survey interview methods. Specifically, health survey items related to education, economic activity, morbidity, and healthcare utilization, as well as all items from the nutrition survey, were collected through interviews. Health behavior items were collected using self-report methods. Additionally, health examinations were carried out through direct measurements, observations, and specimen analyses.
Over the 24-year period, KNHANES used the most recent available data from the Population and Housing Census at the time of sample design as the primary sampling frame. This approach enabled us to draw a representative sample of the target population, which includes all residents of South Korea aged 1 year and older. A two-stage stratified cluster sampling method was employed, with the primary and secondary sampling units being survey districts and households, respectively. Using this method, we initially sampled 231,264 participants and 159,896 participants were included in the study after removing missing values for education level, household income, and weighting variables used in the statistics. The number of participants was as follows: 76,829 in 1998–2005; 16,646 in 2007–2009; 17,484 in 2010–2012; 15,144 in 2013–2015; 23,208 in 2016–2019; 5331 in 2020; and 5254 in 2021. Through these participants, we investigated and compared the prevalence of thyroid disease before and during the COVID-19 pandemic.
The research protocol was approved by the Institutional Review Boards of the KDCA (2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C, 2018-01-03-P-A, 2018-01-03-C-A, 2018-01-03-2C-A, 2018-01-03-5C-A, 2018-01-03-4C-A) in accordance with the Act (Article 2, Paragraph 1) and the Enforcement Regulation (Article 2, Paragraph 2, item 1) of the Bioethics and Safety Act from the Korean government, and by the Institutional Review Boards of Kyung Hee University (KHSIRB-23-384). Written informed consent was obtained from all participants prior to their participation. Written informed consent was obtained from all participants prior to their participation. Our research adhered to the Declaration of Helsinki.
2.2. Ascertainment of thyroid disease
To investigate the prevalence, participants were asked, "Have you ever been diagnosed with thyroid disease by a doctor?”. Utilizing participant responses, we collected data on diverse potential risk factors associated with thyroid disease development. Statistical analyses were performed to identify patterns or trends over the 24-year period.
2.3. Covariates
Covariates proposed in the analysis include age (20–39, 40–59, and ≥60 years), sex (male and female), region of residence (urban and rural) [18], household income (lowest, second, third, and highest quartile), level of education (elementary school or lower, middle school, high school, and college or higher), perceived stress level (hardly at all, a little, quite a lot, and greatly), weight change (no weight change, weight loss, and weight gain), occupation category (white collar job, blue collar job, and others), and BMI. BMI was stratified following the Asian-Pacific guidelines: underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), obese (≥25.0 kg/m2), and unknown (missing data) [19].
2.4. Statistical analyses
Results from the present study were presented using qualitative data expressed in the form of proportions or percentages. We employed a weighted multivariate regression model to analyze the data using weighted odds ratios (OR) and 95 % confidence intervals (CI) to compare estimates of various factors before and during the COVID-19 pandemic [20]. This model, unlike standard regression analysis, is used when there are two or more explanatory variables. It was selected for our study to incorporate variables such as age group, sex, and region of residence in the analysis, helping to elucidate the relationships between thyroid disease and various factors. By applying sample weights, the model reduces sampling bias, ensuring that the sample better represents the population.
The prevalence of thyroid disease was assessed using KNHANES data categorized by year from 1998 to 2021. Weighted complex sampling analysis was employed to guarantee accurate estimation. The OR with 95 % CI or β-coefficients with 95 % CI were determined using linear and logistic regression models. The weights used in KNHANES data are categorized into household weights and individual weights. Household weights are designed to ensure that participating households represent the entire population of households in South Korea, while individual weights are assigned to ensure that participating individuals represent the entire population of South Korea. Additionally, individual weights are further subdivided to reflect differences in the number of participants across different survey sections, resulting in separate weights for each section. To improve the reliability of the findings, analysis considering various variables such as age, sex, region of residence, education level, household income, and BMI was applied to all regression models. In addition, interaction terms for each risk factor were estimated, and OR ratios were calculated to determine changes in OR before and during the pandemic [[21], [22], [23]]. Overall, the primary objective of this approach was to conduct a thorough and comprehensive evaluation of the impact of the COVID-19 pandemic on thyroid disease prevalence while also identifying the factors contributing to vulnerability.
Our statistical analyses utilized SAS software (version 9.4; SAS Institute, Cary, NC, USA) with a two-sided test, and a p-value of ≤0.05 was deemed statistically significant.
3. Results
From 1998 to 2021, a total of 231,264 participants were involved in the KNHANES. Out of these, we incorporated 159,896 in the final study population with the following distribution of characteristics: age (20–39 years, 35.69 % [95 % CI, 35.45–35.92]; 40–59 years, 37.14 % [36.90–37.37]; and ≥60 years, 27.18 % [26.96–27.39]) and sex (male, 44.89 % [44.65–45.13] and female, 55.11 % [54.87–55.35]). Information pertaining to the baseline characteristics of the study population, including both crude and weighted rates, is available in Table 1.
Table 1.
Baseline characteristics of Koreans from 1998 to 2021, based on KNHANES data (N = 159,896), including both crude and weighted rates.
| Total | Pre-pandemic | During the pandemic | ||||||
|---|---|---|---|---|---|---|---|---|
| 1998–2021 | 1998–2005 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2019 | 2020 | 2021 | |
| Overall, n | 159,896 | 76,829 | 16,646 | 17,484 | 15,144 | 23,208 | 5331 | 5254 |
| Crude rate, n(%) | ||||||||
| Age (years), n(%) | ||||||||
| 20-39 | 57,064 (35.69) | 33,303 (43.35) | 5473 (32.88) | 5154 (29.48) | 4193 (27.69) | 6260 (26.97) | 1450 (27.20) | 1231 (23.43) |
| 40-59 | 59,378 (37.14) | 28,375 (36.93) | 6145 (36.92) | 6521 (37.30) | 5784 (38.19) | 8752 (37.71) | 1925 (36.11) | 1876 (35.71) |
| ≥60 | 43,454 (27.18) | 15,151 (19.72) | 5028 (30.21) | 5809 (33.22) | 5167 (34.12) | 8196 (35.32) | 1956 (36.69) | 2147 (40.86) |
| Sex, n(%) | ||||||||
| Men | 71,776 (44.89) | 36,071 (46.95) | 7054 (42.38) | 7414 (42.4) | 6381 (42.14) | 10,130 (43.65) | 2408 (45.17) | 2318 (44.12) |
| Women | 88,120 (55.11) | 40,758 (53.05) | 9592 (57.62) | 10,070 (57.60) | 8763 (57.86) | 13,078 (56.35) | 2923 (54.83) | 2936 (55.88) |
| Region of residence, n(%) | ||||||||
| Urban | 122,366 (76.53) | 56,893 (74.05) | 12,201 (73.30) | 13,820 (79.04) | 12,250 (80.89) | 18,850 (81.22) | 4266 (80.02) | 4086 (77.77) |
| Rural | 37,530 (23.47) | 19,936 (25.95) | 4445 (26.70) | 3664 (20.96) | 2894 (19.11) | 4358 (18.78) | 1065 (19.98) | 1168 (22.23) |
| BMI groupa, n(%) | ||||||||
| Underweight | 4389 (2.74) | 936 (1.22) | 751 (4.51) | 779 (4.46) | 637 (4.21) | 861 (3.71) | 199 (3.73) | 226 (4.30) |
| Normal weight | 40,314 (25.21) | 8221 (10.70) | 6543 (39.31) | 6993 (40.00) | 5965 (39.39) | 8896 (38.33) | 1845 (34.61) | 1851 (35.23) |
| Overweight | 23,959 (14.98) | 4619 (6.01) | 3970 (23.85) | 4072 (23.29) | 3578 (23.63) | 5313 (22.89) | 1205 (22.60) | 1202 (22.88) |
| Obese | 33,540 (20.98) | 5757 (7.49) | 5292 (31.79) | 5572 (31.87) | 4949 (32.68) | 8055 (34.70) | 2018 (37.85) | 1897 (36.11) |
| Unknown | 57,694 (36.08) | 57,296 (74.58) | 90 (0.54) | 68 (0.39) | 15 (0.10) | 83 (0.36) | 64 (1.20) | 78 (1.48) |
| Education, n(%) | ||||||||
| Elementary school or lower | 36,391 (22.76) | 18,697 (24.34) | 4524 (27.18) | 4159 (23.79) | 3217 (21.24) | 4138 (17.83) | 766 (14.37) | 890 (16.94) |
| Middle school | 18,870 (11.80) | 9566 (12.45) | 1980 (11.89) | 2037 (11.65) | 1741 (11.50) | 2457 (10.59) | 552 (10.35) | 537 (10.22) |
| High school | 50,443 (31.55) | 27,230 (35.44) | 4818 (28.94) | 4927 (28.18) | 4258 (28.12) | 6258 (26.96) | 1485 (27.86) | 1467 (27.92) |
| College or higher | 54,192 (33.89) | 21,336 (27.77) | 5324 (31.98) | 6361 (36.38) | 5928 (39.14) | 10,355 (44.62) | 2528 (47.42) | 2360 (44.92) |
| Household income, n(%) | ||||||||
| Lowest quartile | 33,377 (20.87) | 17038 (22.18) | 3565 (21.42) | 3435 (19.65) | 2944 (19.44) | 4456 (19.20) | 923 (17.31) | 1016 (19.34) |
| Second quartile | 39,557 (24.74) | 18855 (24.54) | 4161 (25.00) | 4509 (25.79) | 3829 (25.28) | 5705 (24.58) | 1254 (23.52) | 1244 (23.68) |
| Third quartile | 43,197 (27.02) | 20711 (26.96) | 4424 (26.58) | 4753 (27.18) | 4117 (27.19) | 6245 (26.91) | 1507 (28.27) | 1440 (27.41) |
| Highest quartile | 43,765 (27.37) | 20225 (26.32) | 4496 (27.01) | 4787 (27.38) | 4254 (28.09) | 6802 (29.31) | 1647 (30.89) | 1554 (29.58) |
| Perceived stress level, n(%) | ||||||||
| Greatly | 5411 (3.38) | 1540 (2.00) | 877 (5.27) | 750 (4.29) | 656 (4.33) | 1083 (4.67) | 264 (4.95) | 241 (4.59) |
| Quite a lot | 25,034 (15.66) | 6928 (9.02) | 3861 (23.19) | 3847 (22.00) | 3026 (19.98) | 5078 (21.88) | 1205 (22.60) | 1089 (20.73) |
| A little | 58,414 (36.53) | 11,552 (15.04) | 9011 (54.13) | 10,065 (57.57) | 8647 (57.10) | 13,089 (56.40) | 3041 (57.04) | 3009 (57.27) |
| Hardly at all | 17,813 (11.14) | 3858 (5.02) | 2875 (17.27) | 2787 (15.94) | 2659 (17.56) | 3904 (16.82) | 820 (15.38) | 910 (17.32) |
| Unknown | 53,224 (33.29) | 52,951 (68.92) | 22 (0.13) | 35 (0.20) | 156 (1.03) | 54 (0.23) | 1 (0.02) | 5 (0.10) |
| Weight change, n(%) | ||||||||
| No weight change | 66,081 (41.33) | 12,743 (16.59) | 10,915 (65.57) | 11,813 (67.56) | 9477 (62.58) | 14,753 (63.57) | 3235 (60.68) | 3145 (59.86) |
| Weight loss | 16,379 (10.24) | 4769 (6.21) | 2517 (15.12) | 2504 (14.32) | 2153 (14.22) | 2958 (12.75) | 730 (13.69) | 748 (14.24) |
| Weight gain | 24,176 (15.12) | 6347 (8.26) | 3186 (19.14) | 3127 (17.88) | 3358 (22.17) | 5440 (23.44) | 1366 (25.62) | 1352 (25.73) |
| Unknown | 53,260 (33.31) | 52,970 (68.95) | 28 (0.17) | 40 (0.23) | 156 (1.03) | 57 (0.25) | 0 (0.00) | 9 (0.17) |
| Occupation category, n(%) | ||||||||
| White collar job | 22,024 (13.77) | 3601 (4.69) | 3040 (18.26) | 3638 (20.81) | 3352 (22.13) | 5834 (25.14) | 1319 (24.74) | 1240 (23.60) |
| Blue collar job | 55,855 (34.93) | 24,833 (32.32) | 6683 (40.15) | 6698 (38.31) | 5507 (36.36) | 8307 (35.79) | 1899 (35.62) | 1928 (36.70) |
| Others | 55,674 (34.82) | 22,109 (28.78) | 6907 (41.49) | 7139 (40.83) | 6274 (41.43) | 9049 (38.99) | 2110 (39.58) | 2086 (39.70) |
| Unknown | 26,343 (16.48) | 26,286 (34.21) | 16 (0.10) | 9 (0.05) | 11 (0.07) | 18 (0.08) | 3 (0.06) | 0 (0.00) |
| Weighted rate (95 % CI) | ||||||||
| Age (years), weighted % (95 % CI) | ||||||||
| 20–39 | 39.37 (38.89–39.85) | 45.17 (44.41–45.94) | 41.94 (40.49–43.39) | 39.29 (37.94–40.65) | 37.08 (35.79–38.36) | 34.89 (33.76–36.02) | 34.30 (31.88–36.72) | 33.14 (30.87–35.41) |
| 40–59 | 39.36 (38.96–39.77) | 37.01 (36.36–37.65) | 39.32 (38.09–40.55) | 40.54 (39.46–41.62) | 40.87 (39.80–41.95) | 40.49 (39.53–41.45) | 40.16 (37.96–42.36) | 38.69 (36.90–40.48) |
| ≥60 | 21.27 (20.88–21.66) | 17.82 (17.27–18.36) | 18.74 (17.80–19.69) | 20.17 (19.17–21.16) | 22.05 (21.01–23.09) | 24.62 (23.56–25.68) | 25.54 (23.12–27.95) | 28.17 (25.76–30.58) |
| Sex, weighted % (95 % CI) | ||||||||
| Men | 49.14 (48.89–49.40) | 48.37 (48.07–48.68) | 49.55 (48.83–50.27) | 49.41 (48.66–50.16) | 48.78 (48.01–49.56) | 49.50 (48.86–50.15) | 50.21 (49.09–51.33) | 49.86 (48.53–51.18) |
| Women | 50.86 (50.60–51.11) | 51.63 (51.32–51.93) | 50.45 (49.73–51.17) | 50.59 (49.84–51.34) | 51.22 (50.44–51.99) | 50.50 (49.85–51.14) | 49.79 (48.67–50.91) | 50.14 (48.82–51.47) |
| Region of residence, weighted % (95 % CI) | ||||||||
| Urban | 82.27 (81.28–83.25) | 81.05 (80.27–81.83) | 80.57 (77.48–83.66) | 79.96 (76.70–83.23) | 82.89 (80.02–85.76) | 84.89 (82.46–87.32) | 84.89 (79.78–90.00) | 84.09 (79.07–89.11) |
| Rural | 17.73 (16.75–18.72) | 18.95 (18.17–19.73) | 19.43 (16.34–22.52) | 20.04 (16.77–23.30) | 17.11 (14.24–19.98) | 15.11 (12.68–17.54) | 15.11 (10.00–20.22) | 15.91 (10.89–20.93) |
| BMI groupa, weighted % (95 % CI) | ||||||||
| Underweight | 3.49 (3.36–3.63) | 1.04 (0.91–1.17) | 4.71 (4.29–5.14) | 4.73 (4.32–5.13) | 4.63 (4.21–5.05) | 3.94 (3.63–4.25) | 3.91 (3.20–4.61) | 4.33 (3.70–4.95) |
| Normal weight | 30.64 (30.23–31.04) | 9.06 (8.24–9.89) | 39.35 (38.46–40.24) | 39.71 (38.73–40.70) | 39.87 (38.95–40.79) | 38.26 (37.48–39.04) | 33.65 (32.06–35.23) | 35.81 (34.17–37.45) |
| Overweight | 18.17 (17.88–18.47) | 5.52 (5.00–6.04) | 23.61 (22.88–24.34) | 22.76 (21.98–23.54) | 23.01 (22.21–23.81) | 22.50 (21.86–23.14) | 22.94 (21.72–24.17) | 21.66 (20.25–23.08) |
| Obese | 26.63 (26.26–27.00) | 7.09 (6.43–7.75) | 31.81 (30.91–32.70) | 32.36 (31.40–33.33) | 32.39 (31.49–33.29) | 34.93 (34.13–35.74) | 38.50 (36.89–40.11) | 37.05 (35.23–38.86) |
| Unknown | 21.07 (20.45–21.70) | 77.29 (75.29–79.28) | 0.52 (0.36–0.68) | 0.44 (0.30–0.58) | 0.11 (0.04–0.17) | 0.36 (0.26–0.46) | 1.00 (0.71–1.30) | 1.16 (0.79–1.52) |
| Education, weighted % (95 % CI) | ||||||||
| Elementary school or lower | 16.04 (15.69–16.39) | 20.08 (19.43–20.73) | 18.56 (17.57–19.56) | 17.17 (16.17–18.17) | 14.94 (14.01–15.87) | 12.39 (11.62–13.16) | 9.48 (7.93–11.03) | 10.43 (8.77–12.09) |
| Middle school | 9.87 (9.64–10.09) | 11.3 (10.92–11.68) | 10.71 (10.09–11.34) | 10.35 (9.74–10.97) | 9.40 (8.82–9.98) | 8.60 (8.10–9.11) | 7.77 (6.70–8.83) | 7.56 (6.56–8.56) |
| High school | 31.13 (30.73–31.53) | 36.2 (35.52–36.87) | 31.39 (30.28–32.50) | 30.54 (29.43–31.66) | 29.20 (28.12–30.29) | 27.40 (26.51–28.30) | 28.71 (26.73–30.68) | 28.93 (27–30.87) |
| College or higher | 42.96 (42.38–43.55) | 32.42 (31.53–33.3) | 39.33 (37.77–40.89) | 41.93 (40.42–43.45) | 46.46 (44.95–47.96) | 51.6 (50.15–53.05) | 54.05 (50.75–57.35) | 53.08 (49.99–56.17) |
| Household income, weighted % (95 % CI) | ||||||||
| Lowest quartile | 16.85 (16.44–17.27) | 20.78 (19.95–21.61) | 16.26 (15.16–17.35) | 16.00 (14.97–17.03) | 15.28 (14.19–16.36) | 15.47 (14.53–16.41) | 13.81 (11.70–15.91) | 13.52 (11.53–15.51) |
| Second quartile | 24.80 (24.34–25.25) | 25.15 (24.41–25.89) | 25.09 (23.80–26.37) | 27.22 (25.89–28.54) | 24.63 (23.33–25.93) | 23.90 (22.89–24.90) | 21.73 (19.57–23.89) | 22.72 (20.59–24.85) |
| Third quartile | 28.62 (28.17–29.07) | 26.95 (26.24–27.66) | 28.78 (27.49–30.06) | 29.18 (27.97–30.39) | 29.55 (28.17–30.92) | 28.89 (27.88–29.91) | 29.86 (27.74–31.98) | 30.42 (28.21–32.64) |
| Highest quartile | 29.72 (29.09–30.35) | 27.12 (26.11–28.14) | 29.88 (28.06–31.70) | 27.60 (26.14–29.05) | 30.55 (28.80–32.30) | 31.74 (30.25–33.24) | 34.60 (31.11–38.09) | 33.34 (29.49–37.18) |
| Perceived stress level, weighted % (95 % CI) | ||||||||
| Hardly at all | 11.87 (11.61–12.13) | 4.63 (4.16–5.10) | 14.89 (14.13–15.65) | 13.32 (12.67–13.98) | 15.4 (14.69–16.12) | 14.71 (14.15–15.26) | 13.23 (11.98–14.48) | 15.2 (13.98–16.42) |
| A little | 46.10 (45.59–46.62) | 15.23 (13.86–16.61) | 55.66 (54.72–56.60) | 58.93 (58.03–59.83) | 57.55 (56.55–58.55) | 57.03 (56.25–57.82) | 57.58 (55.85–59.3) | 58.53 (57.03–60.03) |
| Quite a lot | 19.10 (18.74–19.45) | 8.59 (7.81–9.38) | 24.12 (23.36–24.88) | 23.10 (22.27–23.93) | 21.51 (20.68–22.33) | 23.20 (22.50–23.90) | 23.85 (22.13–25.57) | 21.7 (20.24–23.16) |
| Greatly | 4.05 (3.91–4.20) | 1.99 (1.75–2.23) | 5.21 (4.79–5.63) | 4.45 (4.10–4.81) | 4.61 (4.19–5.04) | 4.89 (4.55–5.22) | 5.33 (4.53–6.12) | 4.52 (3.85–5.18) |
| Unknown | 18.88 (18.11–19.65) | 69.55 (66.91–72.20) | 0.12 (0.06–0.19) | 0.19 (0.11–0.28) | 0.93 (0.73–1.13) | 0.18 (0.12–0.23) | 0.01 (0.00–0.04) | 0.05 (0.00–0.09) |
| Weight change, weighted % (95 % CI) | ||||||||
| No weight change | 49.53 (48.99–50.08) | 16.59 (15.12–18.05) | 63.22 (62.30–64.13) | 64.48 (63.45–65.50) | 60.32 (59.27–61.36) | 61.36 (60.51–62.20) | 58.12 (56.31–59.94) | 57.83 (56.03–59.63) |
| Weight loss | 11.72 (11.46–11.99) | 5.52 (4.99–6.06) | 14.94 (14.28–15.61) | 14.29 (13.58–15.00) | 14.52 (13.76–15.28) | 12.97 (12.44–13.50) | 13.93 (12.85–15.01) | 13.69 (12.55–14.82) |
| Weight gain | 19.85 (19.48–20.22) | 8.30 (7.52–9.09) | 21.70 (20.89–22.52) | 21.04 (20.15–21.94) | 24.24 (23.32–25.17) | 25.48 (24.73–26.23) | 27.95 (26.33–29.56) | 28.39 (26.80–29.97) |
| Unknown | 18.90 (18.13–19.67) | 69.59 (66.94–72.23) | 0.14 (0.07–0.20) | 0.19 (0.10–0.28) | 0.92 (0.72–1.12) | 0.20 (0.14–0.26) | NA | 0.09 (0.03–0.15) |
| Occupation category, weighted % (95 % CI) | ||||||||
| White collar job | 20.35 (19.95–20.75) | 5.43 (5.07–5.79) | 22.03 (20.99–23.06) | 23.68 (22.65–24.71) | 25.76 (24.68–26.84) | 28.64 (27.56–29.72) | 28.27 (25.90–30.64) | 27.28 (25.14–29.43) |
| Blue collar job | 35.54 (35.10–35.98) | 28.95 (28.37–29.53) | 39.46 (38.17–40.75) | 41.21 (39.93–42.50) | 37.11 (35.87–38.35) | 36.40 (35.33–37.46) | 35.38 (32.95–37.8) | 36.38 (34.19–38.57) |
| Others | 34.20 (33.82–34.58) | 28.91 (28.25–29.57) | 38.40 (37.34–39.46) | 35.05 (34.02–36.07) | 37.04 (36.03–38.05) | 34.88 (34.01–35.75) | 36.29 (34.34–38.23) | 36.34 (34.60–38.08) |
| Unknown | 9.91 (9.68–10.13) | 36.71 (36.12–37.30) | 0.12 (0.04–0.20) | 0.06 (0.00–0.11) | 0.09 (0.03–0.16) | 0.09 (0.04–0.13) | 0.07 (0.00–0.15) | NA |
Abbreviations: BMI, body mass index; CI, confidence interval; KNHANES, Korea National Health and Nutrition Examination Survey.
According to the Asian–Pacific guidelines, the BMI is divided into four groups: underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥25.0 kg/m2).
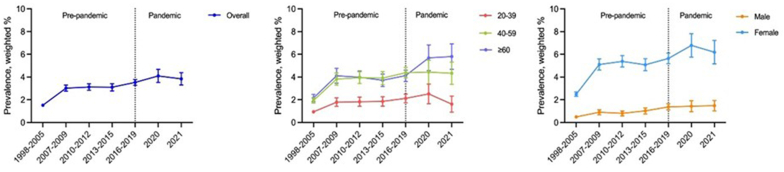
Fig. 1 and Table 2 depict the prevalence of thyroid disease in the overall population, categorized by age group and sex, both pre-pandemic (1998–2019) and during the pandemic (2020–2021). The weighted prevalence of thyroid disease showed a sustained increase until 2020, with a slight decrease in 2021. Nevertheless, in 2021, the prevalence was significantly higher than in 1998 and seems to have generally increased over time. First, the weighted prevalence for the overall population increased from 1.52 % (95 % CI, 1.41–1.64) in 1998–2005 to 4.10 % (3.53–4.67) in 2020. Second, within age groups, the weighted prevalence for the 20–39 age group increased from 0.94 % (0.80–1.08) in 1998–2005 to 2.51 % (1.65–3.37) in 2020. The 40–59 age group showed an increase from 1.94 % (1.71–2.16) to 4.44 % (3.39–5.49), and the ≥60 age group showed an increase from 2.15 % (1.83–2.47) in 1998–2005 to 5.69 % (4.56–6.83) in 2020. Third, regarding perceived stress levels, the prevalence in the ‘hardly at all’ category increased from 1.46 % (0.95–1.98) in 1998–2005 to 2.66 % (1.56–3.75) in 2020. Whereas the ‘a little’ category showed an increase from 1.69 % (1.38–2.00) to 3.53 % (2.86–4.20), ‘quite a lot’ from 1.55 % (1.17–1.94) to 5.65 % (4.28–7.01); and ‘greatly’ from 1.55 % (0.73–2.38) to 6.92 % (3.66–10.19). Last, in the sex groups, the weighted prevalence for males increased from 0.49 % (0.39–0.59) in 1998–2005 to 1.43 % (0.94–1.92) in 2020. For females, the prevalence increased from 2.49 % (2.30–2.69) in 1998–2005 to 6.79 % (5.76–7.81) in 2020. Additionally, the prevalence of thyroid disease did not change significantly during the COVID-19 pandemic. However, specific groups, such as those ≥60 years, those underweight, and those with high school or low education, exhibited significant differences in the rate of prevalence increase during the pandemic compared to before. The βdiff were 0.52 (0.37–0.68) for ≥60 years, 0.69 (0.06–1.32) for underweight, and 0.44 (0.26–0.63) for lower education, indicating a significantly higher rate of increase in prevalence during the pandemic compared to before.
Fig. 1.
The nationwide trend in thyroid disease prevalence over 24 years (1998–2021) among 159,896 Korean adults, stratified by age and sex groups, 1998–2021.
Table 2.
National trends in the prevalence of thyroid disease and odds ratios β-coefficients before and during the COVID-19 pandemic, presented as weighted percentages with 95 % confidence intervals, using data obtained from KNHANES.
| Year | Pre-pandemic |
During the pandemic |
Trends in the pre-pandemic era, β (95 % CI) | Trends in the pandemic era, β (95 % CI) | βdiff between 1998-2019 and 2019–2021 (95 % CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1998–2005 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2019 | 2020 | 2021 | ||||
| Overall, weighted % (95 % CI) | 1.52 (1.41–1.64) | 3.02 (2.75–3.29) | 3.12 (2.84–3.4) | 3.10 (2.78–3.41) | 3.53 (3.27–3.79) | 4.10 (3.53–4.67) | 3.84 (3.30–4.39) | 0.38 (0.30 to 0.45) | 0.15 (−0.14 to 0.45) | −0.22 (−0.53 to 0.08) |
| Age group, weighted % (95 % CI) | ||||||||||
| 20-39 | 0.94 (0.80–1.08) | 1.78 (1.41–2.16) | 1.82 (1.42–2.23) | 1.85 (1.44–2.26) | 2.12 (1.73–2.50) | 2.51 (1.65–3.37) | 1.61 (0.91–2.31) | 0.23 (0.13 to 0.34) | −0.25 (−0.65 to 0.15) | −0.48 (−0.90 to −0.07) |
| 40-59 | 1.94 (1.71–2.16) | 3.82 (3.28–4.35) | 3.94 (3.43–4.46) | 3.90 (3.32–4.48) | 4.38 (3.92–4.85) | 4.44 (3.39–5.49) | 4.33 (3.36–5.30) | 0.45 (0.31 to 0.59) | −0.03 (−0.56 to 0.51) | −0.47 (−1.02 to 0.08) |
| ≥60 | 2.15 (1.83–2.47) | 4.11 (3.46–4.76) | 3.98 (3.43–4.54) | 3.71 (3.16–4.26) | 4.13 (3.66–4.60) | 5.69 (4.56–6.83) | 5.80 (4.68–6.92) | 0.29 (0.14 to 0.45) | 0.81 (0.19 to 1.44) | 0.52 (0.37 to 0.68) |
| Sex, weighted % (95 % CI) | ||||||||||
| Male | 0.49 (0.39–0.59) | 0.90 (0.67–1.12) | 0.80 (0.58–1.01) | 1.02 (0.75–1.29) | 1.37 (1.10–1.65) | 1.43 (0.94–1.92) | 1.48 (1.02–1.94) | 0.19 (0.11 to 0.26) | 0.05 (−0.21 to 0.32) | −0.13 (−0.41 to 0.14) |
| Female | 2.49 (2.30–2.69) | 5.10 (4.61–5.60) | 5.38 (4.88–5.89) | 5.08 (4.55–5.61) | 5.64 (5.18–6.10) | 6.79 (5.76–7.81) | 6.19 (5.16–7.22) | 0.57 (0.44 to 0.70) | 0.27 (−0.28 to 0.82) | −0.30 (−0.87 to 0.27) |
| Region of residence, weighted % (95 % CI) | ||||||||||
| Urban | 1.54 (1.41–1.67) | 3.04 (2.73–3.34) | 3.09 (2.77–3.41) | 3.15 (2.80–3.51) | 3.61 (3.32–3.90) | 4.06 (3.44–4.69) | 3.82 (3.22–4.42) | 0.40 (0.31 to 0.48) | 0.10 (−0.23 to 0.44) | −0.29 (−0.63 to 0.05) |
| Rural | 1.47 (1.21–1.73) | 2.95 (2.36–3.53) | 3.24 (2.60–3.88) | 2.83 (2.16–3.51) | 3.07 (2.52–3.63) | 4.30 (3.06–5.54) | 3.94 (2.74–5.14) | 0.28 (0.12 to 0.44) | 0.42 (−0.23 to 1.08) | 0.14 (−0.09 to 0.37) |
| BMI groupa, weighted % (95 % CI) | ||||||||||
| Underweight | 2.03 (0.82–3.24) | 1.81 (0.76–2.86) | 2.69 (1.44–3.94) | 2.23 (1.06–3.41) | 2.36 (1.35–3.37) | 4.48 (1.31–7.64) | 4.03 (1.10–6.95) | 0.12 (−0.28 to 0.52) | 0.81 (−0.74 to 0.23) | 0.69 (0.06 to 1.32) |
| Normal or overweight | 1.65 (1.33–1.97) | 3.26 (2.89–3.63) | 3.14 (2.79–3.49) | 3.29 (2.91–3.67) | 3.82 (3.47–4.17) | 4.11 (3.36–4.86) | 3.99 (3.24–4.74) | 0.27 (0.14 to 0.41) | 0.09 (−0.32 to 0.50) | −0.18 (−0.61 to 0.25) |
| Obese | 2.00 (1.53–2.47) | 2.78 (2.31–3.24) | 3.10 (2.63–3.58) | 2.85 (2.33–3.38) | 3.14 (2.71–3.58) | 4.10 (3.17–5.02) | 3.52 (2.62–4.42) | 0.13 (−0.04 to 0.31) | 0.18 (−0.31 to 0.67) | 0.04 (−0.48 to 0.57) |
| Education, weighted % (95 % CI) | ||||||||||
| High school or lower | 2.16 (1.93–2.39) | 4.43 (3.83–5.03) | 4.82 (4.19–5.45) | 3.54 (2.91–4.16) | 3.96 (3.42–4.50) | 5.52 (4.07–6.97) | 5.31 (3.87–6.75) | 0.25 (0.10 to 0.40) | 0.69 (−0.07 to 1.46) | 0.44 (0.26 to 0.63) |
| College or higher | 1.23 (1.10–1.36) | 2.44 (2.14–2.74) | 2.47 (2.17–2.78) | 2.96 (2.6–3.31) | 3.42 (3.12–3.72) | 3.8 (3.17–4.43) | 3.52 (2.95–4.09) | 0.47 (0.38 to 0.56) | 0.05 (−0.27 to 0.37) | −0.42 (−0.75 to −0.09) |
| Household income, weighted % (95 % CI) | ||||||||||
| Lowest and second quartile | 1.59 (1.43–1.76) | 3.24 (2.79–3.69) | 3.03 (2.60–3.45) | 2.88 (2.43–3.34) | 3.55 (3.15–3.96) | 4.26 (3.38–5.15) | 3.84 (2.96–4.73) | 0.34 (0.23 to 0.45) | 0.15 (−0.33 to 0.63) | −0.19 (−0.68 to 0.31) |
| Third and highest quartile | 1.47 (1.31–1.62) | 2.86 (2.50–3.23) | 3.19 (2.81–3.57) | 3.24 (2.82–3.65) | 3.52 (3.17–3.86) | 4.01 (3.27–4.74) | 3.84 (3.18–4.50) | 0.41 (0.31 to 0.51) | 0.16 (−0.21 to 0.52) | −0.25 (−0.63 to 0.13) |
| Perceived stress level, weighted % (95 % CI) | ||||||||||
| Hardly at all | 1.46 (0.95–1.98) | 2.77 (2.12–3.42) | 2.54 (1.84–3.23) | 2.99 (2.24–3.74) | 2.76 (2.20–3.32) | 2.66 (1.56–3.75) | 3.86 (2.63–5.08) | 0.14 (−0.08 to 0.36) | 0.55 (−0.14 to 1.24) | 0.41 (−0.31 to 1.13) |
| A little | 1.69 (1.38–2.00) | 2.86 (2.50–3.23) | 3.13 (2.76–3.49) | 3.10 (2.70–3.50) | 3.63 (3.28–3.97) | 3.53 (2.86–4.20) | 3.73 (3.00–4.46) | 0.30 (0.17 to 0.43) | 0.06 (−0.34 to 0.45) | −0.24 (−0.66 to 0.18) |
| Quite a lot | 1.55 (1.17–1.94) | 3.04 (2.47–3.62) | 3.33 (2.69–3.97) | 3.08 (2.44–3.72) | 3.65 (3.09–4.22) | 5.65 (4.28–7.01) | 4.02 (2.77–5.27) | 0.29 (0.09 to 0.49) | 0.19 (−0.49 to 0.88) | −0.10 (−0.81 to 0.61) |
| Greatly | 1.55 (0.73–2.38) | 5.13 (3.61–6.66) | 3.65 (2.18–5.11) | 3.14 (1.64–4.64) | 4.05 (2.66–5.45) | 6.92 (3.66–10.19) | 4.23 (1.29–7.18) | −0.02 (−0.52 to 0.47) | 0.11 (−1.51 to 1.74) | 0.13 (−1.57 to 1.83) |
| Weight change, weighted % (95 % CI) | ||||||||||
| No weight change | 1.40 (1.12–1.69) | 3.01 (2.67–3.35) | 3.06 (2.72–3.40) | 2.92 (2.54–3.29) | 3.48 (3.15–3.81) | 4.17 (3.44–4.90) | 4.04 (3.29–4.78) | 0.24 (0.11 to 0.36) | 0.28 (−0.12 to 0.68) | 0.04 (−0.38 to 0.46) |
| Weight loss | 1.83 (1.29–2.37) | 3.15 (2.36–3.93) | 2.64 (1.98–3.30) | 2.36 (1.69–3.03) | 2.90 (2.26–3.53) | 3.67 (2.06–5.28) | 3.26 (1.87–4.64) | 0.01 (−0.23 to 0.26) | 0.17 (−0.58 to 0.92) | 0.16 (−0.63 to 0.95) |
| Weight gain | 1.87 (1.46–2.28) | 2.93 (2.37–3.50) | 3.64 (2.89–4.40) | 3.95 (3.23–4.66) | 3.95 (3.36–4.55) | 4.16 (2.96–5.36) | 3.67 (2.57–4.77) | 0.41 (0.21 to 0.62) | −0.15 (−0.77 to 0.48) | −0.56 (−1.22 to 0.10) |
| Occupation category, weighted % (95 % CI) | ||||||||||
| White collar job | 1.30 (0.85–1.75) | 1.90 (1.38–2.42) | 2.18 (1.70–2.66) | 2.78 (2.16–3.40) | 2.88 (2.38–3.37) | 3.22 (2.21–4.23) | 3.69 (2.60–4.77) | 0.36 (0.17 to 0.56) | 0.40 (−0.18 to 0.99) | 0.04 (−0.58 to 0.66) |
| Blue collar job | 1.07 (0.90–1.25) | 2.11 (1.71–2.51) | 2.72 (2.30–3.15) | 2.44 (1.95–2.92) | 3.03 (2.64–3.42) | 3.42 (2.50–4.35) | 2.87 (2.10–3.63) | 0.38 (0.26 to 0.50) | −0.08 (−0.52 to 0.35) | −0.46 (−0.91 to −0.01) |
| Others | 1.21 (1.03–1.39) | 4.61 (4.08–5.14) | 4.21 (3.69–4.74) | 3.98 (3.42–4.55) | 4.57 (4.08–5.06) | 5.45 (4.32–6.57) | 4.94 (3.86–6.01) | 0.47 (0.32 to 0.61) | 0.17 (−0.41 to 0.76) | −0.30 (−0.90 to 0.30) |
Abbreviations: BMI, body mass index; CI, confidence interval; KNHANES, Korea National Health and Nutrition Examination Survey.
The numbers in bold indicate a significant difference (p < 0.05).
According to the Asian–Pacific guidelines, the BMI is divided into four groups: underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥25.0 kg/m2).
Table 3 and Table S1 display disease-related factors associated with vulnerable groups among thyroid disease patients, along with weighted OR from 1998 to 2021. From the results, it is observed that those who are older, females, those with a higher BMI, those with lower educational levels, those with high perceived stress levels and those who gained weight are more vulnerable to thyroid disease as follows: 40–59 year age group (OR, 2.20 [95 % CI: 1.99–2.44]; reference 20–39 age group), ≥60 years old (OR, 2.33 [2.10–2.59]; reference 20–39 age group); female (OR, 5.27 [4.74–5.86]; reference male); normal and overweight (OR, 4.59 [3.71–5.69]; reference underweight); obesity (OR, 4.55 [3.64–5.68]; reference underweight); low level of education (OR, 1.16 [1.08–1.25]; reference higher educational); a little of perceived stress (OR, 1.18 [1.05–1.33]; reference hardly at all); quite a lot of perceived stress (OR, 1.24 [1.08–1.41]; reference hardly at all); greatly perceived stress (OR, 1.49 [1.22–1.82]; reference hardly at all); weight gain (OR, 1.11 [1.01–1.22]). The groups susceptible to thyroid disease remained equally vulnerable during the COVID-19 pandemic.
Table 3.
Disparities in thyroid disease prevalence before and during the COVID-19 (weighted % [95 % CI]) using data collected from KNHANES.
| Variable | Overall (1998–2021) |
Pre-pandemic era (1998–2019) |
During pandemic era (2020–2021) |
Ratio of OR (95 % CI), |
||||
|---|---|---|---|---|---|---|---|---|
| during the pandemic versus pre-pandemic (reference) | ||||||||
| Weighted OR (95 % CI) | P-value | Weighted OR (95 % CI) | P-value | Weighted OR (95 % CI) | P-value | Ratio of OR (95 % CI) | P-value | |
| Age group | ||||||||
| 20–39 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| 40–59 | 2.20 (1.99 to 2.44) | <0.001 | 2.20 (1.98 to 2.45) | <0.001 | 2.11 (1.51 to 2.95) | <0.001 | 0.96 (0.67–1.36) | 0.816 |
| ≥60 | 2.33 (2.10 to 2.59) | <0.001 | 2.23 (1.99 to 2.49) | <0.001 | 2.65 (1.93 to 3.64) | <0.001 | 1.19 (0.85–1.66) | 0.315 |
| Sex | ||||||||
| Male | 1.00 (ref) | 1.00(ref) | 1.00(ref) | 1.00 (ref) | ||||
| Female | 5.27 (4.74 to 5.86) | <0.001 | 5.38(4.80 to 6.04) | <0.001 | 4.69 (3.59 to 6.14) | <0.001 | 0.87 (0.65–1.17) | 0.357 |
| Region of residence | ||||||||
| Rural | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Urban | 1.04 (0.94–1.14) | 0.456 | 1.05 (0.94–1.16) | 0.396 | 0.94 (0.73–1.21) | 0.616 | 0.90 (0.68–1.18) | 0.428 |
| BMI groupa | ||||||||
| Underweight | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Normal or overweight | 4.59 (3.71 to 5.69) | <0.001 | 5.05 (4.01 to 6.36) | <0.001 | 2.73 (1.60 to 4.64) | <0.001 | 0.54 (0.30–0.97) | 0.038 |
| Obese | 4.55 (3.64 to 5.68) | <0.001 | 4.94 (3.88 to 6.28) | <0.001 | 2.75 (1.58 to 4.79) | <0.001 | 0.56 (0.30 to 1.02) | 0.058 |
| Education | ||||||||
| College or higher | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| High school or lower | 1.16 (1.08 to 1.25) | <0.001 | 1.18 (1.09 to 1.28) | <0.001 | 1.30 (1.04 to 1.64) | 0.024 | 1.10 (0.87–1.40) | 0.432 |
| Household income | ||||||||
| Lowest and second quartile | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Third and highest quartile | 1.03 (0.96–1.11) | 0.405 | 1.03 (0.95–1.11) | 0.548 | 1.00 (0.81–1.23) | 0.983 | 0.97 (0.78–1.21) | 0.795 |
| Perceived stress level | ||||||||
| Hardly at all | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| A little | 1.18 (1.05 to 1.33) | <0.001 | 1.18 (1.04 to 1.34) | 0.011 | 1.18 (0.87 to 1.59) | 0.285 | 1.00 (0.72–1.39) | 1.000 |
| Quite a lot | 1.24 (1.08 to 1.41) | <0.001 | 1.18 (1.02 to 1.37) | 0.025 | 1.58 (1.14 to 2.19) | 0.007 | 1.34 (0.94–1.92) | 0.110 |
| Greatly | 1.49 (1.22 to 1.82) | <0.001 | 1.42 (1.15 to 1.77) | <0.001 | 1.89 (1.14 to 3.11) | 0.013 | 1.33 (0.77–2.30) | 0.305 |
| Weight change | ||||||||
| No weight change | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Weight loss | 0.90 (0.80–1.02) | 0.100 | 0.91 (0.80–1.04) | 0.175 | 0.84 (0.60–1.17) | 0.302 | 0.92 (0.64–1.32) | 0.662 |
| Weight gain | 1.11 (1.01 to 1.22) | 0.041 | 1.13 (1.02 to 1.25) | 0.023 | 0.97 (0.74–1.26) | 0.796 | 0.86 (0.65–1.14) | 0.294 |
| Occupation category | ||||||||
| White collar job | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Blue collar job | 0.89 (0.79–1.01) | 0.061 | 0.91 (0.80–1.03) | 0.141 | 0.90 (0.66–1.22) | 0.501 | 0.99 (0.71–1.38) | 0.948 |
| Others | 0.86 (0.77 to 0.96) | 0.008 | 0.86 (0.76 to 0.97) | 0.014 | 0.98 (0.75–1.29) | 0.897 | 1.14 (0.85–1.53) | 0.389 |
Abbreviations: BMI, body mass index; CI, confidence interval; KNHANES, Korea National Health and Nutrition Examination Survey; OR, odds ratios.
The numbers in bold indicate significant differences (p < 0.05).
According to the Asian–Pacific guidelines, the BMI is divided into four groups: underweight (<18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥25.0 kg/m2).
4. Discussion
4.1. Key findings
Our study analyzed the prevalence of thyroid disease before and during the COVID-19 pandemic in 159,896 Korean adults using the KNHANES database over 24 years from 1998 to 2021. The overall prevalence of thyroid disease showed an increasing trend from the initial observation period up to 2020 then slightly decreasing in 2021. During the COVID-19 pandemic, the prevalence of thyroid disease among those aged ≥60 years significantly increased. Moreover, the prevalence of thyroid disease in those experiencing high levels of perceived stress and weight gain increased up to 2020. While this suggests that the pandemic may have influenced the prevalence of thyroid disease in certain populations, the increase in overall prevalence during the pandemic is ambiguous. Therefore, further study is needed to verify this hypothesis. Additionally, subgroups including those 40–59 and ≥ 60 years, female, lower educated, overweight or obese, high levels of perceived stress, and weight gain were found to be more vulnerable to thyroid disease.
4.2. Plausible underlying mechanisms
During the period from 1998 to 2020, the prevalence of thyroid disease has shown an increasing trend [9]. This elevation may be due to environmental factors such as radiation exposure, viral infections, and changes in iodine intake that may increase the risk of developing thyroid disease [8]. Additionally, as South Korea's population ages, the prevalence of thyroid disease appears to have increased due to hormonal changes that make older individuals more susceptible [24,25]. The advancement in thyroid disease testing and the increased frequency of examinations may have also contributed to this rise in prevalence [26]. For instance, advancements in gene expression classifiers have significantly improved the diagnostic accuracy of thyroid nodules [27]. Additionally, public health campaigns aimed at raising awareness about thyroid disease, such as Thyroid Awareness Month, may influence the increase in thyroid disease prevalence [28]. These campaigns promote diagnosis and screening, leading to the detection of more cases. This does not mean that the actual incidence rate has increased, but it can be interpreted as an increase in detected cases due to improved diagnostic capabilities. Although previous studies have suggested a relationship between thyroid disease and the COVID-19 pandemic, our findings did not show a significant relationship between the COVID-19 pandemic and thyroid disease [29]. However, among those aged ≥60 years, prevalence rates have increased significantly during the pandemic compared to before the pandemic. Previous studies have shown that the older population are more vulnerable to both COVID-19 and thyroid disease [30], but further research is needed to determine whether the association between COVID-19 and thyroid disease is significant only for people aged ≥60 years.
4.3. Comparison of previous study
In a previous research study on thyroid disease in South Korea, there was a continuous increase from 2006 to 2012, followed by a decrease in 2015 [31]. These findings support the conclusions of the present study. However, while previous research focused on three specific types of thyroid disease, this study comprehensively investigated the prevalence of all types. Moreover, the present research spanning from 1998 to 2021, including the COVID-19 pandemic, provides a more long-term observation period.
Similar trends were observed in the preliminary studies in the United States. The prevalence of hypothyroidism continued to rise from 2012 to 2019, aligning with our research findings [9]. However, this study also focused only on hypothyroidism, one of the thyroid diseases, and comprehensive research considering both pre-pandemic and pandemic periods is rare. Therefore, the significance of our study is underscored in bridging such gaps.
4.4. Clinical and policy implications
As the population ages, the prevalence of thyroid disease is increasing. This is emerging as a serious problem in our society. To prepare for this, more active prevention policies are essential. In particular, because females have a high vulnerability to thyroid disease, there is a need to strengthen regular thyroid screening programs focusing on females and to develop customized health policies that emphasize the impact on female's reproductive function. Additionally, there is an urgent need to routinely integrate thyroid testing into comprehensive health assessments for older populations over 60 years of age.
Our study shows a significant increase in the prevalence of thyroid disease among those 60 years and over during the COVID-19 pandemic. This highlights that the older population are more vulnerable to unexpected situations, suggesting that public health and healthcare systems should prioritize the safety and well-being of the older population [32]. The integration of telemedicine for remote monitoring, which can help with regular screening for the older population, is important [33]. In addition, the COVID-19 pandemic experience emphasizes the importance of having the flexibility of healthcare systems to continue to provide services in times of emergency. Continuous research is needed to understand the specific factors of increasing thyroid disease in the older population.
In clinical practice, developing protocols for the early detection and prevention of thyroid disease is crucial. Raising awareness among healthcare professionals through education about thyroid disease can lead to better patient outcomes [34]. From a public health policy perspective, sustainable health programs must be established to meet the health needs of the aging population, with particular attention given to supporting vulnerable groups. Future research should comprehensively analyze the impact of various factors on thyroid disease and develop prevention and management strategies based on these insights.
Cooperation between public health agencies and those responsible for projects focusing on older adults is crucial to developing a comprehensive approach to aged care, including thyroid health. In conclusion, it is important to strengthen healthcare systems to respond to the changing needs of aging societies as well as the current challenges highlighted in the present study.
4.5. Strengths and limitations
A clear strength of the present study is leveraging extensive data spanning 24 years, from 1998 to 2021, based on the population of South Korea. This data allows us to systematically compare the prevalence of thyroid disease before and during the COVID-19 pandemic. The extensive duration of the study enables the observation of long-term trends and potential changes in thyroid disease prevalence, providing valuable insights into the evolving landscape of these conditions over two decades. However, several noteworthy limitations are present.
First, while the total population of South Korea is approximately 50 million, our sample consists of about 5000 samples per year. Nevertheless, we utilized a complex sampling method to represent the entire nation, allowing for a robust estimation of thyroid disease prevalence in South Korea. Second, although this study was conducted on a large scale over an extended period, it does not fully account for the changes in medical practices, diagnostic criteria, public health interventions, or the impact of genetic factors and family history. This may have resulted in the exclusion of critical variables from our research. Nonetheless, the analysis of thyroid disease trends in South Korea over 24 years provides an important foundation for subsequent studies that consider these changes. Future research is now needed to accurately identify the various factors influencing thyroid disease trends to better understand the evolving medical environment and population characteristics. Third, thyroid disease includes multiple subtypes, such as hyperthyroidism and hypothyroidism. However, the dataset used in this study only allows for determining whether individuals have ever been diagnosed with a thyroid disease without specifying the exact subtype. Nonetheless, given the potential complications that these conditions may induce, it is essential not to overlook them [5,6,35,36]. Therefore, confirming the overall prevalence of thyroid disease is crucial, particularly as this study has proven significantly helpful in examining changes, especially those stemming from the pandemic. Fourth, although the study identifies an increase in prevalence among individuals aged 60 years and above since the COVID-19 pandemic emerged, establishing a causal relationship remains challenging. Nevertheless, the discovery of such trends holds significance. Last, this study relies on secondary data gathered through a combination of self-reported questionnaires and interviews. As such, the accuracy of self-reported responses may be subject to limitations. Additionally, factors such as iodine status in the geographical area and other influencing variables may not be fully accounted for in relation to thyroid disease. Despite these potential limitations, the reliability of the KNHANES dataset has been demonstrated in numerous studies [37,38]. Therefore, we believe that while these factors warrant consideration, they do not significantly compromise the overall validity of our findings.
5. Conclusion
While the impact of the pandemic on the overall prevalence of thyroid disease remains inconclusive, we found a significant increasing trend in the prevalence of thyroid disease from 1998 to 2020 followed by a slight decrease in 2021. Particularly vulnerable groups include individuals aged 40–59 and ≥ 60 years, females, those with lower education levels, those overweight or obese, those with high levels of perceived stress, and those who gained weight. The present results indicate that the impact of the pandemic on overall disease prevalence is unclear, with a significant increase in prevalence only observed in those ≥60 years during the pandemic. Therefore, additional study is needed to validate our hypothesis. In addition, policies related to thyroid disease should be consistently developed.
CRediT authorship contribution statement
Kyeongmin Lee: Writing – original draft, Data curation. Jaeyu Park: Writing – original draft, Data curation. Myeongcheol Lee: Writing – review & editing. Hojae Lee: Writing – review & editing. Yejun Son: Writing – review & editing. Hyejun Kim: Writing – review & editing. Jiseung Kang: Writing – review & editing. Yujin Choi: Writing – review & editing. Sang Youl Rhee: Writing – review & editing. Masoud Rahmati: Writing – review & editing. Ai Koyanagi: Writing – review & editing. Lee Smith: Writing – review & editing. Guillermo F. López Sánchez: Writing – review & editing. Elena Dragioti: Writing – review & editing. Selin Woo: Supervision. Dong Keon Yon: Supervision.
Data accessibility statement
The data are available upon request. Study protocol and statistical code: Available from DKY (yonkkang@gmail.com). Dataset: Available from the Korea Disease Control and Prevention Agency through a data use agreement.
Ethical statement
The research protocol was approved by the Institutional Review Boards of the KDCA (2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C, 2018-01-03-P-A, 2018-01-03-C-A, 2018-01-03-2C-A, 2018-01-03-5C-A, 2018-01-03-4C-A) in accordance with the Act (Article 2, Paragraph 1) and the Enforcement Regulation (Article 2, Paragraph 2, item 1) of the Bioethics and Safety Act from the Korean government, and by the Institutional Review Boards of Kyung Hee University (KHSIRB-23-384). Written informed consent was obtained from all participants prior to their participation. Written informed consent was obtained from all participants prior to their participation. Our research adhered to the Declaration of Helsinki.
Funding information
This research was supported by the MSIT (Ministry of Science and ICT), South Korea, under the ITRC (Information Technology Research Center) support program (IITP-2024-RS-2024-00438239) supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2024-00460379). The funders played no role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to express our sincere gratitude to Dr Soeun Kim (Department of Precision Medicine, Kyung Hee University, Seoul, South Korea) for their valuable feedback and insightful comments on our manuscript. Their thorough evaluation and constructive suggestions have significantly enhanced the quality and clarity of our research. We deeply appreciate their time and effort in reviewing our work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39401.
Contributor Information
Lee Smith, Email: Lee.Smith@aru.ac.uk.
Selin Woo, Email: dntpfls@naver.com.
Dong Keon Yon, Email: yonkkang@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhang X., Wang X., Hu H., Qu H., Xu Y., Li Q. Prevalence and trends of thyroid disease among adults, 1999-2018. Endocr. Pract. 2023;29:875–880. doi: 10.1016/j.eprac.2023.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Chaker L., Bianco A.C., Jonklaas J., Peeters R.P. Hypothyroidism. Lancet. 2017;390:1550–1562. doi: 10.1016/s0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bathgate G., Karra E., Khoo B. New diagnosis of hyperthyroidism in primary care. BMJ. 2018;362 doi: 10.1136/bmj.k2880. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y., Li H., Wang M., Li N., Tian T., Wu Y., Xu P., Yang S., Zhai Z., Zhou L., et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skov J., Kuja-Halkola R., Magnusson P.K.E., Gudbjörnsdottir S., Kämpe O., Bensing S. Shared etiology of type 1 diabetes and Hashimoto's thyroiditis: a population-based twin study. Eur. J. Endocrinol. 2022;186:677–685. doi: 10.1530/eje-22-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daya N.R., Fretz A., Martin S.S., Lutsey P.L., Echouffo-Tcheugui J.B., Selvin E., Juraschek S.P. Association between subclinical thyroid dysfunction and fracture risk. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.40823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollerits E., Zsila Á., Matuszka B. Quality of life, social support, and adherence in female patients with thyroid disorders. BMC Wom. Health. 2023;23:567. doi: 10.1186/s12905-023-02718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari S.M., Fallahi P., Antonelli A., Benvenga S. Environmental issues in thyroid diseases. Front. Endocrinol. 2017;8:50. doi: 10.3389/fendo.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyne K.L., Nair L., Schneiderman C.P., Pinsky B., Antunez Flores O., Guo D., Barger B., Tessnow A.H. Hypothyroidism prevalence in the United States: a retrospective study combining national health and nutrition examination survey and claims data, 2009-2019. Journal of the Endocrine Society. 2022;7 doi: 10.1210/jendso/bvac172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J., Kim H.J., Kim T., Lee H., Kim M., Lee S.W., Kim M.S., Koyanagi A., Smith L., Fond G., et al. Prenatal opioid exposure and subsequent risk of neuropsychiatric disorders in children: nationwide birth cohort study in South Korea. BMJ. 2024;385 doi: 10.1136/bmj-2023-077664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Zhu M., Zhang R., Zhang J., Zhang C., Liu P., Feng Z., Chen Z. Public mental health problems during COVID-19 pandemic: a large-scale meta-analysis of the evidence. Transl. Psychiatry. 2021;11:384. doi: 10.1038/s41398-021-01501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks C.G., Spencer J.R., Sprafka J.M., Roehl K.A., Ma J., Londhe A.A., He F., Cheng A., Brown C.A., Page J. Pediatric BMI changes during COVID-19 pandemic: an electronic health record-based retrospective cohort study. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke S.A., Phylactou M., Patel B., Mills E.G., Muzi B., Izzi-Engbeaya C., Choudhury S., Khoo B., Meeran K., Comninos A.N., et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J. Clin. Endocrinol. Metab. 2021;106:2208–2220. doi: 10.1210/clinem/dgab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biondi B. Subclinical hypothyroidism in patients with obesity and metabolic syndrome: a narrative review. Nutrients. 2023;16 doi: 10.3390/nu16010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivgoulis G., Fragkou P.C., Karofylakis E., Paneta M., Papathanasiou K., Palaiodimou L., Psarros C., Papathanasiou M., Lachanis S., Sfikakis P.P., Tsiodras S. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case-control study. J. Neurol. Neurosurg. Psychiatry. 2021 doi: 10.1136/jnnp-2021-326587. [DOI] [PubMed] [Google Scholar]
- 16.Yoon S.Y., Park H.W., Kim H.J., Kronbichler A., Koyanagi A., Smith L., Shin J.I., Rhee S.Y., Lee S.W., Kim J.S., et al. National trends in the prevalence of chronic kidney disease among Korean adults, 2007-2020. Sci. Rep. 2023;13:5831. doi: 10.1038/s41598-023-33122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H.J., Park H., Yon D.K., Rahmati M. National trends in influenza vaccination coverage rates in South Korea between 2007-2020, including the COVID-19 pandemic: a longitudinal nationwide serial study. Life Cycle. 2023;3 doi: 10.54724/lc.2023.e9. [DOI] [Google Scholar]
- 18.Kim S.Y. Nationwide COVID-19 vaccination coverage and COVID-19 incidence in South Korea, January 2022: a national official report. Life Cycle. 2022;2:e2. doi: 10.54724/lc.2022.e2. [DOI] [Google Scholar]
- 19.Eum S., Rhee S.Y. Age, ethnic, and sex disparity in body mass index and waist circumference: a bi-national large-scale study in South Korea and the United States. Life Cycle. 2023;3 doi: 10.54724/lc.2023.e4. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.W. Regression analysis for continuous independent variables in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e3. doi: 10.54724/lc.2022.e3. [DOI] [Google Scholar]
- 21.Kim S., Lee H., Lee J., Lee S.W., Kwon R., Kim M.S., Koyanagi A., Smith L., Fond G., Boyer L., et al. Short- and long-term neuropsychiatric outcomes in long COVID in South Korea and Japan. Nat. Human Behav. 2024 doi: 10.1038/s41562-024-01895-8. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y., Kim H.J., Park J., Lee M., Kim S., Koyanagi A., Smith L., Kim M.S., Rahmati M., Lee H., Kang J., Yon D.K. Acute and post-acute respiratory complications of SARS-CoV-2 infection: population-based cohort study in South Korea and Japan. Nat. Commun. 2024;15:4499. doi: 10.1038/s41467-024-48825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H., Kyung S., Park J., Lee H., Lee M., Smith L., Rahmati M., Shin J.Y., Kang J., Jacob L., et al. Risks of cutaneous immune-related adverse events in long COVID: multinational cohort studies in South Korea, Japan, and the UK. J. Med. Virol. 2024;96 doi: 10.1002/jmv.29740. [DOI] [PubMed] [Google Scholar]
- 24.Biondi B., Cappola A.R., Cooper D.S. Subclinical hypothyroidism: a review. JAMA. 2019;322:153–160. doi: 10.1001/jama.2019.9052. [DOI] [PubMed] [Google Scholar]
- 25.Chaker L., Cappola A.R., Mooijaart S.P., Peeters R.P. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. 2018;6:733–742. doi: 10.1016/s2213-8587(18)30028-7. [DOI] [PubMed] [Google Scholar]
- 26.Kiel S., Ittermann T., Völzke H., Chenot J.F., Angelow A. Frequency of thyroid function tests and examinations in participants of a population-based study. BMC Health Serv. Res. 2020;20:70. doi: 10.1186/s12913-020-4910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noureldine S.I., Olson M.T., Agrawal N., Prescott J.D., Zeiger M.A., Tufano R.P. Effect of gene expression classifier molecular testing on the surgical decision-making process for patients with thyroid nodules. JAMA Otolaryngol Head Neck Surg. 2015;141:1082–1088. doi: 10.1001/jamaoto.2015.2708. [DOI] [PubMed] [Google Scholar]
- 28.Canaris G.J., Tape T.G., Wigton R.S. Thyroid disease awareness is associated with high rates of identifying subjects with previously undiagnosed thyroid dysfunction. BMC Publ. Health. 2013;13:351. doi: 10.1186/1471-2458-13-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugan A.K., Alzahrani A.S. SARS-CoV-2: emerging role in the pathogenesis of various thyroid diseases. J. Inflamm. Res. 2021;14:6191–6221. doi: 10.2147/jir.S332705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amore S., Puppo E., Melara J., Terracciano E., Gentili S., Liotta G. Impact of COVID-19 on older adults and role of long-term care facilities during early stages of epidemic in Italy. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-91992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon H., Jung J.H., Han K.D., Park Y.G., Cho J.H., Lee D.Y., Han J.M., Park S.E., Rhee E.J., Lee W.Y. Prevalence and annual incidence of thyroid disease in Korea from 2006 to 2015: a nationwide population-based cohort study. Endocrinol Metab (Seoul) 2018;33:260–267. doi: 10.3803/EnM.2018.33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J., Seib C.D. Operative management of thyroid disease in older adults. J Endocr Soc. 2023;7 doi: 10.1210/jendso/bvad070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haimi M., Gesser-Edelsburg A. Application and implementation of telehealth services designed for the elderly population during the COVID-19 pandemic: a systematic review. Health Inf. J. 2022;28 doi: 10.1177/14604582221075561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar P., Khandelwal D., Mittal S., Dutta D., Kalra S., Katiyar P., Aggarwal V. Knowledge, awareness, practices and adherence to treatment of patients with primary hypothyroidism in Delhi. Indian J Endocrinol Metab. 2017;21:429–433. doi: 10.4103/ijem.IJEM_49_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitahara C.M., D K.R.F., Jørgensen J.O.L., Cronin-Fenton D., Sørensen H.T. Benign thyroid diseases and risk of thyroid cancer: a nationwide cohort study. J. Clin. Endocrinol. Metab. 2018;103:2216–2224. doi: 10.1210/jc.2017-02599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappola A.R., Desai A.S., Medici M., Cooper L.S., Egan D., Sopko G., Fishman G.I., Goldman S., Cooper D.S., Mora S., et al. Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention, and treatment. Thyroid. 2019;29:760–777. doi: 10.1089/thy.2018.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J., Lee M., Lee H., Kim H.J., Kwon R., Yang H., Lee S.W., Kim S., Rahmati M., Koyanagi A., et al. National trends in rheumatoid arthritis and osteoarthritis prevalence in South Korea, 1998-2021. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-46279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh J., Kim S., Lee M., Rhee S.Y., Kim M.S., Shin J.Y., Lim H., Lee S.W., Rahmati M., Kim S., Yon D.K. National and regional trends in the prevalence of type 2 diabetes and associated risk factors among Korean adults, 2009-2021. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-43353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request. Study protocol and statistical code: Available from DKY (yonkkang@gmail.com). Dataset: Available from the Korea Disease Control and Prevention Agency through a data use agreement.