Abstract
Hypericum perforatum L. (St. John's Wort) extract (HPE), powdered H. perforatum (PHP), and selenium (Se) on growth, intestinal flora, and immunity of broiler chicks were investigated. In total, 504 one-day-old broiler chicks were randomly allocated into 6 dietary treatments, which were then denoted as negative control (NC) group (basal diet), containing organic Se 0.2% in the starter and grower period as positive control (PC), containing 1% PHP in the starter and grower period, and HPE I, HPE II, and HPE III groups containing respectively, 1.5, 3.0, and 4.5 mL / kg HPE in the starter and grower period. The results on performance showed that a significant (P < 0.05) higher body weight of chickens in the HPE III group was observed when compared with that of the NC and PHP groups. Although average daily weight gain and feed intake are significant in the HPE III group, the difference in terms of total feed conversion rate was insignificant (P > 0.05). The liver weights in PC and HPE III were lower compared to HPE I (P < 0.05). The difference in total lactic acid bacteria count (TLABC) between the NC group and all HPE groups was found to be significant (P ˂ 0.05), in addition to TLABC was higher in the HPE III group than other groups (P = 0.001). The highest serum antibody titers to the Newcastle disease vaccine were determined in the HPE III group on the 24th, 35th, and 42nd days of age. IL-1B and IL-6 were found to be insignificant between the groups in chickens (P ˃ 0.05). TNF-α in the HPE III group was greatly increased than the other groups and significant compared to the NC and HPE I groups (P = 0.018). In conclusion, 4.5 mL / kg HPE, which has a low production cost and is easy to extract and without causing environmental problems, varied significantly in their impact on growth performance, intestinal microflora, and immunity of growing broilers.
Keywords: Hypericum perforatum (St. John's Wort), Broiler, Growth performance, Intestinal microflora, Immunity
Introduction
Herbal feed additives have different bioactive substances; thus, they could be used as essential additives in poultry feeds (Rashid et al., 2020; Phillips et al., 2023). Hypericum perforatum L. (St. John's wort), one of the most well-known species worldwide belonging to the Hypericaceae family, is a plant that can be used medicinally and feed (Caldeira et al., 2022; Kwiecień et al., 2023). It is claimed that this product is used in many diseases such as icterus, liver and bile disorders, insomnia, gastrointestinal system diseases, skin wounds, eczema, and burns (Kıyan et al., 2015). The chemical composition of this plant in medicinal applications includes naphthodiantrone compounds (e.g. hypericin), monomeric flavonoids (e.g. quercetin derivatives), bioflavonoids (e.g. amentoflavone) and procyanidin. It also contains phenolic compounds such as tannins (catechin polymers) and essential fatty acids (Kwiecień et al. 2023).
Much attention has been attracted to extracts, and organic component in recent years which has roots in use for overall improvement in growth performance, intestinal microflora, and immunity sensory characteristics of broilers (Davoodi et al., 2014; Kogut and Fernandez-Miyakawa, 2022; Zengin et al., 2022; Sur et al., 2023). In the poultry industry such substances have been used for many purposes, from antibacterial, antiviral, and antioxidant activities, and improving overall performance (Axarlis et al., 1998). When feeding diets to animals, interactions between microbiota and host axis systems need to be understood and scientific studies that bridge the gaps between feed-gut microbial ecology-chicken physiology (Kogut, 2022). Some studies have reported that H. perforatum had significant therapeutic efficacy and could improve immunologic functions for chickens infected experimentally with infectious bursal disease virus (IBDV) and avian influenza virus (AIV), respectively (Landy et al., 2012). It was stated that the immunostimulating activity of ethanol extraction fraction of H. perforatum with concerning the system of mononuclear phagocyte system, cellular, and humoral immunity (Evstifeeva and Sibiriak, 1996).
Selenium (Se) is a trace mineral that is essential for both humans and animals. Se is important for optimum immune response and is considered as a functional part of the antioxidant properties through selenoproteins with selenocysteine such as glutathione peroxidase, thioredoxin reductase in chickens (Chen and Berry, 2003; Rao et al., 2013; Hou et al., 2020). Supplementation of Se increases antibody titers to Newcastle disease (ND) in chicken diet (Hegazy and Adachi, 2000). Besides, Se influences immune responses through its incorporation into selenoproteins such as selenocysteine (Hoffman and Berry, 2008).
Some studies were performed to evaluate the effect of various plants and their derivatives as alternatives to nutritional antibiotics on animal productive performance and quality of products of animal origin during the last years. H. perforatum has been well studied from a pharmacological perspective, especially in human medicine, but little is known regarding the broiler growth performance, intestinal microflora, and immunity up to now. In this study, it was aimed to investigate the effect of H. perforatum extract (HPE) and, powdered H. perforatum (PHP) on the dietary inclusion of different levels of extract, powdered leaves, and organic Se on body weight, intestinal microflora, and immunity of broiler chickens.
Materials and methods
Ethical approval
All applications performed on animals within of the study were carried out within the scope of the work permit approved by Balıkesir University, Local Ethics Committee for Animal Experiments (Approval number: 2021/11-1).
Bird management, diets and experimental design
504 one-day-old-mixed gender chicks (Ross-308) were obtained from Kula Hatchery Company (Kula A.Ş., Balıkesir, Türkiye). After hatching, the chicks were brought to Balıkesir University, Livestock Application and Research Center. The chicks were divided into 6 main groups consisting of a total of 84 birds. A total of 504 one day old broiler chicks were divided to 6 experimental groups with 6 replicates. 1st group negative control (NC), 2nd positive control (organic Se 0.2%, 2,000.00 mg/kg Se yeast, YES Selenio, YesSinergy®, Lucélia/SP, Brazil) (PC), 3rd 1% powdered H. perforatum (PHP), 4th H. perforatum extract (HPE) HPE I (1.5 mL / kg), 5th HPE II (3 mL / kg), and the 6th was formed as HPE III (4.5 mL / kg). The coop where the animals were housed was divided into compartments with wire fences of 1.5×1.0 m in size, and 10 cm wooden floor was laid on the floor. Animals were housed in a ground system and a lighting program of 20 h of light and 4 h of darkness was applied. The temperature was initially set at 33°C on d 1 and gradually reduced average by 2.5°C per week until reaching 24°C to 25°C. Feed and water were given ad-libitum. All chicks were free to access tap water and their formulated diets throughout the experiment. In the trial, a basic compound feed based on corn and soybean meal was prepared, and this diet constituted the basal feed of all animals. Ration components were mixed homogeneously in the feed mixing unit according to NRC (1994). The basal diet composition and nutrient contents are presented in Table 1.
Table 1.
Basal diet composition and nutrient contents#.
| Ingredients | d 1-21 | d 22-42 | Nutritional composition | g 1-21 | g 22-42 |
|---|---|---|---|---|---|
| Corn grain | 52.05 | 59.33 | Dry matter, g/kg | 902.0 | 902.0 |
| Soybean meal, 48% CP | 32.42 | 25.50 | ME kcal/kg | 3003 | 3191 |
| Sunflower meal, 28% | 7.50 | 6.50 | Crude protein, g/kg DM | 225.0 | 194.0 |
| Vegetable oil | 3.50 | 5.00 | Ether extract, g/kg DM | 50.8 | 66.7 |
| Dicalcium phosphate | 1.94 | 1.50 | Crude ash, g/kg DM | 66.7 | 56.5 |
| DL-Methionine | 0.33 | 0.25 | Calcium, % | 0.96 | 0.78 |
| L-Lysine | 0.34 | 0.22 | Available phosphorus, % | 0.49 | 0.39 |
| L-Threonine | 0.14 | 0.07 | Lysine, % | 1.44 | 1.15 |
| Calcium carbonate | 1.00 | 0.85 | |||
| Sodium chloride | 0.34 | 0.34 | |||
| Mineral premix | 0.22 | 0.22 | |||
| Vitamin premix | 0.22 | 0.22 | |||
| Total | 100.00 | 100.00 |
Vitamin premix provided per kg, vitamin D3: 5,000 IU, vitamin E: 30 IU, vitamin A: 12,000 IU, vitamin B1 (thiamine): 3 mg, vitamin B3 (niacin): 45 mg. Mineral premix provided per kg, manganese: 100 mg, iron: 60 mg, zinc: 60 mg, copper: 5 mg, cobalt: 0.3 mg, iodine: 1 mg, inorganic selenium: 0.10 mg.
Calculated according to NRC (1994).
Growth performance and internal organ weight
The body weight (BW) and feed intake (FI) were recorded on days 2, 21, and 42 to average daily weight gain (ADWG) and feed conversion ratio (FCR). Internal organs (liver, heart, gizzard + proventriculus, spleen, and intestine) were carefully removed and weighed. Organ weights of chickens were calculated as a percentage according to their live weight and group proportions. The weights of the liver, heart, gizzard, spleen, and intestine were calculated g / 100 g BW.
Plant material and preparation of H. perforatum extract (HPE)
HPE was prepared from the H. perforatum plant that grows naturally in Mount Ida (Atapharma, Balıkesir, Türkiye). Dried H. perforatum was ground into powder using a 1.0 mm sieve (Retsch ZM 200 Haan, Germany). The extraction process was carried out with extra-virgin olive oil provided by Balıkesir University, Edremit Olive Cultivation Institute. Macerate was prepared by mixing dried H. perforatum with olive oil in a glass bottle (Eroğlu and Girgin, 2021). The volatile fatty acid profile in HPE was determined at Bezmialem University. The conventional folin ciocalteu method was applied to determine total phenolic compounds (Singleton and Rossi, 1965) and gallic acid (149-91-7, Sigma-Aldrich) was used as a control. The aluminum chloride colorimetric method was applied to determine total flavonoid compounds (Pourmorad et al., 2006) and quercetin (117-39-5, Sigma-Aldrich) was used as a control. The amount of hypericin was determined according to the LC–DAD–MS method (Isacchi et al. 2007). Biologically active compounds of HPE are given in Table 2.
Table 2.
Biologically active compounds of the H. perforatum extract.
| Ingredients | Compound levels |
|---|---|
| Total phenolic compounds | 921.74 mg / L |
| Total flavonoid compounds | 3607.79 mg / L |
| Hypericin | 0.0034% |
Microbiological analysis of intestinal contents
At the end of the experiment (42 d), 8 chickens were randomly selected from each group. Their gastrointestinal tracts were quickly excised and part of the ileum (5 cm above the ileum-cecal junction) and cecum (end part of left cecum) were removed aseptically, and microbiological analyses were performed immediately. Homogenized pooled ileal and cecum digesta were analyzed to determine the number of total aerobic bacteria count (TABC), total coliforms and E. coli count (TCECC), total lactic acid bacteria count (TLABC), total Enterococcus spp. count (TEC), and total yeast and mold count (TYMC). 10 g of intestinal homogenate and 90 mL of 0.9% sterile saline solution (SSS) (pH: 7.2) were mixed in a sterile tube. For each intestinal homogenate, 10-fold serial dilutions (from 10−1 to 10−10) were made and 100 µL of them were plated in duplicate plates on a specific medium for each microorganism group. TABC, TCECC, and TEC were enumerated using the pour plate technique on nutrient agar (NA) (Oxoid Ltd., CM0003, Basingstoke, UK), eosin methylene blue agar (EMBA) (Oxoid Ltd., CM0069, UK), and kanamycin aesculin azide agar (KAAA) (Oxoid Ltd., CM0591, UK), respectively. NA and EMBA plates were incubated at 37°C for 24 h and KAAA for 48 h. Colonies were enumerated and presented as log10 colony-forming units per g (cfu/g) of digesta (Proietti et al., 2009; ISO, 2017; Bortoluzzi et al., 2018). Sabouraud dextrose agar (SDA) (Merck, 1.05438, Darmstadt, Germany) and dichloran-rose bengal chloramphenicol agar (DRBCA) (Merck, 1.00466, Germany) were used to determine the TYMC and the plates were incubated at 25°C for 5 d. The enumeration results were expressed as cfu/g (ISO, 2008; Proietti et al., 2009). The detection of TLABC was performed according to ISO (1998) with some modifications. Briefly, the intestinal homogenate was diluted with SSS (from 10−1 to 10−10) and 100 μL of inoculated was transferred on De Man, Rogosa and Sharpe (MRS) agar (Merck, 1.10660, Germany). The plates were incubated for 48 h at 37°C. Lactic acid bacteria (LAB) and Enterococcus spp. suspected colonies were analyzed using BD Phoenix M50™ System (BD, Baltimore, Maryland).
Serological analysis
All chickens were vaccinated with ND live vaccine (Fatro, Bio-Vac La Sota, Bologna, Italia) on 10th and 24th d of age by eye drop vaccination. To determine the antibody titers against the ND vaccine, 8 broilers were randomly selected from each group and their blood samples (approximately 300 µL) were collected from the wing vein at the 24 th, 35th, and 42nd d of age. Sera samples were tested by haemagglutination inhibition test (HIT) described by Allan and Gough (1974).
To determine the antibody titers against sheep red blood cell (SRBC), blood samples were washed three times with SSS, then diluted and injected into 6 chickens from each group in two doses, on the 18th and 25th d of the age. Serum samples were taken from 8 broilers on the 28th and 42nd d of the age and tested by micro-hemagglutination inhibition (m-HIT) test as previously described (Delhanty and Solomon, 1966). Before the test, the complement was inactivated by heating the sera samples at 56°C for 30 min. Total [presumably IgM and IgY(IgG)] and mercaptoethanol (ME) (Merck, 8.05740.0250, Hohenbrunn, Germany) resistant (presumably IgY) anti-SRBC antibodies were determined. Antibody titers were expressed as the log2 of the reciprocal of the last dilution in which agglutination was observed macroscopically.
For the detection of cytokines, serum samples taken from 8 chickens on 42 d from each group during slaughter were tested for interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) by commercial ELISA kits. IL-1β (ECH0040, Fine Test, Wuhan Fine Biotech Co., Ltd., China) and IL-6 (ECH0046, Fine Test, Wuhan Fine Biotech Co., Ltd., China) were detected by sandwich ELISA method, while TNF-α (EA0010Ch, BT LAB, Jiaxing Korain Biotech Co., Zhejiang, China) were tested by competitive ELISA method. The kits were used in by the manufacturer's recommendations.
Statistical analysis
Data were analyzed using one-way analyses (ANOVA) using SPSS 25.0. The data were expressed as means ± standard error mean (SEM), accompanied by Tukey's test as post-hoc test. P < 0.05 was considered difference among the treatment groups at a significant level, P values between 0.05 and 0.10 were classified. As a statistical model:
Yij = the perceived value of the treatment, μ = it means observed for the treatment, Ti= treatment impact, and eij = error related to an individual observation.
Results
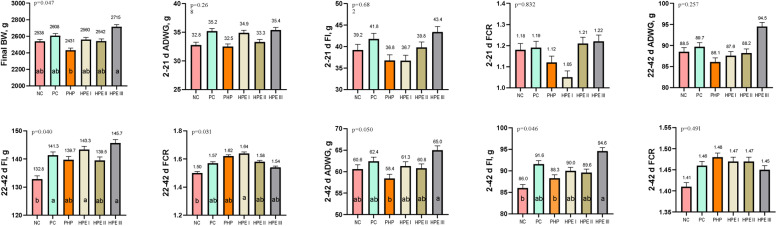
The means of BW in 42 d of the experiment were determined as 2.538, 2.608, 2.431, 2.560, 2.542, and 2.715 g in the NC, PC, PHP, HPE I, HPE II, and HPE III groups, respectively, and the lowest (P < 0.05) BW mean was seen in the PHP group (Fig. 1).
Fig. 1.
Growth performance (Mean ± SEM) of experimental broiler (n = 14/group).
The means with different superscripts in the columns are significantly different from each other (p < 0.05). SEM: Standard Mean Error, p: Significance. BW: Body weight, ADWG: Average daily weight gain, FI: Feed intake, FCR: feed conversion ratio. NC: Negative control consuming basal diet, PC: Positive control selenium added the basal diet, PHP: Powdered H. perforatum added the basal diet, HPE I: 1.5 mL / kg H. perforatum extract (HPE) added the basal diet, HPE II: 3 mL / kg HPE added the basal diet, HPE III: 4.5 mL / kg HPE added the basal diet.
The FCR between 22∼42 d of the study was determined as 1.50, 1.57, 1.62, 1.64, 1.58, and 1.54 in the NC, PC, PHP, HPE I, HPE II, and HPE III groups, respectively (Fig. 1). FCR was highest in the NC group and lowest in the HPE I group, and the difference was found to be significant (P < 0.031).
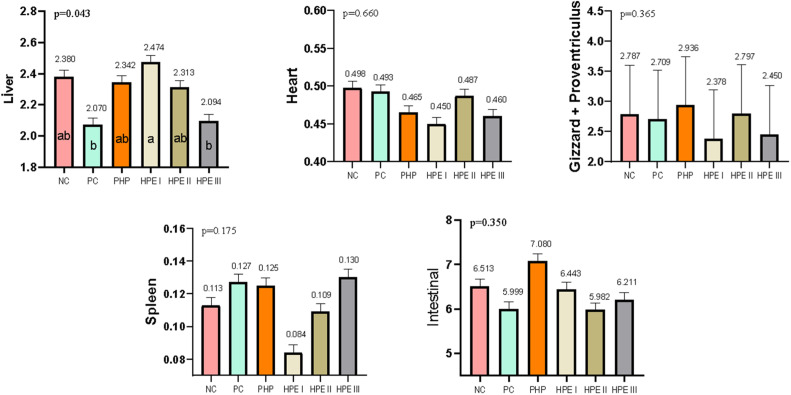
Data on organ weights of the trial groups are presented in Fig. 2. Organ weights of chickens were calculated as a percentage according to their BW. Liver, heart, gizzard + proventriculus, spleen, and intestine weights were calculated, differences in liver weights were observed between the groups, and the data were found to be statistically significant. Liver weight ratios were determined as 2.380, 2.070, 2.342, 2.474, 2.313 and 2.094 in the NC, PC, PHP, HPE I, HPE II, and HPE III groups, respectively, and the differences between the groups were found to be significant (P ≤ 0.043).
Fig. 2.
Internal organ weight (Mean ± SEM) of experimental broilers (n = 8/group) #.
The means with different superscripts in the columns are significantly different from each other (p < 0.05). SEM: Standard Mean Error, p: Significance NC: Negative control consuming basal diet, PC: Positive control selenium added the basal diet, PHP: Powdered H. perforatum added the basal diet, HPE I: 1.5 mL / kg H. perforatum extract (HPE) added the basal diet, HPE II: 3 mL / kg HPE added the basal diet, HPE III: 4.5 mL / kg HPE added the basal diet.
#Internal organ weight presented as g/100 g BW.
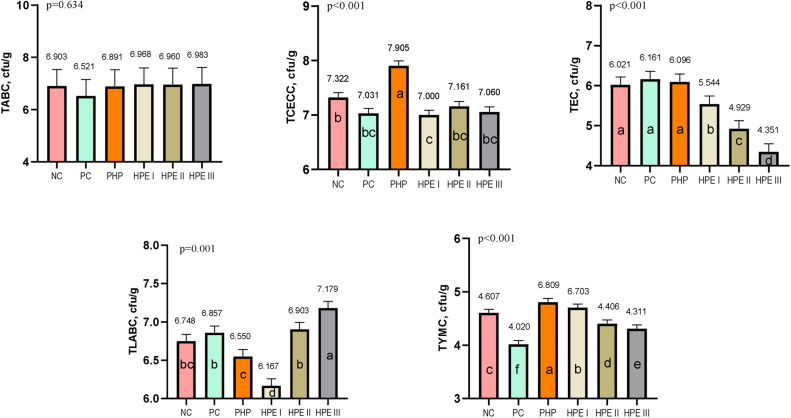
Microbiological analysis
The difference between groups in terms of TABC was found to be insignificant (P ˃ 0.05). TCECC was detected as highest in the PHP group and lowest in the HPE I group. TEC was determined to be highest in the NC group and lowest in the HPE III, HP II, and HPE I groups, respectively. TLABC was detected as highest in the HPE III group, followed by the HPE II and HPE I groups, respectively. The difference between the NC group and HPE III, HPE II, and HPE I groups in terms of TLABC was found to be significant (P < 0.05). Whereas TEC (P < 0.001) and TYMC (P < 0.05) in the HPE III group were lower in compared to the NC group (Fig. 3).
Fig. 3.
Bacteria count of intestinal contents (Mean ± SEM) of the groups (cfu/g).
TABC: Total aerobic bacteria count, TCECC: Total coliforms and E. coli count, TEC: Total Enterococcus spp. count, TLABC: Total lactic acid bacteria count, TYMC: Total yeast and mold count.
The means with different superscripts in the columns are significantly different from each other (p < 0.05). SEM: Standard Mean Error, p: Significance NC: Negative control consuming basal diet, PC: Positive control selenium added the basal diet, PHP: Powdered H. perforatum added the basal diet, HPE I: 1.5 mL / kg H. perforatum extract (HPE) added the basal diet, HPE II: 3 mL / kg HPE added the basal diet, HPE III: 4.5 mL / kg HPE added the basal diet.
Serological analysis
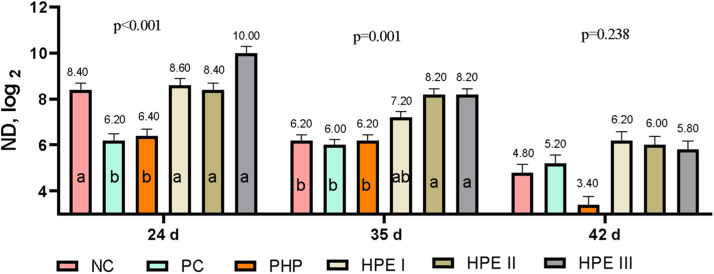
The highest serum antibody titer (log2) to the ND vaccine by HIT was determined in the HPE III group on both the 24th, 35th, and 42nd d of age (Fig. 4). In addition, it was determined that the ND antibody titers in all groups except the HPE III group fell below the protective antibody titer (7 log2) at the time of slaughter (42 d).
Fig. 4.
Newcastle disease vaccine antibody titers (Mean ± SEM) of groups by hemagglutination inhibition test (7 log2).
The means with different superscripts in the columns are significantly different from each other (p < 0.05). SEM: Standard Mean Error, p: Significance NC: Negative control consuming basal diet, PC: Positive control selenium added the basal diet, PHP: Powdered H. perforatum added the basal diet, HPE I: 1.5 mL / kg H. perforatum extract (HPE) added the basal diet, HPE II: 3 mL / kg HPE added the basal diet, HPE III: 4.5 mL / kg HPE added the basal diet.
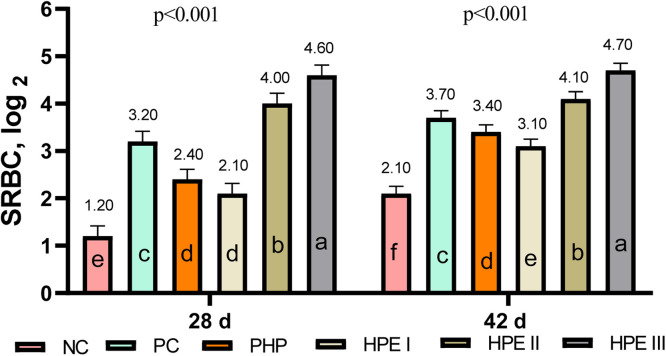
In the study conducted with SRBC, it was determined that the IgM + IgY titer was higher than the IgY titer in all groups on the 28th and 42nd d. Both IgM + IgY and IgY titers were determined to be highest in the HPE III group, both in the periods after the first and second injection (Fig. 5).
Fig. 5.
Micro-Haemagglutination inhibition test results (Mean ± SEM) of immunization with sheep red blood cells in broilers (log2).
The means with different superscripts in the columns are significantly different from each other (p < 0.05). SEM: Standard Mean Error, p: Significance NC: Negative control consuming basal diet, PC: Positive control selenium added the basal diet, PHP: Powdered H. perforatum added the basal diet, HPE I: 1.5 mL / kg H. perforatum extract (HPE) added the basal diet, HPE II: 3 mL / kg HPE added the basal diet, HPE III: 4.5 mL / kg HPE added the basal diet.
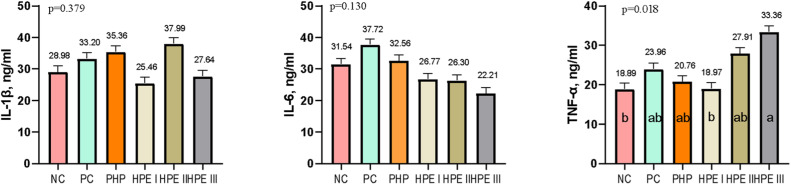
At the end of the study, IL-1B and IL-6 (Fig. 6) were found to be insignificant between the groups in chickens (P ˃ 0.05). TNF-α was found to be similar between the NC and HPE I groups, but lower compared with HPE III (P = 0.018).
Fig. 6.
The concentration of serum cytokine (Mean ± SEM) of broiler chickens (ng / mL).
The means with different superscripts in the columns are significantly different from each other (p < 0.05). SEM: Standard Mean Error, p: Significance NC: Negative control consuming basal diet, PC: Positive control selenium added the basal diet, PHP: Powdered H. perforatum added the basal diet, HPE I: 1.5 mL / kg H. perforatum extract (HPE) added the basal diet, HPE II: 3 mL / kg HPE added the basal diet, HPE III: 4.5 mL / kg HPE added the basal diet.
Discussion
Broiler sector is in great development world in the last decades. Broiler chicken's growth performance in terms of some parameters within the growth period is linked to intestinal microbiota, such as various other factors, including chicken management, environment, and vaccines (Rashid et al., 2020; Kiarie et al., 2013). In poultry diets, there are studies on improving intestinal microflora, increasing absorption and strengthening immunity in poultry diets (Mandal et al., 2015; Kogut, 2022; Ge et al., 2023; Liu et al. 2024). Türkiye hosts a large number of indigenous species of the Hyprecium genus (Güner et al., 2012). While there are some studies on the effects of H. perforatum, the main components of which are hypericin and flavonoids, on the performance of broiler chickens, there is less research on the intestinal microflora and immune systems (Axarlis et al., 1998; Behboodi et al., 2021; Chauveau et al., 2023; Hristakieva et al., 2023).
The acquired results showed that a beneficial effect of H. perforatum supplementation on BW, DWG, and FI without affecting the FCR of broilers. Landy et al. (2012) reported that adding the dried form of H. perforatum to the diet of broiler chickens has a positive effect on BW at slaughter and feed conversion, but drying the plant and adding it to the diet in powder form had no significant effect on live weight and feed utilization. On the contrary, our study determined that there was an increase in the BW of the HPE III group. This was evaluated as adding 4.5 mL / kg of HPE to the diet had positive effects on the growth performance of the poultry. Furthermore, Banisharif et al. (2016) indicated that H. perforatum in the poultry diet increased FI and BW in treatments compared to the control group. Hristakieva et al. (2023) investigated the effect of adding 2% dry H. perforatum and other dry herbs to the diets of broiler chickens on growth performance and observed that it did not affect final weight and FCR compared to the control. In their study, the difference between the performance values of the groups consuming extract and dry form of H. perforatum showed that the use of different forms of plants could make a difference in performance. In a study examining the effects of different HPE and neomycin concentrations in chicken diets, it was reported that HPE did not affect FCR (Beheshti Moghadam et al., 2015). Davoodi et al. (2014), the addition of HPE and virginamycin to broiler drinking water have significant in terms of live weight gain compared to the control group.
The chickens that consumed feed with the PC and the highest of HPE level had the lower liver weight, compared to the chickens consumed the lowest of HPE level. The liver weight of the other groups was not affected by the treatments. Seraji et al. (2015) stated that the addition of HPE to broiler chicken water increased the liver relative weight on the 24th d of the study, but at the end of the study there was no change.
The researches carried out by Davoodi et al., (2014), Hernandez et al., (2004), and Landy et al., (2012) were similar to the present study.
Although medical studies on H. perforatum have been extensively investigated (Coşkun et al., 2004; Kıyan et al., 2015; Eroğlu and Girgin, 2021), its effects on the intestinal microflora in poultry have been lower investigated. In a study on rats investigating the effect of HPE on irritable bowel syndrome, Mozaffari et al. (2011) used fluoxetine and loperamide as positive controls and found that HPE, the trial created by loperamide, inhibited small bowel syndrome and colonic transit acceleration, but had minimal effect on gastric emptying. However, in a study conducted on rats, it was stated that HPE made a significant contribution to the reestablish of metabolite profiles and the intestinal microbiota composition (Chen et al., 2021). The mechanism of the gastroprotective activity of HPE may be attributed to the reduction of vascular permeability and strengthening of the mucosal barrier (Sofi et al., 2020).
When HPE was given to the drinking water of broiler chickens at levels of 150 mg / L, 200 mg / L, and 250 mg / L, respectively, the count of E. coli decreased, while ND vaccine titer increased significantly (Davoodi et al., 2014). Landy et al., (2012) stated that high amounts of HPE in the diet may cause performance loss in broilers due to the possibility of negative effects on some beneficial microbial populations such as Lactobacillus spp. Culture method has been used as the 'gold standard' for the detection of the bacterial counts in intestinal microflora. In the present study, the numbers of microorganisms belonging to 5 different genera (TABC, TCECC, TLABC, TEC, and TYMC) were determined by the 'gold standard' methods (ISO, 1998; ISO, 2008; Proietti et al., 2009; ISO, 2017; Bortoluzzi et al., 2018). The difference in TLABC between the NC and HPE groups was significant, and TLABC in HPE III group was higher than in all groups. Lactic acid bacteria (LAB), which are among the first microorganisms to colonize the intestine, protect against various infections by synthesizing lactic acid, hydrogen peroxide, and bacteriocins. LAB synthesizes adenosine triphosphate by converting carbohydrates into lactic acid as nearly the major end product, and it contributes the performance (Sharma et al., 2020). Besides, the production of lactic acid and acetate by these bacteria helps to keep the pH of the intestine acidic, so many pathogens cannot cause infection at low pH. It could be also thought that Lactobacillus spp. compounds could inhibit the growtht of Enterococcus spp. in broiler systems (Jung et al., 2019). The use of HPE in broiler diets may have positive effects on the intestinal microbiota by increasing the number of LAB. LAB makes significant positive contributions to acquired immunity by contributing positively to antibody synthesis, and to innate or acquired immunity by affecting the synthesis of various cytokines (Perdigón et al., 2002).
Essential oils are an ideal eco-friendly antifungal feed additive due to their antimicrobial activity and gut regulatory effects (Hou and Huang, 2024). In the present study, TYMC in the PC and HPE III group were lower in compared to the NC group (P < 0.05). With the results obtained from this study conducted in a limited number of animals, it could be evaluate that HPE and Se would be useful in combating intestinal fungal infections in poultry (Islam et al., 2024). Further research on this subject would be beneficial.
It has been reported that H. perforatum increases antibody titers against the ND vaccine when administered to chickens via drinking water or increases antibody titers against AIV with the feed (Davoodi et al., 2014; Landy et al. 2012). Shang et al., (2012) showed that HPE has significant therapeutic efficacy and improves immunological functions in chickens in combating IBDV. In this study, the highest serum antibody titer against ND vaccine was determined in HPE III group on both the 24th and 35th d. Therefore, it could be considered that high-level HPE may be important in obtaining a good immune response and maintaining it for a certain period for the prevention of viral diseases in poultry by vaccination. Also, it was reported that immunostimulating activity of polyphenolicfraction of H. perforatum with respect to the mononuclear phagocyte system, cellular and humoral immunity (Evstifeeva and Sibiriak, 1996).
There are some studies using SRBC as antigens to assess the humoral immune response in poultry (Yalçın et al., 2010; Davoodi et al., 2014). In this study, the HPE III group had the highest both IgM + IgY and IgY titers than other groups. The detection of a higher rate of antibodies against both the ND vaccine and SRBC antigens compared to the control and other groups may be related to the positive effect on the immunity when given to broilers at the appropriate level of HPE (Evstifeeva and Sibiriak, 1996; Landy et al., 2012). Regarding the m-HIT, it is also simple and inexpensive, it could be an alternative to expensive and complex techniques for measuring humoral immunity in chickens.
Cytokines are proteins or peptides secreted by cells that play a key role in immune and inflammatory responses through the activation and regulation of other cells and tissues. IL-1β (being to activate the immune system in an acute phase response and to determine activity viral and bacterial infections models in the chickens) and IL-6 (involved in acute-phase responses, immune regulation, and haematopoesis) are among the pro-inflammatory cytokines (Borish and Steinke, 2006; Commins et al., 2010). TNF-α is a primary regulator of both the immune response and inflammation produced by macrophages, T cells, and NK cells, and is synthesis to stimulated by tumor cells, lipopolysaccharides of bacteria, viruses, and parasites antigens, immune complex formation, complement system activation, and interferon-γ. IL-1 and IL-6 are the most investigated cytokines that can be expressed by monocytes and macrophages after invading pathogens are identified (Huang and Li, 2018; Edens et al., 2024). In this study, which continued a total of 42 d, because bacterial, viral, or parasitic infections were not observed throughout the period, no changes were detected in these pro-inflammatory cytokines. There are a wide variety of substances that affect TNF-α synthesis positively or negatively. In studies conducted in humans, it has been stated that some substances such as corticosteroids, vegetable oils, and fish oil suppress TNF synthesis (Caughey et al., 1996). However, avian cytokines have been poorly defined, both in terms of structure and function. Since H. perforatum contains many biochemical ingredients, some of these agents may stimulate TNF-α synthesis in broiler chickens. In present study, TNF-α in HPE III group was higher than others. Therefore, it would be useful to conduct detailed studies under controlled environmental conditions to better understand the effects of HPE on the synthesis of TNF-α and other cytokines in chickens. So, it could be thought that the bioactive substances (hypericin and hyperforin) found in St. John's wort may have potent anti-inflammatory effects to varying degrees in chickens by suppressing cytokine synthesis (Berköz et al., 2018). Long-term usage of HPE in Ross-308 broilers can stimulate nonspecific TNF-α production, relatively. This result may be also important for breeding broilers with longer life spans.
Conclusion
This study revealed that 4.5 mL / kg H. perforatum extract, which has a low production cost and is easy to extract and without causing environmental problems, varied significantly in their impact on growth performance, intestinal microflora, and immunity of growing broiler chickens. Furthermore, it increases total lactic acid bacteria count, and decrease total Enterococcus spp. counts.
Disclosures
The authors declare no conflicts of interest. They declare that they have no personal and/or financial relationships with any other corporation or individual that can inappropriately influence our work.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ziya Ilhan reports financial support was provided by Balikesir University. Ziya Ilhan reports a relationship with Balikesir University that includes:. Ziya Ilhan has patent pending to No. The authors declare no conflicts of interest. They declare that they have no personal and/or financial relationships with any other corporation or individual that can inappropriately influence our work. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by Balıkesir University, Balıkesir Scientific Research Projects Coordination Unit (Project No: 2021-135). The authors would like to also thank Prof. Dr. Abdulkadir Keskin for statistical analysis and Cevat Ekrem Postacı for his contributions.
References
- Allan W.H., Gough R.E. A standard hemagglutination inhibition test for Newcastle disease (1). A comparison of macro and micro methods. Vet. Rec. 1974;95:120–123. doi: 10.1136/vr.95.6.120. [DOI] [PubMed] [Google Scholar]
- Axarlis S., Mentis A., Demetzos C., Mitaku S., Skaltsounis A.L., Marselos A.L., Malamas M. Antiviral in vitro activity of Hypericum perforatum L. extract on the human cytomegalovirus (HCMV) Phytother. Res. 1998;12:507–511. [Google Scholar]
- Banisharif M., Kheiri F., Jalali S.M.A. Hypericum perforatum and probiotic effects on performance, carcass characteristics and intestinal morphology in Japanese quails (Coturnix japonica) J. Herb. Drugs. 2016;7:83–88. [Google Scholar]
- Behboodi H.R., Sedaghat A., Baradaran A., Nazarpak H.H. The effects of the mixture of betaine, vitamin C, St John's wort (Hypericum perforatum L.), lavender, and Melissa officinalis on performance and some physiological parameters in broiler chickens exposed to heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheshti Moghadam S., Ansari Z., Deldar H., Jafarpour S.A. Effects of different concentrations of St. John's Wort (Hypericum perforatum) extract on performance, blood parameters and physical and chemical meat quality of broiler chicks. Anim. Sci. J. 2015;28:229–240. [Google Scholar]
- Berköz M., Allahverdiyev O., Yıldırım M. Investigation of the effect of hyperforin and hypericin on inflammatory response in RAW 264.7 macrophages. Van Med. J. 2018;25:124–131. doi: 10.5505/vtd.2018.07769. [DOI] [Google Scholar]
- Borish L.C., Steinke J.W. 3. Cytokines and chemokines. J. Allergy Clin. Immunol. 2006;117:S441–S445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Barbosa J.G.M., Pereira R., Fagundes N.S., Rafael J.M., Menten J.F.M. Autolyzed yeast (Saccharomyces cerevisiae) supplementation improves performance while modulating the intestinal immune-system and microbiology of broiler chickens. Front. Sustain. Food Syst. 2018;2:85. doi: 10.3389/fsufs.2018.00085. [DOI] [Google Scholar]
- Caldeira G.I., Gouveia L.P., Serrano R., Silva O.D. Hypericum genus as a natural source for biologically active compounds. Plants. 2022;11:2509. doi: 10.3390/plants11192509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey G.E., Mantzioris E., Gibson R.A., Cleland L.G., James M.J. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- Chauveau A., Treyer A., Geirnaert A., Bircher L., Babst A., Abegg V.F., Simões-Wüst A.P., Lacroix C., Potterat O., Hamburger M. Intestinal permeability and gut microbiota interactions of pharmacologically active compounds in valerian and St. John's wort. Biomed. Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114652. [DOI] [PubMed] [Google Scholar]
- Chen J., Berry M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003;86:1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu Y., Tang Z., Shi X., Song Z., Cao F., Wei P., Li M., Li X., Jiang D., Yan Y., Yang N. Improvements in estrogen deficiency-induced hypercholesterolemia by Hypericum perforatum L. extract are associated with gut microbiota and related metabolites in ovariectomized (OVX) rats. Biomed. Pharmacother. 2021;125 doi: 10.1016/j.biopha.2020.111131. [DOI] [PubMed] [Google Scholar]
- Commins S.P., Borish L., Steinke J.W. Immunologic messenger molecules: cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010;125:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Coşkun Ö., Kanter M., Armutçu F., Çetin K., Kaybolmaz B., Yazgan Ö. Protective effects of quercetin, a flavonoid antioxidant, in absolute ethanol-induced acute gastric ulcer. Eur. J. Gen. Med. 2004;1:37–42. [Google Scholar]
- Davoodi S.M., Kheiri F., Rahimian Y. Effect of poultry feed supplemented with Hypericum perforatum extract and virginiamycine on growth performance, some immune responses and intestinal microbial population of broilers. Russ. J. Agric. Socio-Econ. Sci. 2014;36:27–33. [Google Scholar]
- Delhanty J.J., Solomon J.B. The nature of antibodies to goat erythrocytes in the developing chicken. Immunology. 1966;11:103–113. [PMC free article] [PubMed] [Google Scholar]
- Edens F.W., Siegel P.B., Beckstead R.B., Honaker C.F., Hodgson D. Tissue cytokines in chickens from lines selected for high or low humoral antibody responses, given supplemental Limosilactobacillus reuteri and challenged with Histomonas meleagridis. Front. Physiol. 2024;14 doi: 10.3389/fphys.2023.1294560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroğlu E., Girgin S.N. A unique phenolic extraction method from olive oil macerate of Hypericum perforatum using DMSO: Assessment of in vitro anticancer activity, LC-MS/MS profile, total phenolic content and antioxidant capacity. S. Afr. J. Bot. 2021;139:6–11. doi: 10.1016/j.sajb.2021.01.015. [DOI] [Google Scholar]
- Evstifeeva T.A., Sibiriak S.V. The immunotropic properties of biologically active products obtained from Klamath weed (Hypericum perforatum L.) Eksp. Klin. Farmakol. 1996;59:51–54. [PubMed] [Google Scholar]
- Ge C., Luo X., Wu L., Lv Y., Hu Z., Yu D., Liu B. Plant essential oils improve growth performance by increasing antioxidative capacity, enhancing intestinal barrier function, and modulating gut microbiota in Muscovy ducks. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy S.M., Adachi Y. Comparison of the effects of dietary selenium, zinc, and selenium and zinc supplementation on growth and immune response between chick groups that were inoculated with Salmonella and aflatoxin or Salmonella. Poult. Sci. 2000;79:331–335. doi: 10.1093/ps/79.3.331. [DOI] [PubMed] [Google Scholar]
- Hernandez F., Madrid J., Garcia V., Orengo J., Megias M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Qiu H., Sun P., Zhu L., Chen F., Qin S. Selenium-enriched Saccharomyces cerevisiae improves the meat quality of broiler chickens via activation of the glutathione and thioredoxin systems. Poult. Sci. 2020;99:6045–6054. doi: 10.1016/j.psj.2020.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G., Huang T. Essential oils as promising treatments for treating Candida albicans infections: Research progress, mechanisms, and clinical applications. Front. Pharmacol. 2024;15 doi: 10.3389/fphar.2024.1400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristakieva P., Oblakova M., Ivanova I., Mincheva N., Penchev I., Ivanov N., Lalev M. Growth performance, carcass characteristics and meat quality of broilers fed diets supplemented with some dry herbs. Bulg. J. Agric. Sci. 2023;29:102–109. [Google Scholar]
- Huang C.M., Lee T.T. Immunomodulatory effects of phytogenics in chickens and pigs - a review. Asian-Australas J. Anim. Sci. 2018;31:617–627. doi: 10.5713/ajas.17.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Park O.J., Kim A.R., Ahn K.B., Lee D., Kum K.Y., Yun C.H., Han S.H. Lipoteichoic acids of lactobacilli inhibit Enterococcus faecalis biofilm formation and disrupt the preformed biofilm. J. Microbiol. 2019;57:310–315. doi: 10.1007/s12275-019-8538-4. [DOI] [PubMed] [Google Scholar]
- Isacchi B., Bergonzi M.C., Carnevali F., Van der Esch S.A., Vincieri F.F., Bilia A.R. Analysis and stability of the constituents of St. John’s wort oils prepared with different methods. J. Pharm. Biomed. Anal. 2007;45:756–761. doi: 10.1016/j.jpba.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Das P., Samanta I., Roy B., Mukherjee A., Mondal T. Effect of Selenium-Yeast supplementation on gut microbiome, intestinal histomorphology and immunity in broilers. Microbe. 2024;3 doi: 10.1016/j.microb.2024.100071. [DOI] [Google Scholar]
- Güner, A., Asla S., Ekim T., Vural M., Babaç, M.T., 2012. List of Turkish flora (Vascular plants) publication of Nezahat Gökyiğit Botanical Garden and Flora Research Foundation, Istanbul. (Accessed: 06 September 2023). http//www.bizimbitkiler.org.tr/v2/hiyerarsi.php?c=Hypericum.
- ISO. 1998. Microbiology of food and animal feedingstuffs-Horizontal method for the enumeration of mesophilic lactic acid bacteria – Colony count technique at 30 degrees C. ISO 15214:1998. International Organization for Standardization, Geneva, Switzerland.
- ISO. 2008. Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of yeasts and moulds. Part 1: Colony count technique in products with water activity greater than 0.95. Reference number: ISO 21527-1:2008. https://www.iso.org/standard/38275.html.
- ISO. 2017. Microbiology of the food chain. Horizontal method for the detection and enumeration of Enterobacteriaceae. Part 2: colony-count technique. Reference number: 21528-2:2017. https://www.iso.org/standard/63504.html.
- Kıyan S., Uyanıkgil Y., Altuncı Y.A., Çavuşoğlu T., Çetin Uyanıkgil E.Ö., Karabey F. Investigation of acute effects of Hypericum perforatum (St. John's Wort) treatment in experimental thermal burns and comparison with silver sulfadiazine treatment. Ulus. Travma Acil. Cerrahi. Derg. 2015;21:323–336. doi: 10.5505/tjtes.2015.. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. Role of diet-microbiota interactions in precision nutrition of the chicken: Facts, gaps, and new concepts. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Fernandez-Miyakawa M.E. Editorial: Functional mechanisms at the avian gut microbiomeintestinal immunity interface and its regulation of avian physiological responses. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.1063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecień I., Miceli N., Kedzia E., Cavò E., Taviano M.F., Beerhues L., Ekiert H. Different types of Hypericum perforatum cvs. (elixir, helos, topas) ın vitro cultures: A rich source of bioactive metabolites and biological activities of biomass extracts. Molecules. 2023;28:2376. doi: 10.3390/molecules28052376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy N., Ghalamkari G.T., Toghyani M. Evaluation of St John's Wort (Hypericum perforatum L.) as an antibiotic growth promoter substitution on performance, carcass characteristics, some of the immune responses, and serum biochemical parameters of broiler chicks. J. Med. Plants Res. 2012;6:510–515. [Google Scholar]
- Liu M., Chen R., Wang T., Ding Y., Zhang Y., Huang G., Guo S. Dietary Chinese herbal mixture supplementation improves production performance by regulating reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal R.S., Saha S., Das S. Metagenomic surveys of gut microbiota. Genom. Proteom. Bioinform. 2015;13:148–158. doi: 10.1016/j.gpb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari S., Esmaily H., Rahimi R., Baeeri M., Sanai Y., Asadi-Shahmirzadi A., Salehi-Surmaghi M.S., Abdollahi M. Effects of Hypericum perforatum extract on rat irritable bowel syndrome. Pharmacogn. Mag. 2011;7:213–223. doi: 10.4103/0973-1296.84235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. 9th rev. ed. [Google Scholar]
- Perdigón G., Maldonado Galdeano C., Valdez J., Medici M. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 2002;56(Suppl. 4) doi: 10.1038/sj.ejcn.1601658. [DOI] [PubMed] [Google Scholar]
- Pourmorad F., Hosseinimehr S.J., Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006;5:1142–1145. [Google Scholar]
- Phillips C.J.C., Hosseintabar-Ghasemabad B., Gorlov I.F., Slozhenkina M.I., Mosolov A.A., Seidavi A. Immunomodulatory effects of natural feed additives for meat chickens. Life. 2023;13:1287. doi: 10.3390/life13061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti P.C., Dal Bosco A., Hilbert F., Pia Franciosini M., Castellini C. Evaluation of intestinal bacterial flora of conventional and organic broilers using culture-based methods. Ital. J. Anim. Sci. 2009;8:51–63. doi: 10.4081/ijas.2009.51. [DOI] [Google Scholar]
- Rao S.V.R., Prakash B., Raju M.V., Panda A.K., Poonam S., Murthy O.K. Effect of supplementing organic selenium on performance, carcass traits, oxidative parameters and immune responses in commercial broiler chickens. Asian J. Plant Sci. Res. 2013;26:247–252. doi: 10.5713/ajas.2012.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid Z., Mirani Z.A., Zehra S., Gilani S.M.H., Ashraf A., Azhar A., Al-Ghanim K.A., Al-Misned F., Al-Mulahi N., Mahboobi S., Galani S. Enhanced modulation of gut microbial dynamics affecting body weight in birds triggered by natural growth promoters administered in conventional feed. Saudi J. Biol. Sci. 2020;27:2747–2755. doi: 10.1016/j.sjbs.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraji H., Hosseini-Vashan H., Afzali N., Namayi M.H., Allahressan A. Effect of water extract of Hypericum perforatum on performance, abdominal fat and blood biochemical parameters of broiler chicken. Anim. Sci. J. 2015;28:133–146. [Google Scholar]
- Shang R., He C., Chen J., Pu X., Liu Y., Hua L., Wang L., Liang J. Hypericum perforatum extract therapy for chickens experimentally infected with infectious bursal disease virus and its influence on immunity. Can. J. Vet. Res. 2012;76:180–185. [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Gupta G., Ahmad T., Kaur B., Hakeem K.R. Tailoring cellular metabolism in lactic acid bacteria through metabolic engineering. J. Microbiol. Methods. 2020;170 doi: 10.1016/j.mimet.2020.105862. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. doi: 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- Sofi S.H., Nuraddin S.M., Amin Z.A., Al-Bustany H.A., Nadir M.Q. Gastroprotective activity of Hypericum perforatum extract in ethanol-induced gastric mucosal injury in Wistar rats: A possible involvement of Hþ/Kþ ATPase α inhibition. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur A., Zengin M., Bacaksız O.K., Gökçe Z., Yılmaz Ö., Gürses M., Esen V.K., Azman M.A., Esen S. Performance, blood biochemistry, carcass fatty acids, antioxidant status, and HSP70 gene expressions in Japanese quails reared under high stocking density: The effects of grape seed powder and meal. Trop. Anim. Health Prod. 2023:28. doi: 10.1007/s11250-023-03481-y. [DOI] [PubMed] [Google Scholar]
- Yalçın S., Erol H., Özsoy B., Onbaşılar İ., Yalçın S., Üner A. Effects of glycerol on performance, egg traits, some blood parameters and antibody production to SRBC of laying hens. Livest. Sci. 2010;129:129–134. doi: 10.1016/j.livsci.2010.01.014. [DOI] [Google Scholar]
- Zengin M., Sur A., İlhan Z., Azman M.A., Tavşanlı H., Esen S., Bacaksız O.K., Demir E. Effects of fermented distillers grains with solubles, partially replaced with soybean meal, on performance, blood parameters, meat quality, intestinal flora, and immune response in broiler. Res. Vet. Sci. 2022;150:58–64. doi: 10.1016/j.rvsc.2022.06.027. [DOI] [PubMed] [Google Scholar]