Abstract
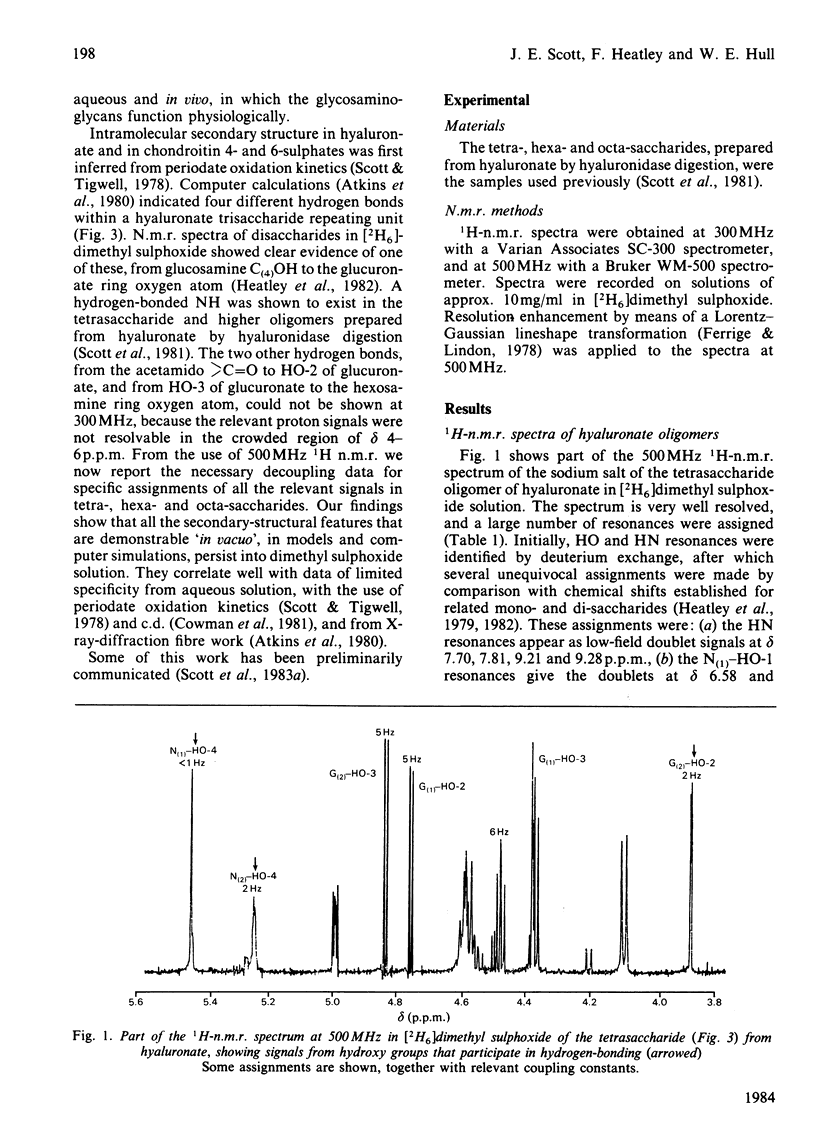
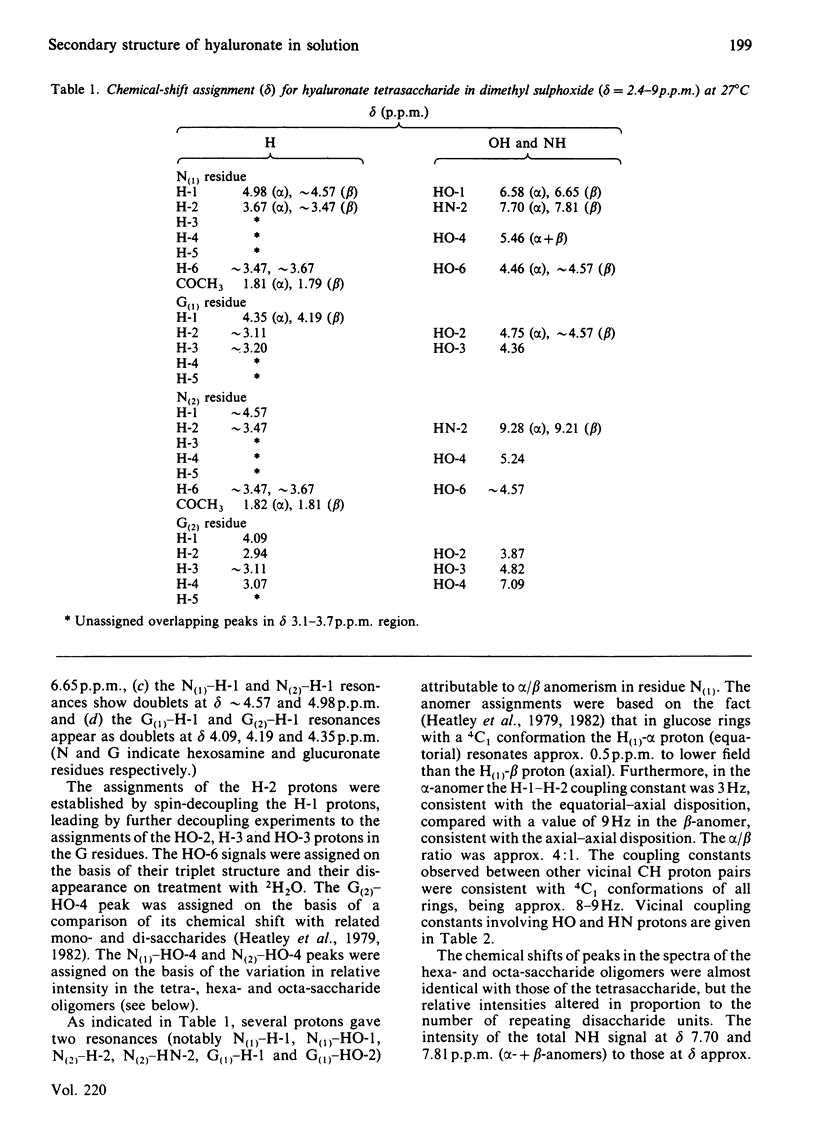
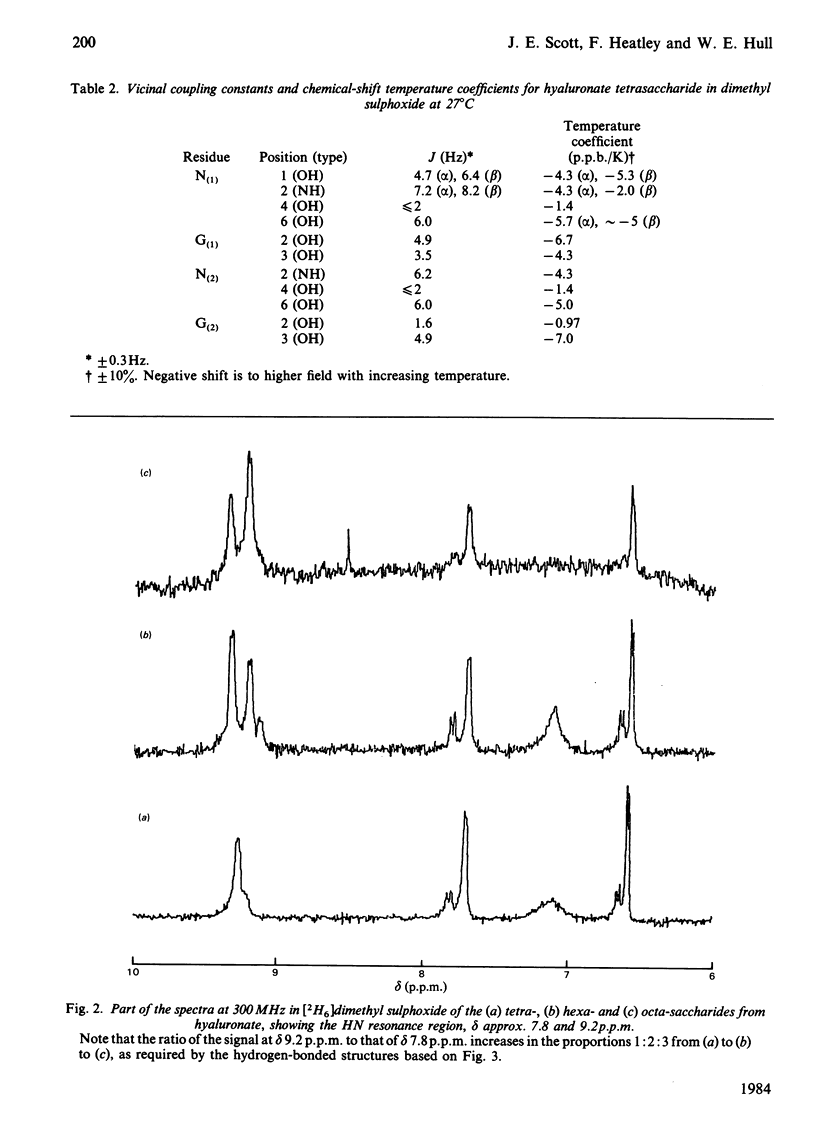
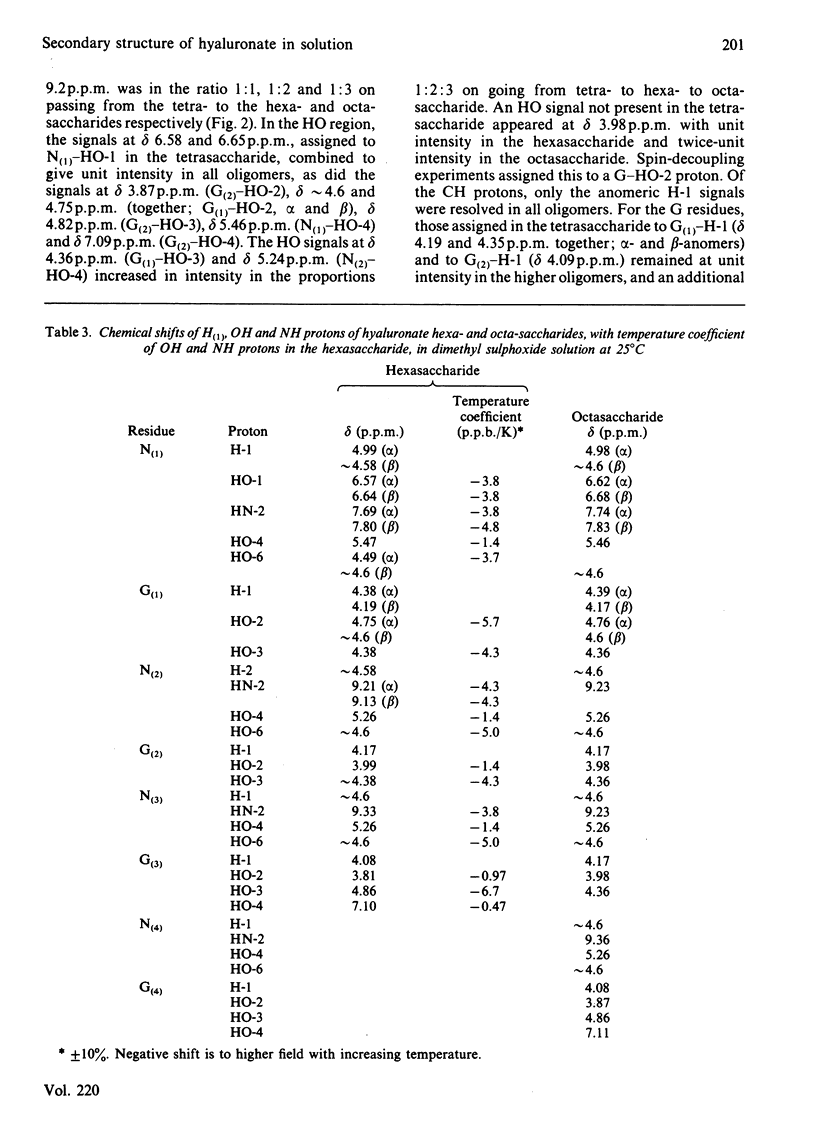
The 1H-n.m.r. spectra of solutions in [2H6]dimethyl sulphoxide of the sodium salts of tetra-, hexa- and octa-saccharides prepared from hyaluronate by testicular-hyaluronidase digestion were examined at 300 and 500 MHz. The signals from hydroxy groups at positions 2 and 3 in the glucuronic acid moiety were assigned. Their chemical shifts and associated temperature-dependencies, as well as their coupling constants, depended on whether or not the uronic acid was at the non-reducing end. Deviations from the 'normal' pattern of hydroxy-group proton n.m.r. behaviour were attributable to participation in hydrogen bonds, either to the acetamido carbonyl oxygen atom or the pyranose ring oxygen atom of neighbouring N-acetylhexosamine moieties. A secondary structure, containing four different hydrogen bonds per trisaccharide unit of glucuronsyl-hexosaminyl-glucuronic acid, was demonstrated. This is the first complete and detailed secondary structure to be established for hyaluronate in any solvent. Hyaluronate is compared with chondroitin sulphate, dermatan sulphate, heparan sulphate and keratan sulphate in their potential to form secondary structures with features in common. The significance of the details of the structure to its overall stability, and the probability of their persistence into aqueous environments, are discussed. The presence of all or most of the secondary structure in glycosaminoglycuronans is correlated with a space-filling function in the tissue, and with a high carbohydrate content in the parent proteoglycan in the case of the chondroitin sulphates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowman M. K., Balazs E. A., Bergmann C. W., Meyer K. Preparation and circular dichroism analysis of sodium hyaluronate oligosaccharides and chondroitin. Biochemistry. 1981 Mar 3;20(5):1379–1385. doi: 10.1021/bi00508a053. [DOI] [PubMed] [Google Scholar]
- Heatley F., Scott J. E., Jeanloz R. W., Walker-Nasir E. Secondary structure in glycosaminoglycuronans: N.M.R. spectra in dimethyl sulphoxide of disaccharides related to hyaluronic acid and chondroitin sulphate. Carbohydr Res. 1982 Jan 1;99(1):1–11. doi: 10.1016/s0008-6215(00)80969-6. [DOI] [PubMed] [Google Scholar]
- Schamper T. J. P.m.r. spectra and conformation of the pyranose amino sugars, 2-acetamido-2-deoxy-alpha-D-glucopyranose, 2-acetamido-2-deoxy-alpha-D-galactopyranose, and 2-acetamido-2-deoxy-beta-D-mannopyranose. Carbohydr Res. 1974 Aug;36(1):233–237. doi: 10.1016/s0008-6215(00)82016-9. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Heatley F. Detection of secondary structure in glycosaminoglycans via the H n.m.r. signal of the acetamido NH group. Biochem J. 1982 Oct 1;207(1):139–144. doi: 10.1042/bj2070139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Jones M. N., Wilkinson A., Olavesen A. H. Secondary structure of chondroitin sulphate in dimethyl sulphoxide. Eur J Biochem. 1983 Feb 15;130(3):491–495. doi: 10.1111/j.1432-1033.1983.tb07177.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Heatley F., Moorcroft D., Olavesen A. H. Secondary structures of hyaluronate and chondroitin sulphates. A 1H n.m.r. study of NH signals in dimethyl sulphoxide solution. Biochem J. 1981 Dec 1;199(3):829–832. doi: 10.1042/bj1990829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Tigwell M. J. Periodate oxidation and the shapes of glycosaminoglycuronans in solution. Biochem J. 1978 Jul 1;173(1):103–114. doi: 10.1042/bj1730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti D., Rees D. A., Welsh E. J. Solution conformation of glycosaminoglycans: assignment of the 300-MHz 1H-magnetic resonance spectra of chondroitin 4-sulphate, chondroitin 6-sulphate and hyaluronate, and investigation of an alkali-induced conformation change. Eur J Biochem. 1979 Mar;94(2):505–514. doi: 10.1111/j.1432-1033.1979.tb12919.x. [DOI] [PubMed] [Google Scholar]


