Abstract
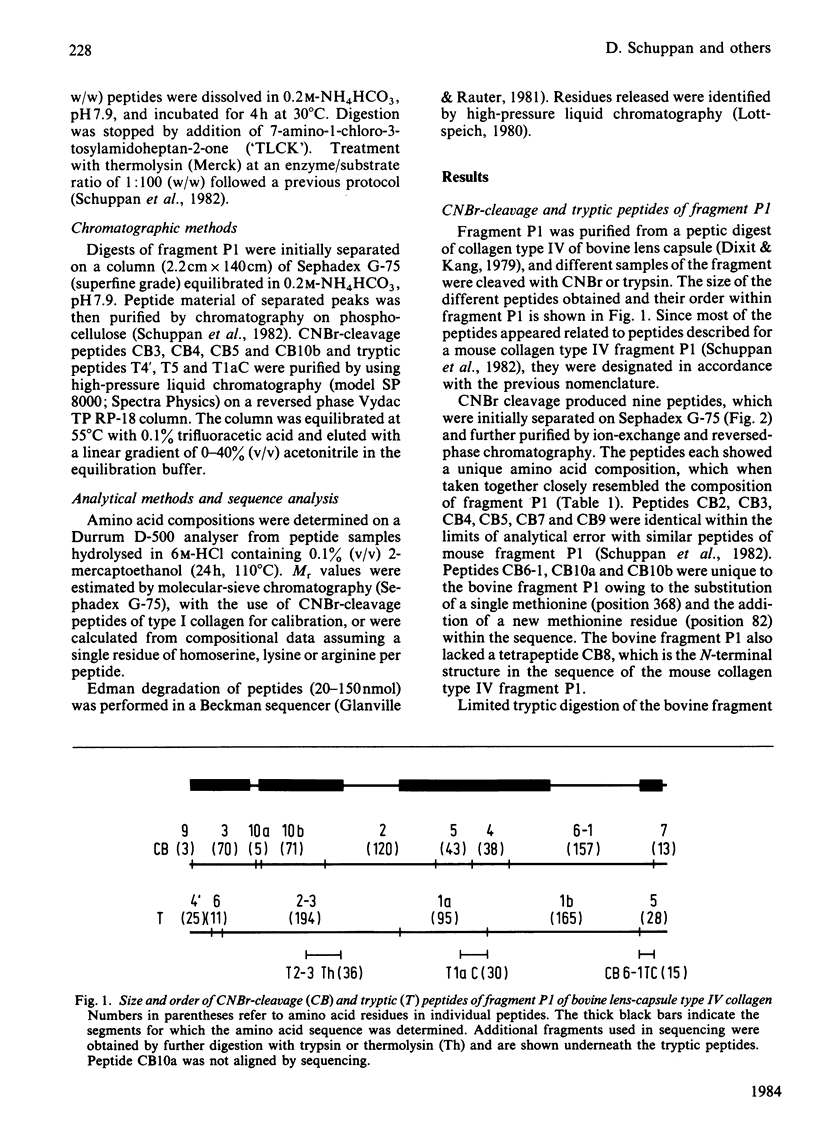
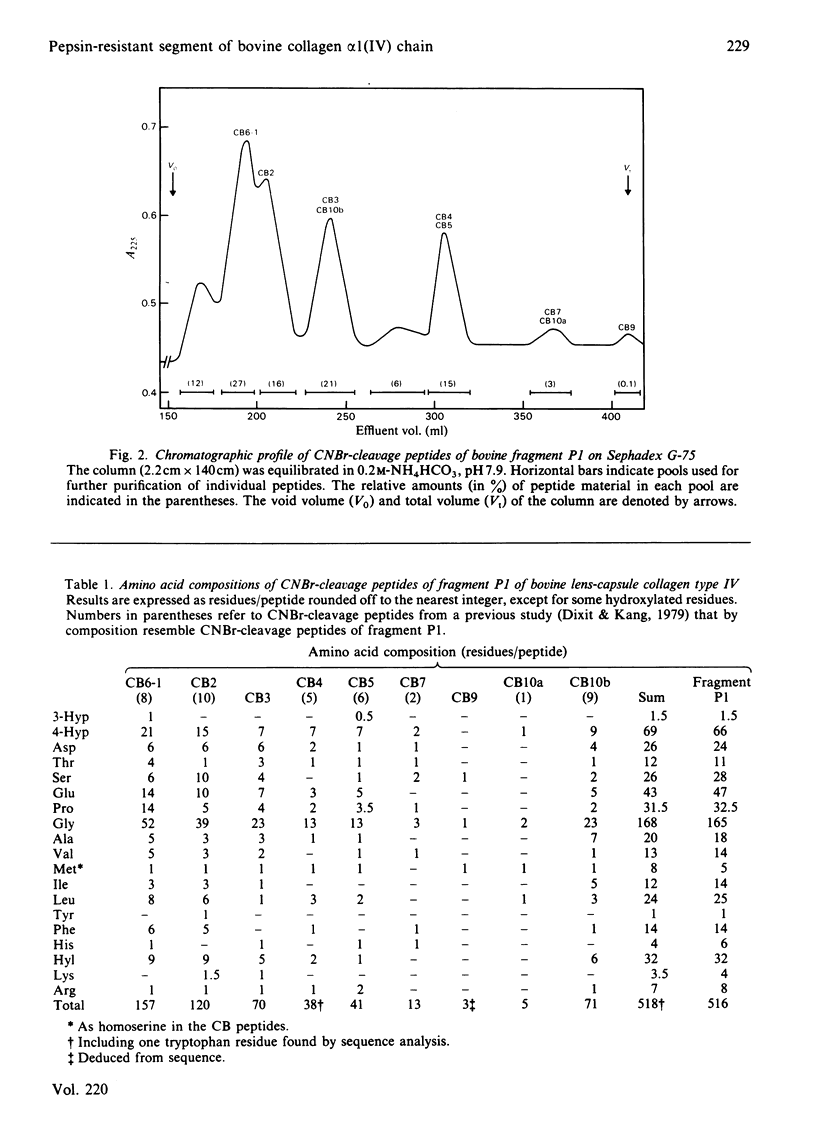
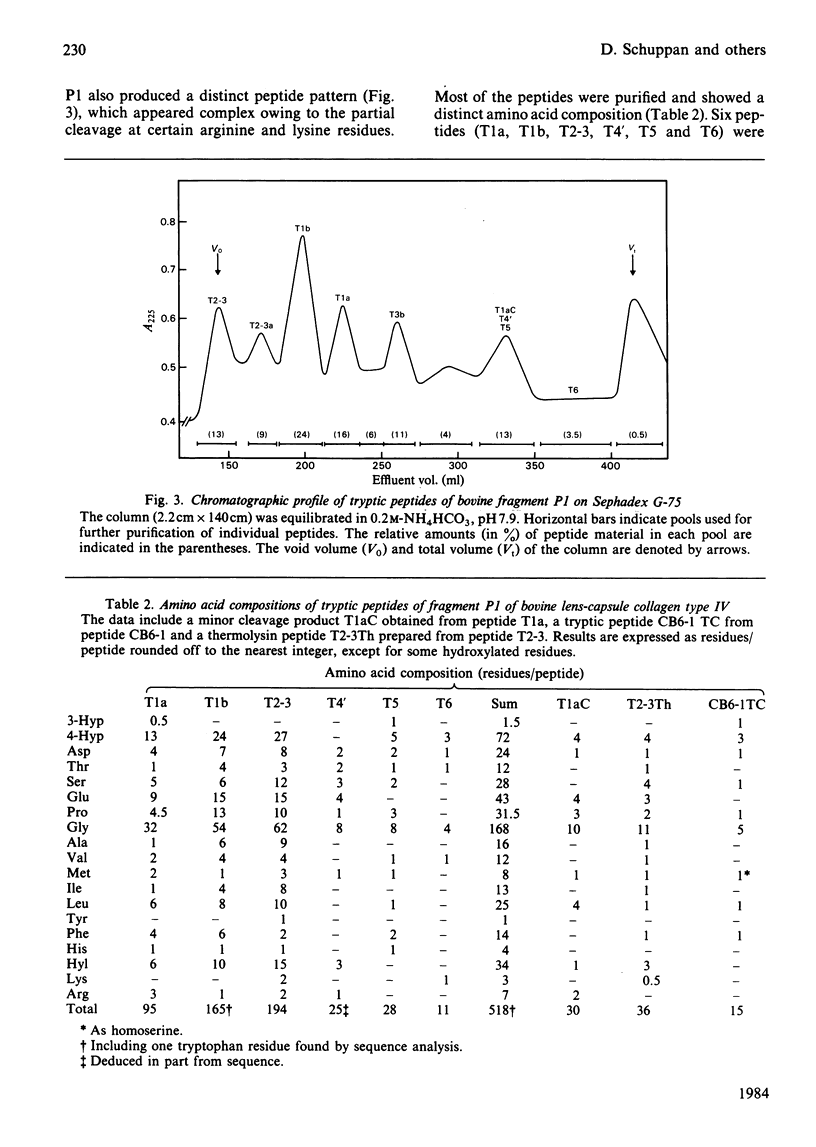
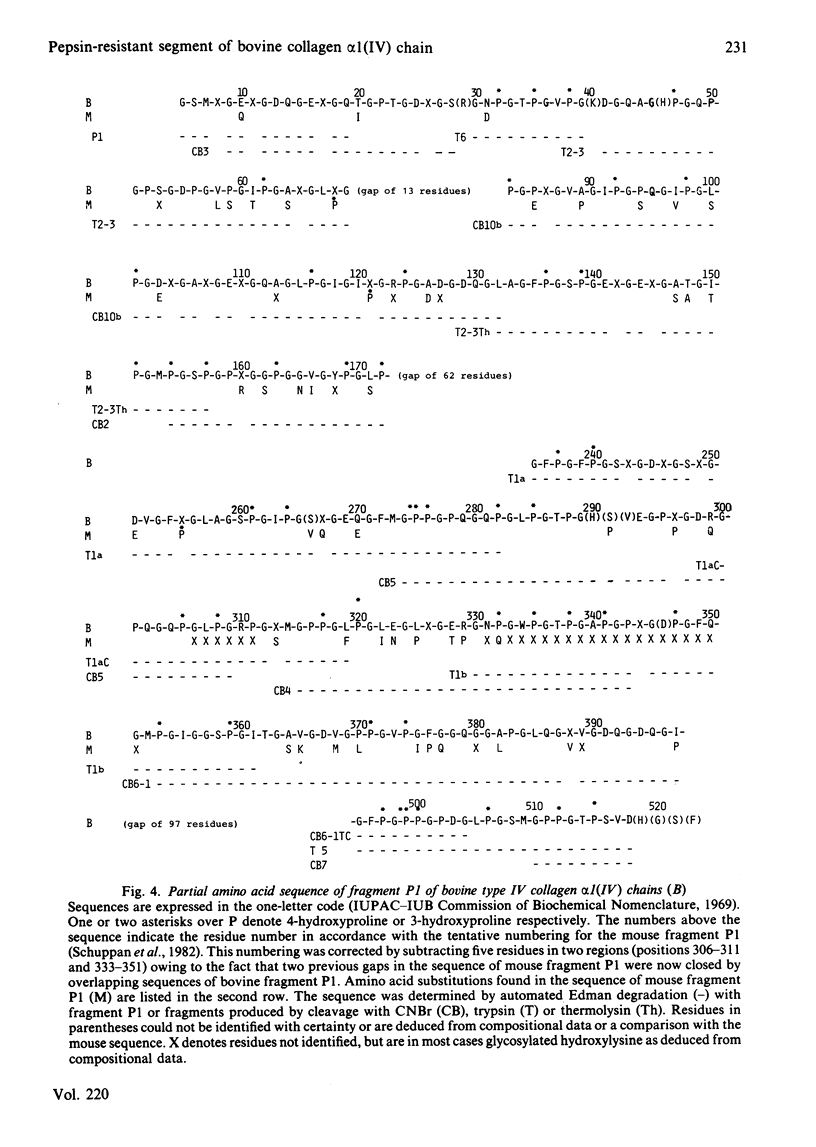
The C-terminal peptic fragment P1 (about 518 amino acid residues) of bovine lens-capsule collagen alpha 1(IV) chain was cleaved with CNBr and trypsin. The peptides were purified and characterized, allowing their ordering within the P1 fragment by comparison with a corresponding section of mouse collagen alpha 1(IV) chain [Schuppan, Glanville & Timpl (1982) Eur. J. Biochem. 123, 505-512]. About 67% of the sequence of bovine collagen fragment P1 was determined by Edman degradation. Comparison with the sequence of the corresponding mouse collagen fragment P1 showed 76% identity for positions Xaa and Yaa of the triplet structures Gly-Xaa-Yaa. Invariance was found for the positions of two non-triplet interruptions and of 3-hydroxyproline residues, pointing to the functional importance of these structures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dehm P., Kefalides N. A. The collagenous component of lens basement membrane. The isolation and characterization of an alpha chain size collagenous peptide and its relationship to newly synthesized lens components. J Biol Chem. 1978 Oct 10;253(19):6680–6686. [PubMed] [Google Scholar]
- Dixit S. N. Isolation and characterization of two collagenous components from anterior lens capsule. FEBS Lett. 1978 Jan 1;85(1):153–157. doi: 10.1016/0014-5793(78)81269-1. [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Kang A. H. Anterior lens capsule collagens: cyanogen bromide peptides of the C chain. Biochemistry. 1979 Dec 11;18(25):5686–5692. doi: 10.1021/bi00592a026. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Gay S., Miller E. J. Characterization of lens capsule collagen: evidence for the presence of two unique chains in molecules derived from major basement membrane structures. Arch Biochem Biophys. 1979 Dec;198(2):370–378. doi: 10.1016/0003-9861(79)90509-5. [DOI] [PubMed] [Google Scholar]
- Glanville R. W., Rauter A. Pepsin fragments of human placental basement-membrane collagens showing interrupted triple-helical amino acid sequences. Hoppe Seylers Z Physiol Chem. 1981 Jul;362(7):943–951. doi: 10.1515/bchm2.1981.362.2.943. [DOI] [PubMed] [Google Scholar]
- Hofmann H., Fietzek P. P., Kühn K. The role of polar and hydrophobic interactions for the molecular packing of type I collagen: a three-dimensional evaluation of the amino acid sequence. J Mol Biol. 1978 Oct 25;125(2):137–165. doi: 10.1016/0022-2836(78)90342-x. [DOI] [PubMed] [Google Scholar]
- Hofmann H., Voss T., Kühn K., Engel J. Localization of flexible sites in thread-like molecules from electron micrographs. Comparison of interstitial, basement membrane and intima collagens. J Mol Biol. 1984 Jan 25;172(3):325–343. doi: 10.1016/s0022-2836(84)80029-7. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A., Parry D. A., Piez K. A., Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol. 1973 Sep 5;79(1):137–148. doi: 10.1016/0022-2836(73)90275-1. [DOI] [PubMed] [Google Scholar]
- Kühn K., Wiedemann H., Timpl R., Risteli J., Dieringer H., Voss T., Glanville R. W. Macromolecular structure of basement membrane collagens. FEBS Lett. 1981 Mar 9;125(1):123–128. doi: 10.1016/0014-5793(81)81012-5. [DOI] [PubMed] [Google Scholar]
- Lottspeich F. Identification of the phenylthiohydantoin derivatives of amino acids by high pressure liquid chromatography, using a ternary, isocratic solvent system. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1829–1834. doi: 10.1515/bchm2.1980.361.2.1829. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Glanville R. W., Timpl R. Covalent structure of mouse type-IV collagen. Isolation, order and partial amino-acid sequence of cyanogen-bromide and tryptic peptides of pepsin fragment P1 from the alpha 1(IV) chain. Eur J Biochem. 1982 Apr;123(3):505–512. [PubMed] [Google Scholar]
- Schuppan D., Timpl R., Glanville R. W. Discontinuities in the triple helical sequence Gly-X-Y of basement membrane (type IV) collagen. FEBS Lett. 1980 Jun 30;115(2):297–300. doi: 10.1016/0014-5793(80)81191-4. [DOI] [PubMed] [Google Scholar]
- Timpl R., Bruckner P., Fietzek P. Characterization of pepsin fragments of basement membrane collagen obtained from a mouse tumor. Eur J Biochem. 1979 Apr 2;95(2):255–263. doi: 10.1111/j.1432-1033.1979.tb12961.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]


