Abstract
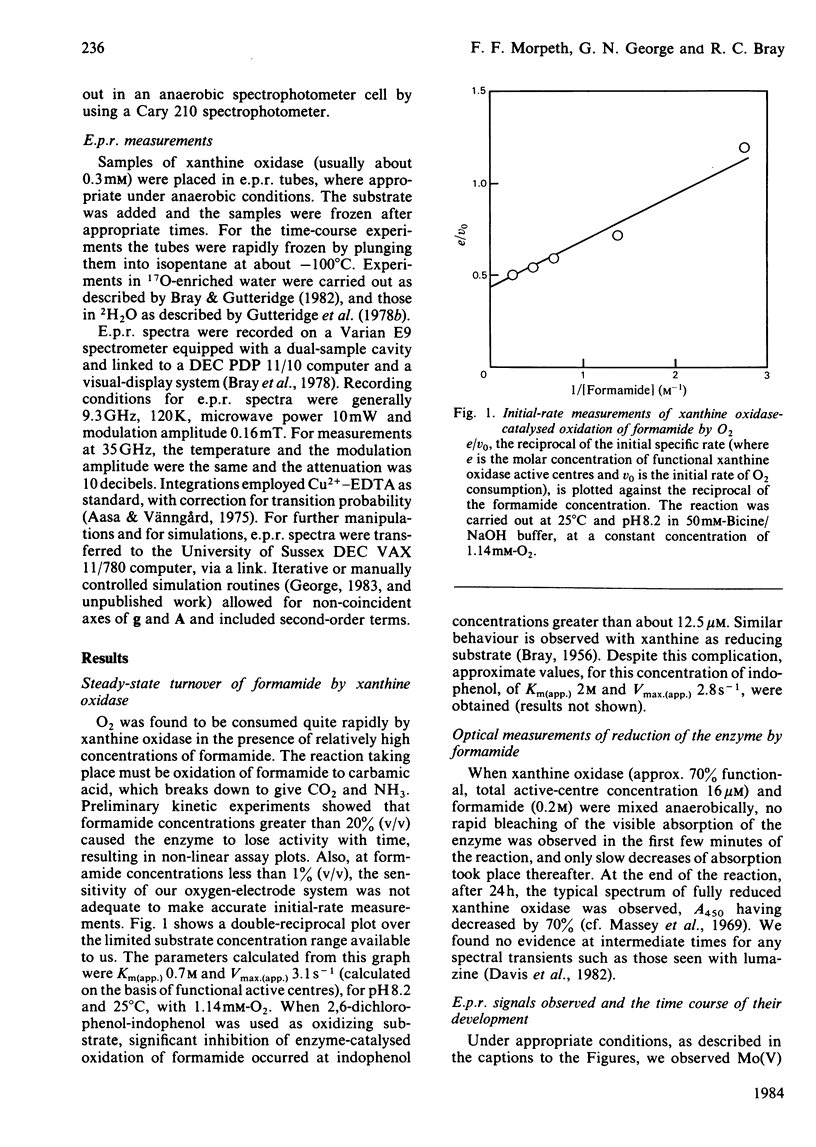
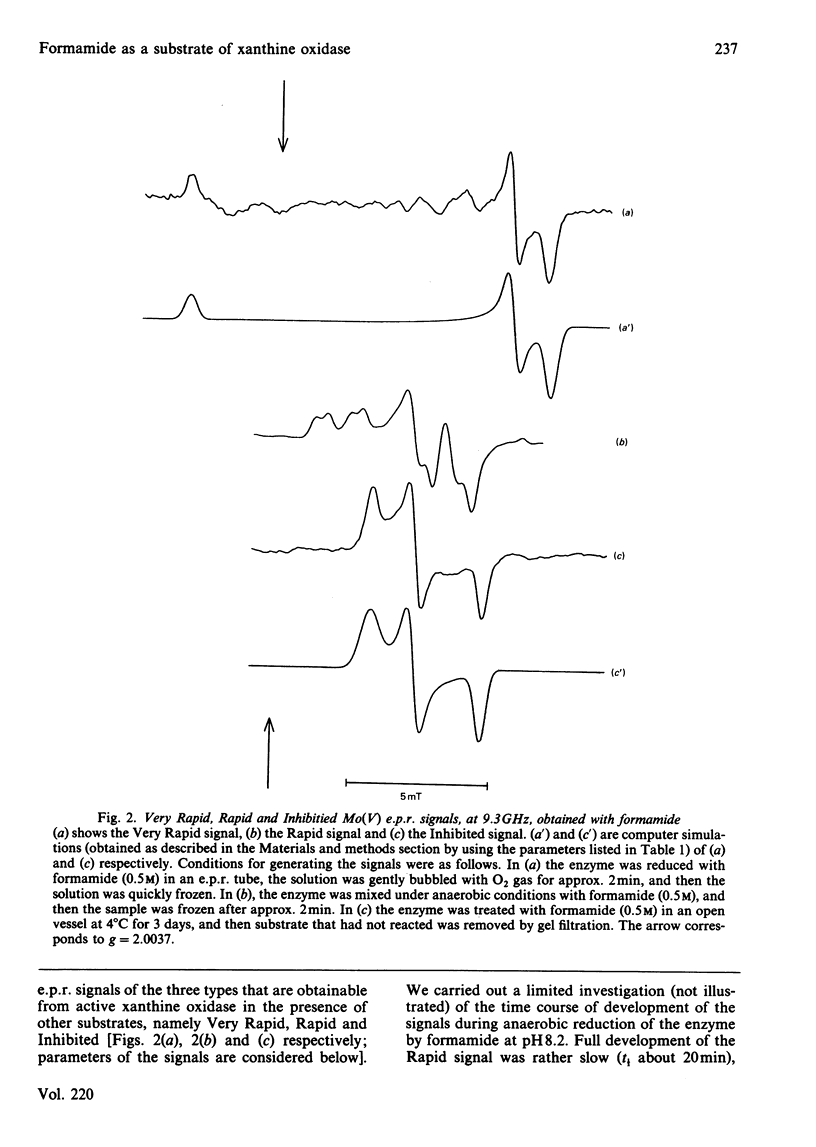
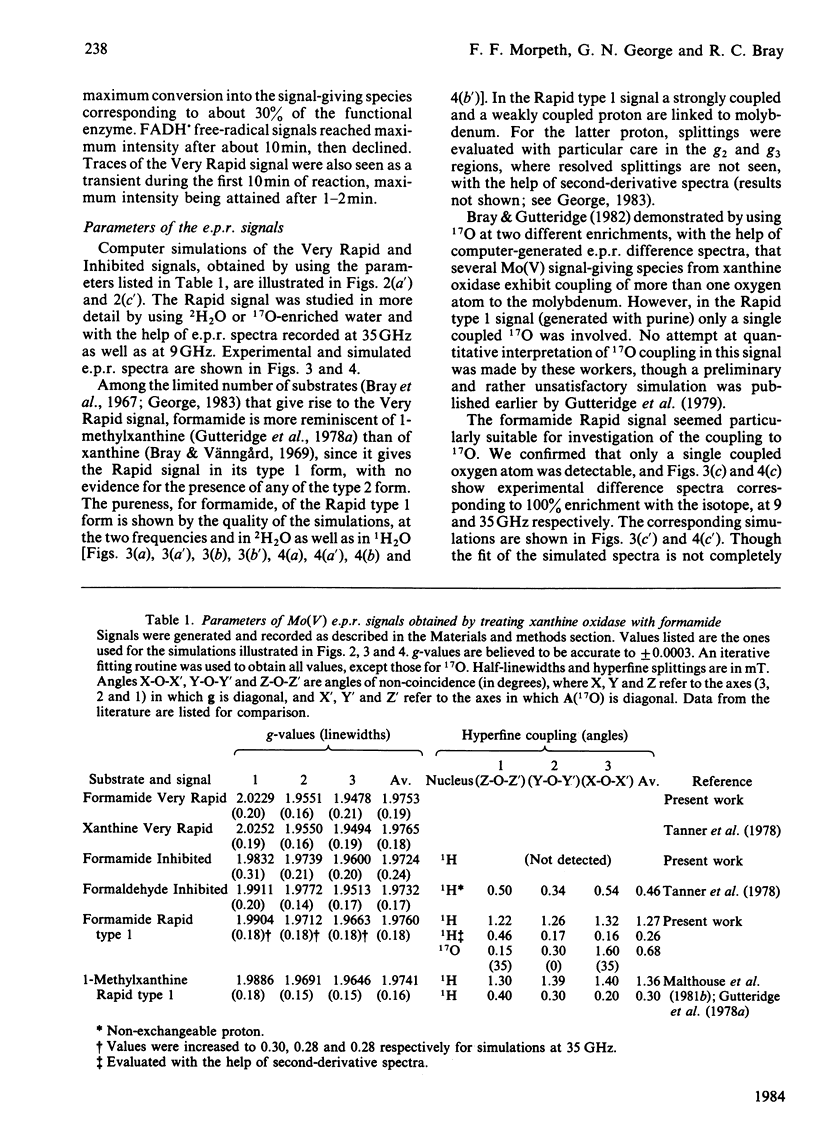
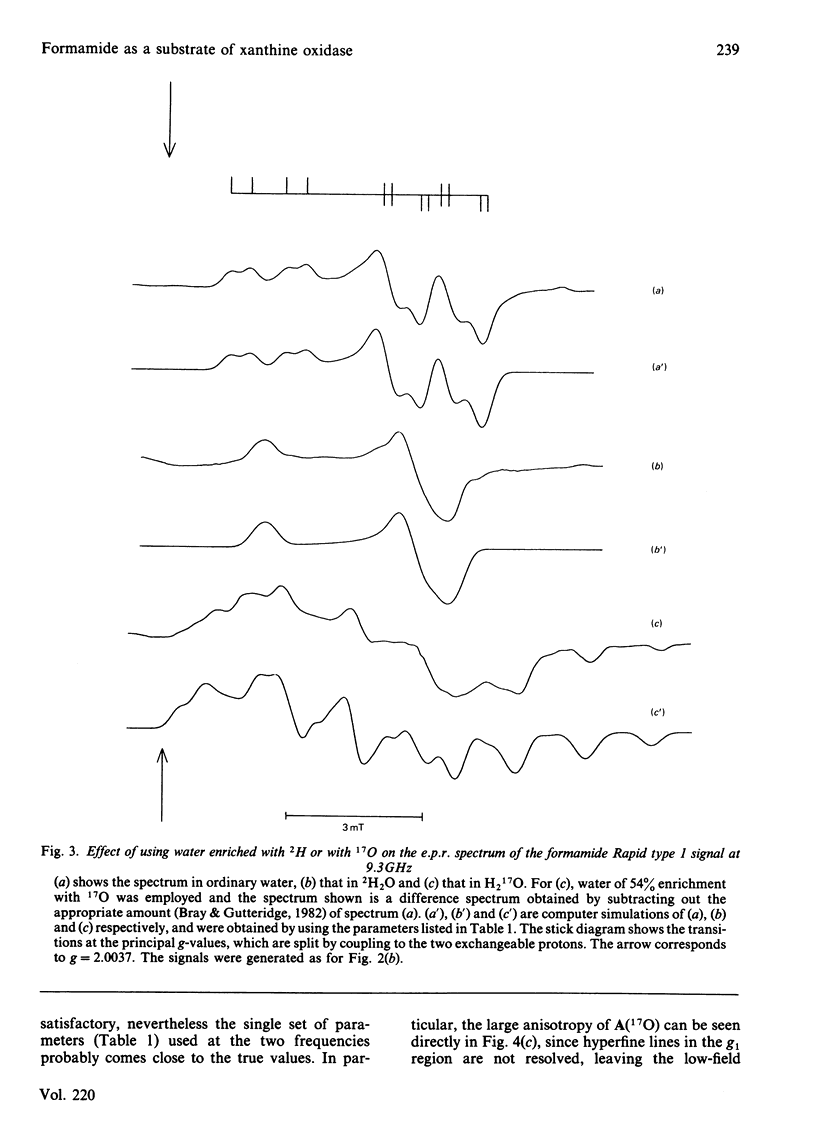
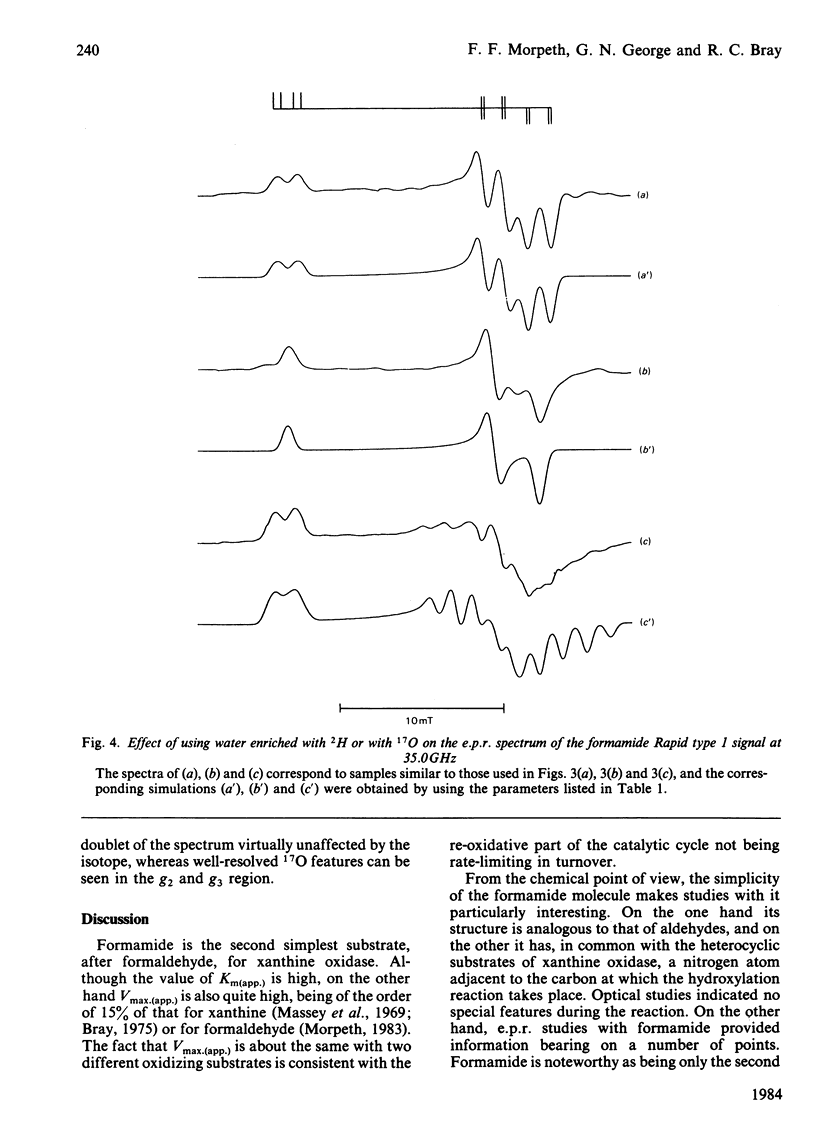
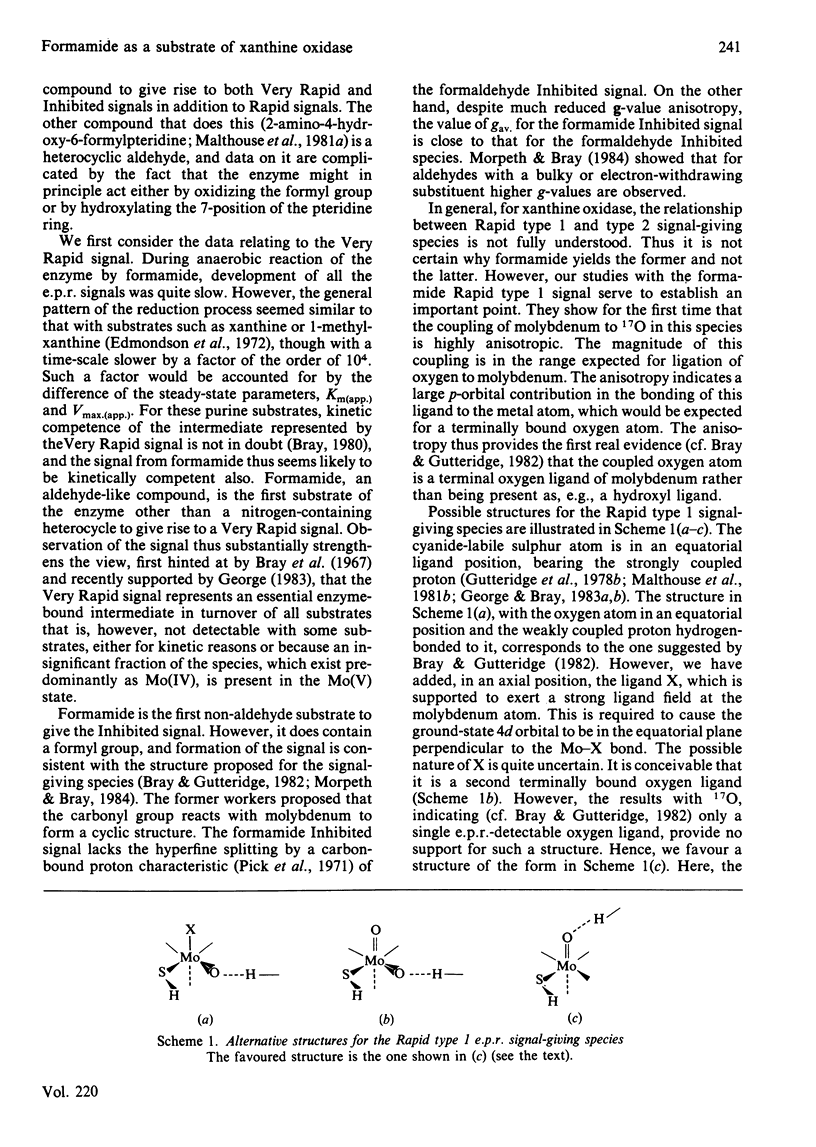
Formamide is a substrate of xanthine oxidase. At pH 8.2 and 1.14 mM-O2, Vmax.(app.) is 3.1 s-1 and Km (app.) is 0.7 M. Mo(V) e.p.r. signals obtained by treating the enzyme with formamide were studied, and these provide new information about the ligation of molybdenum in the enzyme and about the enzymic mechanism. The substrate is the first compound that is not a nitrogen-containing heterocycle to give a Very Rapid signal. This supports the hypothesis that the Very Rapid signal, though it is not detectable with all substrates, represents an essential intermediate in turnover. Formamide also gives the Inhibited signal and is the first non-aldehyde substrate to do so. The Rapid type 1 signal obtained in the presence of formamide was examined in H2O enriched with 2H or with 17O. The single oxygen atom detectable in the signal is shown to be strongly and anisotropically coupled. This indicates that this atom remains as an oxo ligand of molybdenum in this signal-giving species. Other structural features of this species are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth V. H. The specificity of xanthine oxidase. Biochem J. 1938 Mar;32(3):494–502. doi: 10.1042/bj0320494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Barber M. J., Lowe D. J. Electron-paramagnetic-resonance spectroscopy of complexes of xanthine oxidase with xanthine and uric acid. Biochem J. 1978 Jun 1;171(3):653–658. doi: 10.1042/bj1710653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Gutteridge S. Numbers and exchangeability with water of oxygen-17 atoms coupled to molybdenum (V) in different reduced forms of xanthine oxidase. Biochemistry. 1982 Nov 9;21(23):5992–5999. doi: 10.1021/bi00266a041. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Gutteridge S., Stotter D. A., Tanner S. J. The mechanism of action of xanthine oxidase. The relationship between the rapid and very rapid molybdenum electron-paramagnetic-resonance signals. Biochem J. 1979 Jan 1;177(1):357–360. doi: 10.1042/bj1770357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Vänngård T. "Rapidly appearing" molybdenum electron-paramagnetic-resonance signals from reduced xanthine oxidase. Biochem J. 1969 Oct;114(4):725–734. doi: 10.1042/bj1140725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. D., Olson J. S., Palmer G. Charge transfer complexes between pteridine substrates and the active center molybdenum of xanthine oxidase. J Biol Chem. 1982 Dec 25;257(24):14730–14737. [PubMed] [Google Scholar]
- Edmondson D., Ballou D., Van Heuvelen A., Palmer G., Massey V. Kinetic studies on the substrate reduction of xanthine oxidase. J Biol Chem. 1973 Sep 10;248(17):6135–6144. [PubMed] [Google Scholar]
- George G. N., Bray R. C. Reaction of arsenite ions with the molybdenum center of milk xanthine oxidase. Biochemistry. 1983 Mar 1;22(5):1013–1021. doi: 10.1021/bi00274a003. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. Comparison of the molybdenum centres of native and desulpho xanthine oxidase. The nature of the cyanide-labile sulphur atom and the nature of the proton-accepting group. Biochem J. 1978 Dec 1;175(3):887–897. doi: 10.1042/bj1750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. The molybdenum centre of native xanthine oxidase. Evidence for proton transfer from substrates to the centre and for existence of an anion-binding site. Biochem J. 1978 Dec 1;175(3):869–878. doi: 10.1042/bj1750869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch Biochem Biophys. 1972 Jun;150(2):585–599. doi: 10.1016/0003-9861(72)90078-1. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., George G. N., Lowe D. J., Bray R. C. Coupling of [33S]sulphur to molybdenum(V) in different reduced forms of xanthine oxidase. Biochem J. 1981 Dec 1;199(3):629–637. doi: 10.1042/bj1990629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthouse J. P., Williams J. W., Bray R. C. Molybdenum(V) e.p.r. signals obtained from xanthine oxidase on reduction with aldehyde substrates and with 2-amino-4-hydroxy-6-formylpteridine. Biochem J. 1981 Aug 1;197(2):421–425. doi: 10.1042/bj1970421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- Morpeth F. F. Studies on the specificity toward aldehyde substrates and steady-state kinetics of xanthine oxidase. Biochim Biophys Acta. 1983 May 18;744(3):328–334. doi: 10.1016/0167-4838(83)90207-8. [DOI] [PubMed] [Google Scholar]
- Pick F. M., McGartoll M. A., Bray R. C. Reaction of formaldehyde and of methanol with xanthine oxidase. Eur J Biochem. 1971 Jan 1;18(1):65–72. doi: 10.1111/j.1432-1033.1971.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Rosemeyer H., Seela F. Methylated 7-deazahypoxanthines as regiochemical probes of xanthine oxidase. Eur J Biochem. 1983 Aug 15;134(3):513–515. doi: 10.1111/j.1432-1033.1983.tb07596.x. [DOI] [PubMed] [Google Scholar]
- Tanner S. J., Bray R. C., Bergmann F. 13C hyperfine splitting of some molybdenum electron-paramagnetic-resonance signals from xanthine oxidase [proceedings]. Biochem Soc Trans. 1978;6(6):1328–1330. doi: 10.1042/bst0061328. [DOI] [PubMed] [Google Scholar]


