Abstract
When Mg2+ was added to rat oncomodulin, a paravalbumin-like tumour protein, changes in the c.d. spectrum and tyrosine fluorescence intensity were observed. The addition of Ca2+ resulted in even greater changes in these spectra. The fluorescence excitation spectra of apo- and Mg-oncomodulin were superimposable, whereas that of Ca-oncomodulin was markedly different. The u.v.-absorption spectrum of the Ca2+ form also showed major differences from those of the other two forms. These observations indicate that Ca2+ induced a significant and specific conformational change in the protein that was not observed on binding Mg2+. In contrast, the conformational change induced by either Mg2+ or Ca2+ was identical in the homologous rat parvalbumin. This Ca2+-specific conformational change may be the basis for oncomodulin's Ca2+-dependent protein/protein interaction.
Full text
PDF
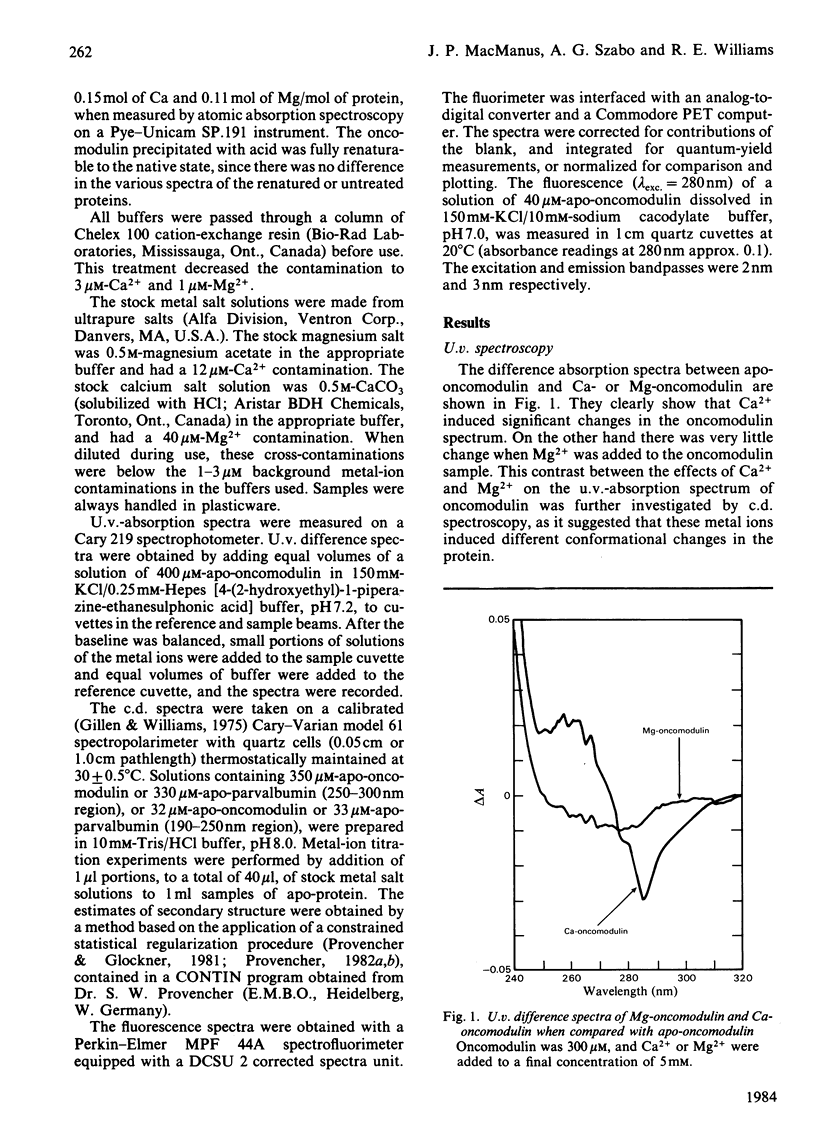
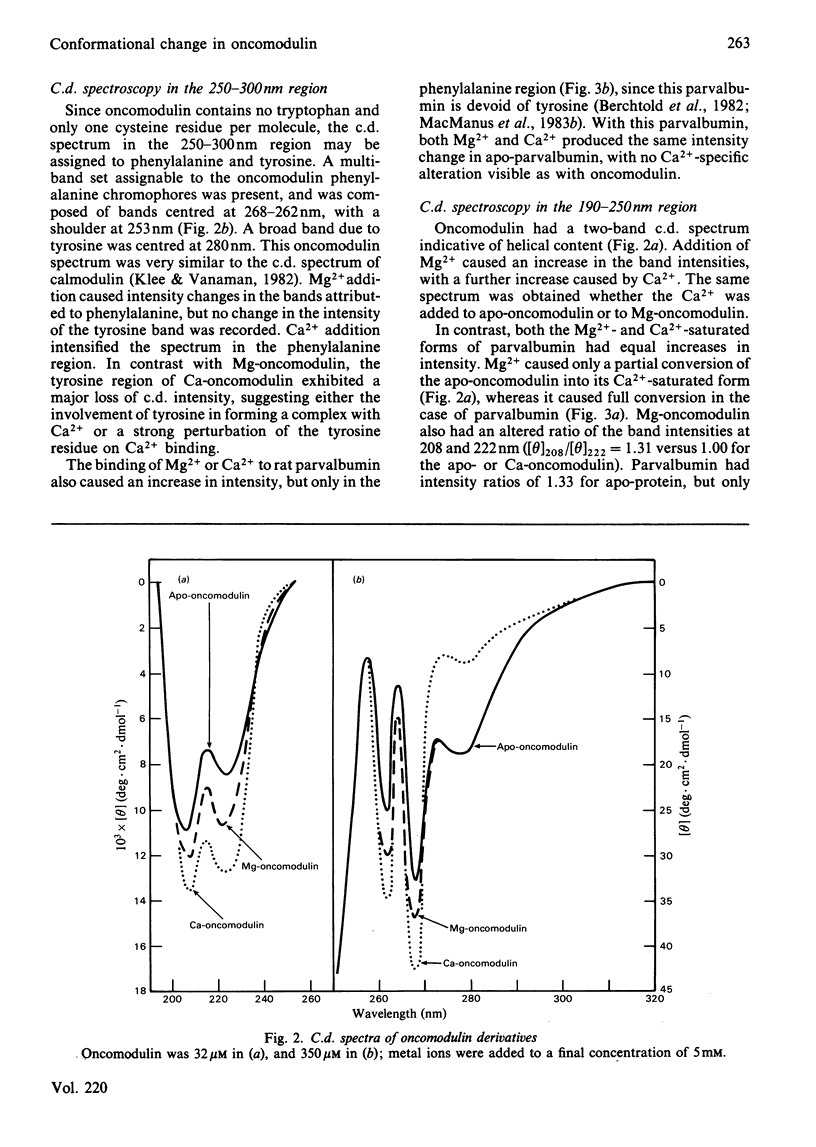
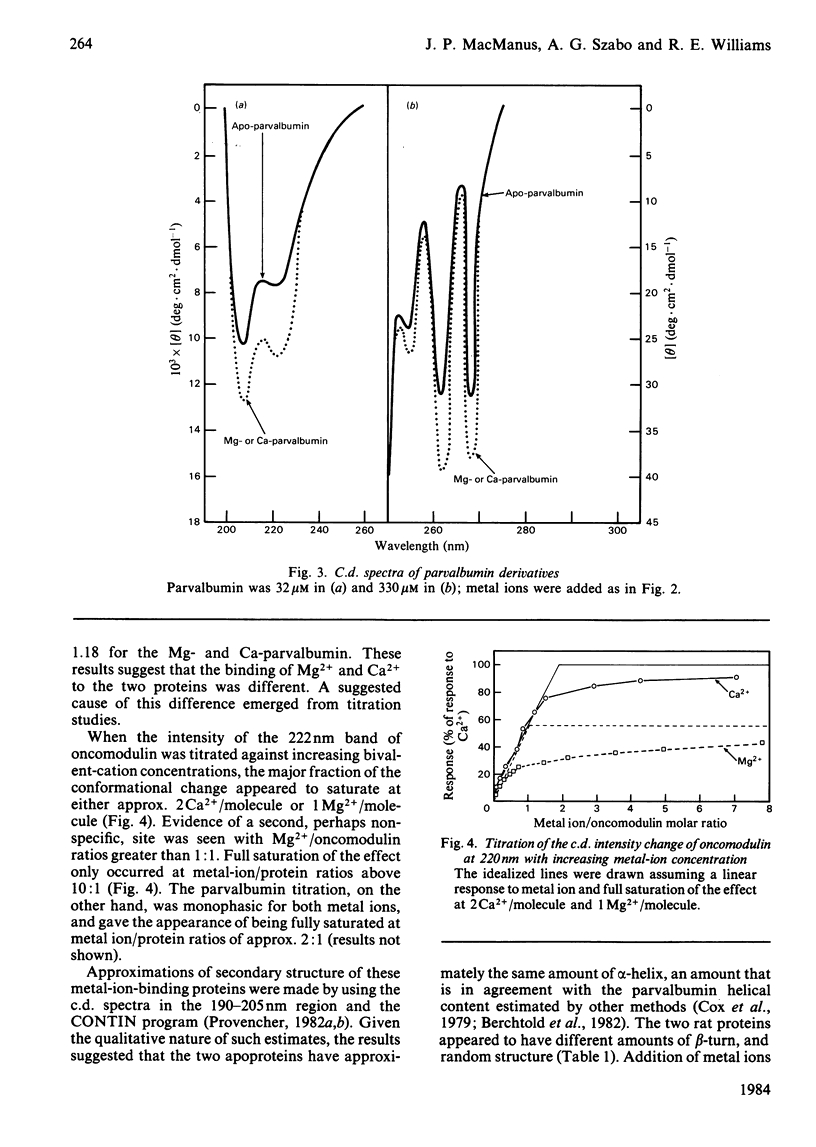
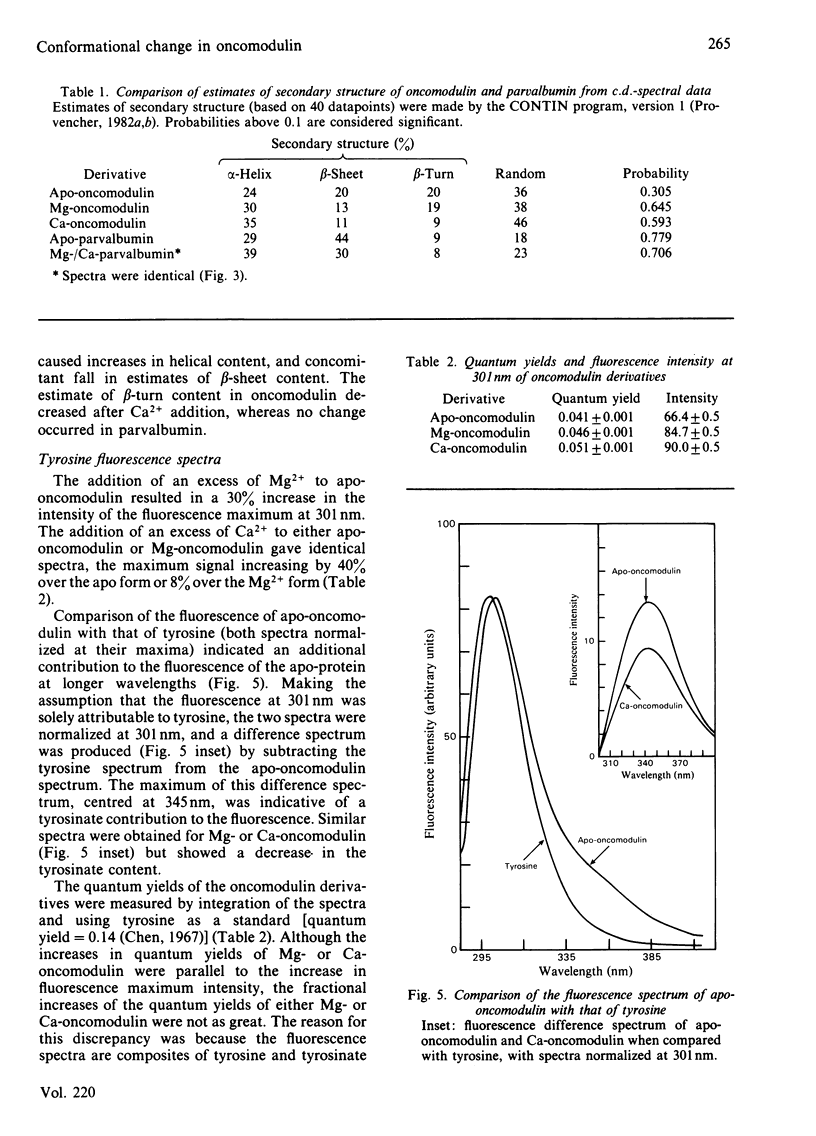


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berchtold M. W., Heizmann C. W., Wilson K. J. Primary structure of parvalbumin from rat skeletal muscle. Eur J Biochem. 1982 Oct;127(2):381–389. doi: 10.1111/j.1432-1033.1982.tb06883.x. [DOI] [PubMed] [Google Scholar]
- Birdsall W. J., Levine B. A., Williams R. J., Demaille J. G., Haiech J., Pechere J. F. Calcium and magnesium binding by parvalbumin. A proton magnetic resonance spectral study. Biochimie. 1979;61(7):741–750. doi: 10.1016/s0300-9084(79)80268-0. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., MacManus J. P., Whitfield J. F. Stimulation of liver cell DNA synthesis by oncomodulin, an MW 11 500 calcium-binding protein from hepatoma. Exp Cell Res. 1982 Apr;138(2):454–457. doi: 10.1016/0014-4827(82)90198-7. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Winge D. R., Stein E. A. Calcium, magnesium and the conformation of parvalbumin during muscular activity. Biochimie. 1979;61(5-6):601–605. doi: 10.1016/s0300-9084(79)80157-1. [DOI] [PubMed] [Google Scholar]
- Criss W. E., Kakiuchi S. Calcium: calmodulin and cancer. Fed Proc. 1982 May;41(7):2289–2291. [PubMed] [Google Scholar]
- Haiech J., Derancourt J., Pechere J. F., Demaille J. G. A new large-scale purification procedure for muscular parvalbumins. Biochimie. 1979;61(5-6):583–587. doi: 10.1016/s0300-9084(79)80155-8. [DOI] [PubMed] [Google Scholar]
- Haiech J., Klee C. B., Demaille J. G. Effects of cations on affinity of calmodulin for calcium: ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry. 1981 Jun 23;20(13):3890–3897. doi: 10.1021/bi00516a035. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- MacManus J. P. Occurrence of a low-molecular-weight calcium-binding protein in neoplastic liver. Cancer Res. 1979 Aug;39(8):3000–3005. [PubMed] [Google Scholar]
- MacManus J. P. The purification of a unique calcium-binding protein from Morris hepatoma 5123 tc. Biochim Biophys Acta. 1980 Feb 27;621(2):296–304. doi: 10.1016/0005-2795(80)90181-6. [DOI] [PubMed] [Google Scholar]
- MacManus J. P. The stimulation of cyclic nucleotide phosphodiesterase by a Mr 11 500 calcium binding protein from hepatoma. FEBS Lett. 1981 Apr 20;126(2):245–249. doi: 10.1016/0014-5793(81)80252-9. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Watson D. C., Yaguchi M. A new member of the troponin C superfamily: comparison of the primary structures of rat oncomodulin and rat parvalbumin. Biosci Rep. 1983 Nov;3(11):1071–1075. doi: 10.1007/BF01121034. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Watson D. C., Yaguchi M. The complete amino acid sequence of oncomodulin--a parvalbumin-like calcium-binding protein from Morris hepatoma 5123tc. Eur J Biochem. 1983 Oct 17;136(1):9–17. doi: 10.1111/j.1432-1033.1983.tb07698.x. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Whitfield J. F., Boynton A. L., Durkin J. P., Swierenga S. H. Oncomodulin--a widely distributed, tumour-specific, calcium-binding protein. Oncodev Biol Med. 1982;3(2-3):79–90. [PubMed] [Google Scholar]
- Moeschler H. J., Schaer J. J., Cox J. A. A thermodynamic analysis of the binding of calcium and magnesium ions to parvalbumin. Eur J Biochem. 1980 Oct;111(1):73–78. doi: 10.1111/j.1432-1033.1980.tb06076.x. [DOI] [PubMed] [Google Scholar]
- Moews P. C., Kretsinger R. H. Refinement of the structure of carp muscle calcium-binding parvalbumin by model building and difference Fourier analysis. J Mol Biol. 1975 Jan 15;91(2):201–225. doi: 10.1016/0022-2836(75)90160-6. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]


