Abstract
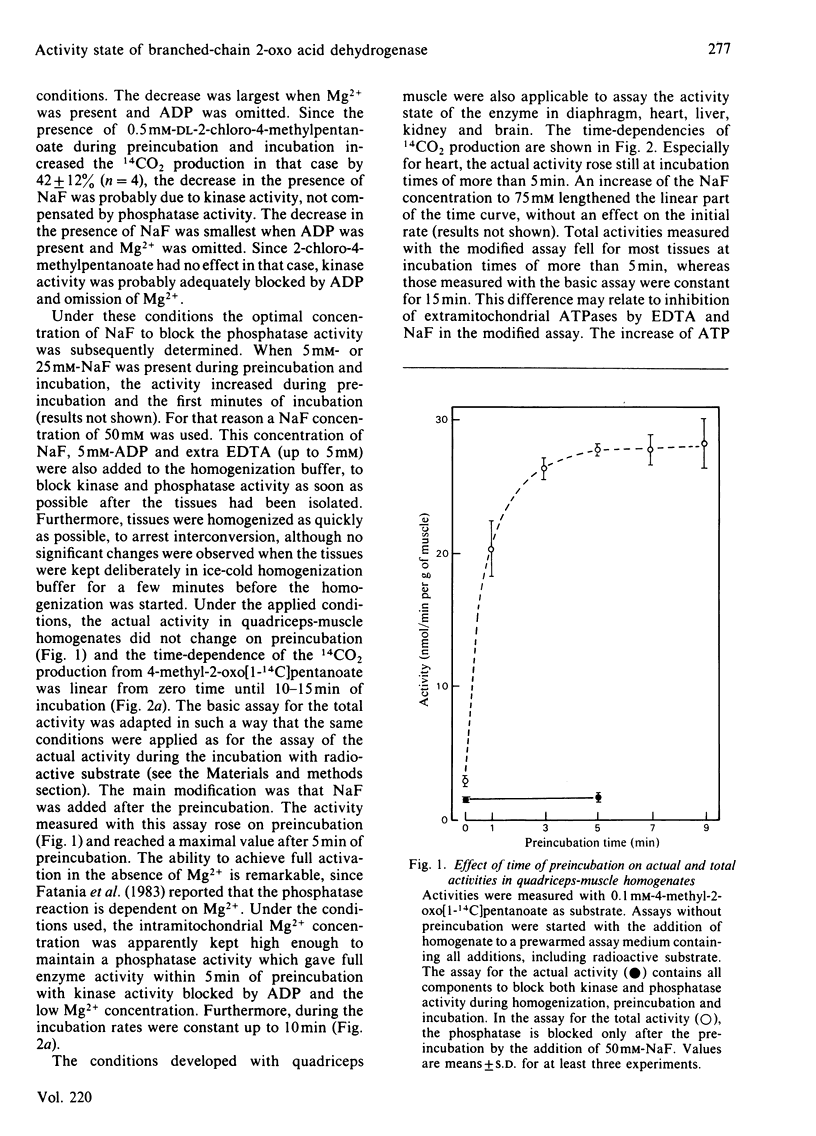
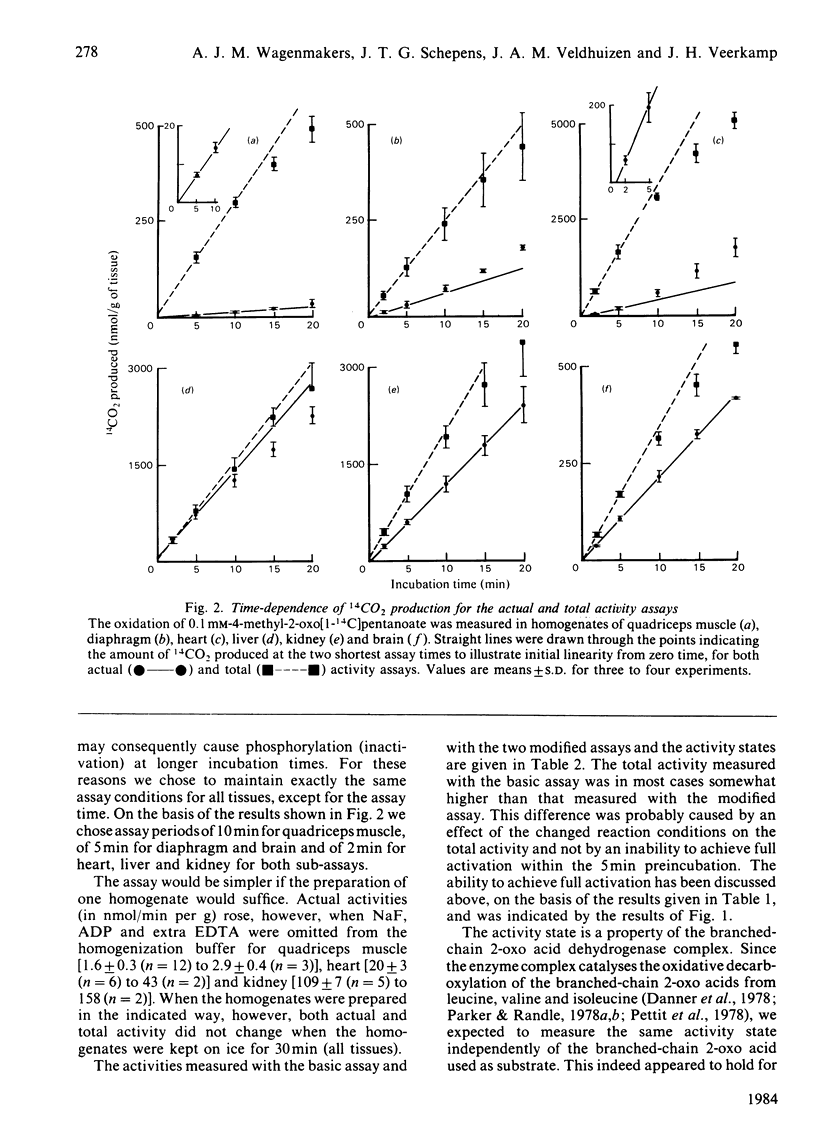
An assay is described to define the proportion of the branched-chain 2-oxo acid dehydrogenase complex that is present in the active state in rat tissues. Activities are measured in homogenates in two ways: actual activities, present in tissues, by blocking both the kinase and phosphatase of the enzyme complex during homogenization, preincubation, and incubation with 1-14C-labelled branched-chain 2-oxo acid, and total activities by blocking only the kinase during the 5 min preincubation (necessary for activation). The kinase is blocked by 5 mM-ADP and absence of Mg2+ and the phosphatase by the simultaneous presence of 50 mM-NaF. About 6% of the enzyme is active in skeletal muscle of fed rats, 7% in heart, 20% in diaphragm, 47% in kidney, 60% in brain and 98% in liver. An entirely different assay, which measures activities in crude tissue extracts before and after treatment with a broad-specificity protein phosphatase, gave similar results for heart, liver and kidney. Advantages of our assay with homogenates are the presence of intact mitochondria, the simplicity, the short duration and the high sensitivity. The actual activities measured indicate that the degradation of branched-chain 2-oxo acids predominantly occurs in liver and kidney and is limited in skeletal muscle in the fed state.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., May M. E., Manos P. N., Buse M. G. Regulation of alpha-ketoisocaproate oxidation in liver mitochondria by adenine nucleotides and calcium. J Biol Chem. 1982 Jun 10;257(11):6156–6163. [PubMed] [Google Scholar]
- Brandt H., Capulong Z. L., Lee E. Y. Purification and properties of rabbit liver phosphorylase phosphatase. J Biol Chem. 1975 Oct 25;250(20):8038–8044. [PubMed] [Google Scholar]
- Brandt H., Killilea S. D., Lee E. Y. Activation of phosphorylase phosphatase by a novel procedure: evidence for a regulatory mechanism involving the release of a catalytic subunit from enxyme-inhibitor complex(es) of higher molecular weight. Biochem Biophys Res Commun. 1974 Nov 27;61(2):598–604. doi: 10.1016/0006-291x(74)90999-1. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Jursinic S., Reid S. S. Regulation of branched-chain amino acid oxidation in isolated muscles, nerves and aortas of rats. Biochem J. 1975 Jun;148(3):363–374. doi: 10.1042/bj1480363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Elsas L. J., 2nd Substrate specificity and stabilization by thiamine pyrophosphate of rat liver branched chain alpha-ketoacid dehydrogenase. Biochem Med. 1978 Feb;19(1):27–38. doi: 10.1016/0006-2944(78)90004-2. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Lau K. S., Randle P. J. Activation of phosphorylated branched chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1982 Oct 4;147(1):35–39. doi: 10.1016/0014-5793(82)81006-5. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Patston P. A., Randle P. J. Dephosphorylation and reactivation of phosphorylated purified ox-kidney branched-chain dehydrogenase complex by co-purified phosphatase. FEBS Lett. 1983 Jul 25;158(2):234–238. doi: 10.1016/0014-5793(83)80585-7. [DOI] [PubMed] [Google Scholar]
- Gillim S. E., Paxton R., Cook G. A., Harris R. A. Activity state of the branched chain alpha-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats. Biochem Biophys Res Commun. 1983 Feb 28;111(1):74–81. doi: 10.1016/s0006-291x(83)80119-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Harris R. A., Paxton R., DePaoli-Roach A. A. Inhibition of branched chain alpha-ketoacid dehydrogenase kinase activity by alpha-chloroisocaproate. J Biol Chem. 1982 Dec 10;257(23):13915–13918. [PubMed] [Google Scholar]
- Harris R. A., Paxton R., Parker R. A. Activation of the branched-chain alpha-ketoacid dehydrogenase complex by a broad specificity protein phosphatase. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1497–1503. doi: 10.1016/s0006-291x(82)80168-x. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982 Jul 19;144(1):57–62. doi: 10.1016/0014-5793(82)80568-1. [DOI] [PubMed] [Google Scholar]
- Livesey G., Lund P. Enzymic determination of branched-chain amino acids and 2-oxoacids in rat tissues. Transfer of 2-oxoacids from skeletal muscle to liver in vivo. Biochem J. 1980 Jun 15;188(3):705–713. doi: 10.1042/bj1880705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Odessey R. Reversible ATP-induced inactivation of branched-chain 2-oxo acid dehydrogenase. Biochem J. 1980 Oct 15;192(1):155–163. doi: 10.1042/bj1920155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Active and inactive forms of branched-chain 2-oxoacid dehydrogenase complex in rat heart and skeletal muscle. FEBS Lett. 1980 Apr 7;112(2):186–190. doi: 10.1016/0014-5793(80)80176-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Branched chain 2-oxo-acid dehydrogenase complex of rat liver. FEBS Lett. 1978 Jun 1;90(1):183–186. doi: 10.1016/0014-5793(78)80325-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Inactivation of rat heart branched-chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1978 Nov 1;95(1):153–156. doi: 10.1016/0014-5793(78)80072-6. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Partial purification and properties of branched-chain 2-oxo acid dehydrogenase of ox liver. Biochem J. 1978 Jun 1;171(3):751–757. doi: 10.1042/bj1710751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J. Mitochondrial 2-oxoacid dehydrogenase complexes of animal tissues. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):47–57. doi: 10.1098/rstb.1983.0037. [DOI] [PubMed] [Google Scholar]
- Shinnick F. L., Harper A. E. Branched-chain amino acid oxidation by isolated rat tissue preparations. Biochim Biophys Acta. 1976 Jul 21;437(2):477–486. doi: 10.1016/0304-4165(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W., Veerkamp J. H., Engelen P. J., Ghijsen W. J. Effect of L-carnitine on the oxidation of leucine and valine by rat skeletal muscle. Biochem Med. 1978 Aug;20(1):115–124. doi: 10.1016/0006-2944(78)90056-x. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W., Veerkamp J. H., Glatz J. F. 4-Methyl-2-oxopentanoate oxidation by rat skeletal-muscle mitochondria. Biochem J. 1979 Aug 15;182(2):353–360. doi: 10.1042/bj1820353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp J. H., van Hinsbergh V. W., Cordewener J. H. Degradation of branched-chain amino acids and 2-oxo acids in human and rat muscle. Biochem Med. 1980 Oct;24(2):118–129. doi: 10.1016/0006-2944(80)90003-4. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Veerkamp J. H. Degradation of branched-chain amino acids and their derived 2-oxo acids and fatty acids in human and rat heart and skeletal muscle. Biochem Med. 1982 Aug;28(1):16–31. doi: 10.1016/0006-2944(82)90051-5. [DOI] [PubMed] [Google Scholar]
- Waymack P. P., DeBuysere M. S., Olson M. S. Studies on the activation and inactivation of the branched chain alpha-keto acid dehydrogenase in the perfused rat heart. J Biol Chem. 1980 Oct 25;255(20):9773–9781. [PubMed] [Google Scholar]
- van Hinsbergh V. W., Veerkamp J. H., Cordewener J. H. Effect of carnitine and branched-chain acylcarnitines on the 2-oxo acid dehydrogenase activity in intact mitochondria of rat muscle. Int J Biochem. 1980;12(4):559–565. doi: 10.1016/0020-711x(80)90007-5. [DOI] [PubMed] [Google Scholar]



