Abstract
目的
本研究回顾性收集进行68镓(68Ga)-前列腺特异性膜抗原(prostate specific membrane antigen, PSMA)和18氟(18F)-代脱氧葡萄糖(flurodeoxyglucose, FDG)正电子发射计算机断层成像(PET/CT)显像的转移性前列腺癌(metastatic PCa, mPCa),分析双示踪剂摄取模式,影响病灶摄取18F-FDG的临床病理学参数及影响前列腺特异性抗原(prostate specific antigen, PSA)-无进展生存期(progression-free survival, PFS)的预后分析。
方法
回顾性纳入2021年9月–2024年1月间于我院行68Ga-PSMA 及18F-FDG PET/CT双示踪剂显像的41例mPCa。除外1例PSMA、FDG双阴性摄取,基于是否存在FDG阳性病灶,将剩余40例分为两组:Group A(PSMA、FDG双阳性组和FDG单阳性组,33例);Group B(PSMA单阳性组,7例)。比较Group A和Group B组间临床病理学特点。通过Kaplan-Meier法分析不同参数与PSA-PFS的关系。
结果
26例(63.4%)患者为转移性去势抵抗性PCa(metastatic castration-resistant PCa, mCRPC),Gleason 评分 8~9 分38例(92.7%),远处转移以骨骼转移(36例,87.8%)为主。骨骼及远处淋巴结转移灶多表现为PSMA、FDG双阳性摄取模式〔85.7%(24/28),81.8%(9/11)〕;在脏器转移灶中,37.5%(3/8)存在FDG单阳性的摄取模式。Group A血清PSA水平高于Group B(P=0.013)。13例特殊病理类型(导管内癌和神经内分泌分化)患者均在Group A。41例患者中,16例患者失访。25例完成随访的患者中9例患者发生PSA进展,PSA中位值为104 ng/mL;16 例患者无PSA进展,PSA中位值为0.34 ng/mL,两组间PSA中位值差异有统计学意义(P<0.001)。Kaplan-Meier生存分析显示特殊病理类型中位PSA-PFS(7个月) 短于经典型PCa(16个月),两组间差异有统计学意义(P=0.043);Group A中位PSA-PFS为30个月,Group B仍有超过一半的个体尚未发生PSA进展,中位PSA-PFS尚未达到(P=0.645)。
结论
mPCa多表现为68Ga-PSMA和18F-FDG双示踪剂摄取,血清PSA水平是预测病灶FDG阳性的可靠指标。存在导管内癌及神经内分泌分化的mPCa病灶易表现为FDG阳性且出现PSA进展。
Keywords: 转移性前列腺癌, 68Ga-PSMA, 18F-FDG, 异质性
Abstract
Objective
In this study, we retrospectively analyzed the imaging characteristics of dual-tracer 68Ga-prostate specific membrane antigen (PSMA) and 18F-flurodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in metastatic prostate cancer (mPCa) patients. We analyzed the uptake modes of the dual tracers, explored clinical pathological parameters affecting the 18F-FDG uptake in the lesions, and evaluated their prognostic implications for prostate specific antigen progression-free survival (PSA-PFS).
Methods
A total of 41 mPCa patients who underwent dual-tracer PET/CT (68Ga-PSMA and 18F-FDG) scans between September 2021 and January 2024 were retrospectively enrolled. One patient had negative uptake of both PSMA and FDG. According to the uptake patterns of the 2 tracers, the other patients, 40 in total, were categorized in 2 groups, including group A consisting of 33 cases who showed PSMA and FDG dual and those who showed FDG only avidity, and group B consisting of 7 cases who showed PSMA avidity only. Comparative analyses of clinical pathological characteristics between group A and group B were conducted. The relationship between various parameters and PSA-PFS was analyzed by the Kaplan-Meier method.
Results
A total of 26 patients (63.4%) were diagnosed with metastatic castration-resistant prostate cancer (mCRPC), and 38 cases (92.7%) had a Gleason score of 8-9. Bone metastasis, the predominant type of distant metastasis, occurred in 36 cases (87.8%). The skeletal and distant lymph node metastases mostly showed a dual positive uptake pattern for both PSMA and FDG (85.7% [24/28] and 81.8% [9/11]). 37.5% (3/8) of the metastases to organs showed FDG only positive uptake pattern. The serum levels of prostate specific antigen (PSA) in group A were significantly higher than those in group B (P=0.013). A total of 13 patients of special pathological classification (intraductal carcinoma and neuroendocrine differentiation) were all found to be in group A. Among the 41 cases, 16 were lost to follow-up. Of the 25 patients who completed follow-up, 9 patients, with a median PSA value of 104 ng/mL, experienced PSA progression, while the 16 other patients, with a median PSA of 0.34 ng/mL, did not incur any PSA progression. There was significant difference in the median PSA between patients showing PSA progression and those who did not show PSA progression (P<0.001). Kaplan-Meier survival analysis revealed that the median PSA-PFS of patients of specific pathological classifications was 7 months, which was shorter than the 16 months of the patients with typical prostate cancer, with the difference between the two groups being statistically meaningful (P=0.043). The median PSA-PFS for group A was 30 months. With more than half of the patients in the group not experiencing any PSA progression, group B did not reach the median PSA-PFS (P=0.645).
Conclusion
Dual-tracer PET/CT imaging with 68Ga-PSMA and 18F-FDG commonly exhibits avidity for both tracers in mPCa. Serum PSA level is a reliable biomarker for predicting FDG-positive lesions. mPCa presented with intraductal carcinoma and neuroendocrine differentiation tends to exhibit FDG avidity and is more susceptible to PSA progression.
Keywords: Metastatic prostate cancer, 68Ga-PSMA, 18F-FDG, Heterogeneity
前列腺癌(prostate cancer, PCa)是男性常见的恶性肿瘤之一[1]。在初始治疗后,仍有约30%~40%患者会发生疾病复发或转移[2]。检测复发或转移病灶对于患者后续临床管理有着重要意义。前列腺特异性膜抗原(prostate specific membrane antigen, PSMA)是一种Ⅱ型跨膜糖蛋白,在PCa细胞膜上高表达。68Ga-PSMA是常用的靶向PSMA的示踪剂,68Ga-PSMA PET已应用于初发中高危患者和生化复发患者诊断[3-4]。一项纳入635例PCa患者的研究显示68Ga-PSMA PET对于生化复发患者病灶检出率随前列腺特异性抗原(prostate specific antigen, PSA)水平增高而显著提高[5]。对于传统影像学检查如CT或骨显像上诊断为非远处转移性去势抵抗性PCa(metastatic castration-resistant PCa, mCRPC)中,PSMA PET可发现额外39%的远处淋巴结转移,24%骨转移,6%内脏转移[6]。
PSMA介导的放射性配体治疗(radioligand therapy, RLT)已获批用于雄激素受体通路抑制剂和紫杉烷类治疗后进展的PSMA阳性的mCRPC,其重要筛选标准为PSMA PET显像中病灶摄取水平高于肝脏[7-8];有些RLT临床试验还要求病灶18F-代脱氧葡萄糖(flurodeoxyglucose, FDG)PET 显像阴性[9]。这是由于多线治疗,肿瘤生物学行为可发生变化,病灶内或病灶间可出现异质性表达,可出现肿瘤PSMA表达水平逐渐丢失而糖酵解增加,从而影响疗效[10]。通过18F-FDG及68Ga-PSMA双示踪剂检查可更好地显示疾病范围并评估肿瘤异质性[11-13]。因此,本研究回顾性描述转移性PCa(metastatic Pca, mPCa)患者的病灶摄取68Ga-PSMA和18F-FDG双示踪剂PET/CT显像特点,分析影响病灶摄取FDG的临床病理学参数及影响PSA-无进展生存期(PSA progression-free survival, PSA-PFS)的预后分析。
1. 资料与方法
1.1. 研究对象
回顾性收集2021年9月–2024年1月在四川大学华西医院行68Ga-PSMA和18F-FDG PET/CT检查的mPCa患者。依照纳入与排除标准进行筛选,最终41例患者纳入本研究。纳入标准:①病理确诊为PCa;②存在转移性病变;③两种示踪剂检查间隔时间小于1周。排除标准:①合并其他恶性肿瘤;②缺少临床资料。转移性病变定义为由临床、影像或病理随访结果证实的远处转移灶,如远处淋巴结、骨骼和脏器转移等。收集患者进行PET显像时的血清PSA水平、治疗方案及激素状态,确诊时的Gleason评分,国际泌尿外科病理学会(International Society of Urological Pathologists, ISUP)分级及病理类型等临床病理学资料。PET检查前治疗方案包括根治性前列腺切除术、放疗、常规内分泌治疗、新型内分泌治疗(novel hormonal agents, NHA)、化疗及其他治疗方案。本研究获四川大学华西医院生物医学伦理审查委员会批准,批准号2021年审(735)号。
1.2. 图像采集
由四川大学华西医院核医学科合成68Ga-PSMA和18F-FDG。患者接受18F-FDG注射前需要禁食至少6 h且血糖值≤11.1 mmol/L,18F-FDG注射剂量为3.7 MBq/kg。患者接受68Ga-PSMA注射前无特殊准备,68Ga-PSMA的注射剂量为1.85 MBq/kg。注射药物后患者于安静舒适的房间休息45~60 min,排空尿液后进行PET/CT检查(联影 uMi780 PET/CT 和荷兰 Philips 公司的 Gemini GXL PET/CT)。采集时患者取仰卧位,双手上举,平静呼吸。首先进行全身低剂量CT扫描用于衰减矫正。在CT扫描后立即行PET扫描,以每床位3 min(68Ga-PSMA)及1.5 min(18F-FDG)的速度从颅顶扫至股骨中段,常规采集6~8个床位。根据病变累及部位加做相应部位的诊断剂量CT。根据不同厂家的标准进行迭代重建 PET 图像。
1.3. 图像分析
由2名经验丰富的核医学医师对68Ga-PSMA PET及18F-FDG PET图像进行判读。当出现意见不一致时,由两者协商后达成共识。68Ga-PSMA或18F-FDG阳性病灶定义为排除生理性摄取后,高于纵隔血池背景或周围正常组织背景的局灶性摄取。根据转移病灶对示踪剂的摄取模式将患者分为以下4组:PSMA单阳性组(PSMA+FDG-)、双阳性组(至少一个病灶表现为PSMA+FDG+)、FDG单阳性组(PSMA-FDG+)、双阴性组(PSMA-FDG-)。排除双阴性病例后,再基于是否存在FDG阳性病灶,分为两组:Group A:双阳性组和FDG单阳性组;Group B:PSMA单阳性组。对于双阳性(至少一个病灶表现为PSMA+FDG+)组,按照不同病灶部位(远处淋巴结、骨骼、脏器)进行摄取特点分类。
1.4. 随访
进行PET检查后,对患者进行随访,收集患者PET检查后的治疗方案、血清PSA变化及影像学检查结果等。主要终点观察指标是PSA-PFS[14]。PSA-PFS定义为从PET检查开始到PSA进展或因PCa死亡的时间。PSA进展定义为PSA较基线至少增加25%。
1.5. 统计学方法
采用IBM SPSS 26.0统计分析软件进行分析。Prism 9.0进行相关图形的绘制。符合正态分布的计量资料用均数及标准差进行描述,不符合者用中位数及四分位数间距(interquartile ranges, IQR)表示。计数资料用频率及百分比进行描述。采用Fisher确切概率法或非参数秩和检验(Mann-Whitney U test及Wilcoxon Z test)比较不同组间参数差异。采用Kaplan-Meier法绘制生存曲线并用log-rank分析各组间PSA-PFS的差异。P<0.05为差异有统计学意义。
2. 结果
2.1. 转移性前列腺癌患者的基本临床特点
共纳入患者41例,中位年龄为64(58~75)岁。检查时PSA水平中位值为3.92(0.51~40.20) ng/mL。mCRPC患者26例(63.4%),转移性激素敏感性前列腺癌(metastatic hormone-sensitive prostate cancer, mHSPC)患者15例(36.6%)。Gleason评分7分患者3例(7.3%), 8分患者16例(39.0%), 9分患者22例(53.7%)。ISUP分级2级1例(2.4%),3级2例(4.9%),4级16例(39.0%),5级22例(53.7%)。病理类型中存在导管内癌的有6例(14.6%),神经内分泌分化7例(17.1%)。远处淋巴结转移15例(36.6%),骨转移36例(87.8%),脏器转移9例(22.0%)。患者基本临床资料见表1。
表 1. Clinicopathological characteristics of patients with metastatic prostate cancer (n=41).
转移性前列腺癌患者临床病理特征 (n=41)
| Clinicopathological characteristic | Case (%) or median (P25-P75) |
| mCRPC: metastatic castration-resistant prostate cancer; mHSPC: metastatic hormone-sensitive prostate cancer; PSA: prostate specific antigen; ISUP: International Society of Urological Pathology; ADT: androgen deprivation therapy; NHA: novel endocrine therapy. # Some patients have multiple organ metastases at the same time, such as liver and lung metastases, while others have liver, or lung, or other organ metastases alone. The table is calculated according to one metastatic site, so patients with both liver and lung metastasis are recorded as 1 liver metastasis and 1 lung metastasis, respectively. * Oligometastasis is defined as 1-5 metastases found on PET imaging. Polymetastasis is defined as PET imaging showing more than 5 metastases. | |
| Hormonal status | |
| mCRPC | 26 (63.4) |
| mHSPC | 15 (36.6) |
| Age/yr. | 64 (58-75) |
| PSA level/(ng/mL) | 3.92 (0.51-40.20) |
| <10 | 25 (61.0) |
| ≥10 | 16 (39.0) |
| Gleason score | |
| 7 | 3 (7.3) |
| 8 | 16 (39.0) |
| 9 | 22 (53.7) |
| ISUP/WHO grade | |
| 2 | 1 (2.4) |
| 3 | 2 (4.9) |
| 4 | 16 (39.0) |
| 5 | 22 (53.7) |
| Specific pathological type | |
| Intraductal carcinoma | 6 (14.6) |
| Neuroendocrinization | 7 (17.1) |
| Metastatic sites | |
| Distant lymph nodes | 15 (36.6) |
| Bone | 36 (87.8) |
| Organs# | 9 (22.0) |
| Liver | 4 (9.7) |
| Lung | 6 (14.6) |
| Other organs | 3 (7.3) |
| Local recurrence | 20 (48.8) |
| Metastasis* | |
| Oligometastasis | 20 (48.8) |
| Polymetastasis | 21 (51.2) |
| Previous systemic treatment | |
| Radical surgery±radiotherapy±ADT | 14 (34.1) |
| Radical surgery±radiotherapy±ADT+NHA | 14 (34.1) |
|
Radical surgery±radiotherapy±ADT+other (chemotherapy) |
13 (31.8) |
2.2. 68Ga-PSMA和18F-FDG PET/CT检查结果
2.2.1. 68Ga-PSMA和18F-FDG PET/CT患者转移灶摄取特点
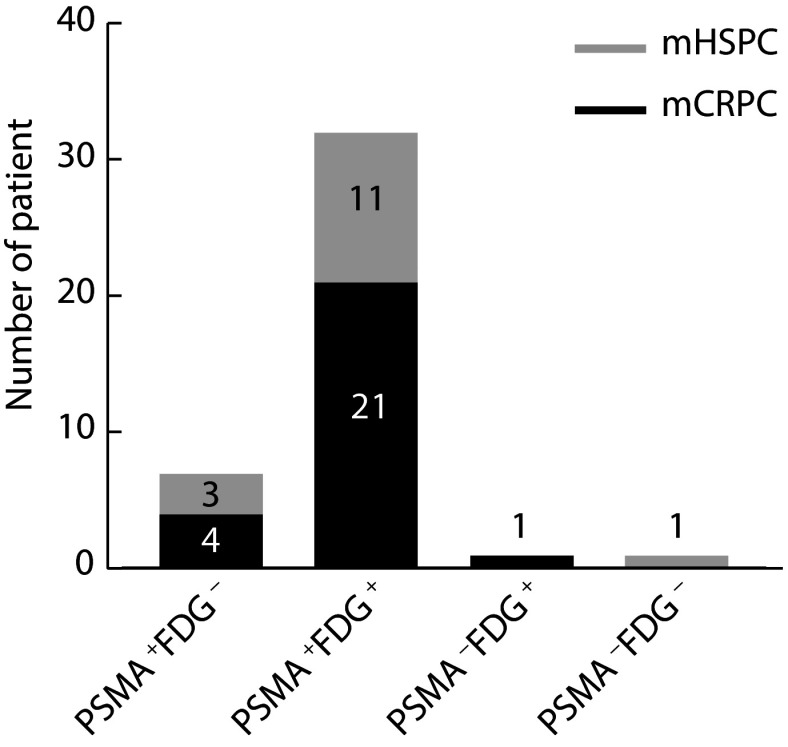
如图1所示,根据双示踪剂PET/CT检查结果,双阴性组1例(2.4%),PSMA单阳性组 7例(17.1%),FDG单阳性组 1例(2.4%),双阳性组32例(78.1%)。其中双阳组mCRPC患者占比65.6%,mHSPC患者占比34.4%。Group A有33例,Group B有7例。FDG单阳性组示例见图2,PSMA单阳性组示例见图3,双阳性组示例见图4。
图 1.
Dual-tracer uptake patterns of metastatic prostate cancer on 68Ga-PSMA and 18F-FDG PET/CT
转移性前列腺癌68Ga-PSMA和18F-FDG PET/CT双示踪剂摄取情况
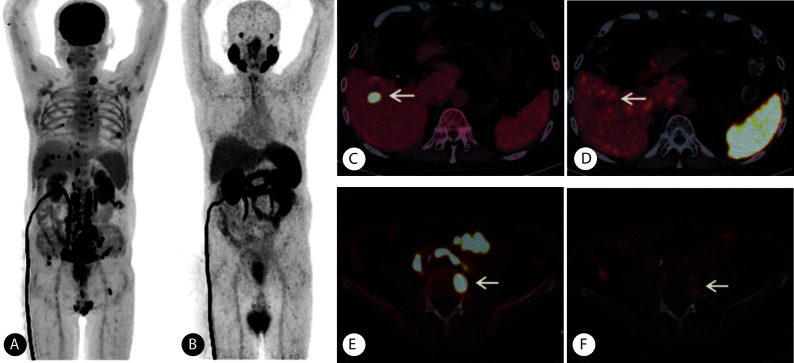
图 2.
PAMS-FDG+ lesions on 68Ga-PSMA and 18F-FDG PET/CT in a patient (Gleason score: 4+5=9, focal adenocarcinoma with neurodocrine differentiation) with metastatic prostate cancer
一例在68Ga-PSMA和18F-FDG PET/CT表现为PSMA-FDG+的前列腺癌患者(Gleason评分4+5=9分,病理类型为腺癌局灶性伴神经内分泌分化 )
A, The maximum intensity projection of 18F-FDG PET; B, the maximum intensity projection of 68Ga-PSMA PET; C, the uptake of 18F-FDG in the hepatic lesion was abnormally increased (arrow), with a SUVmax of 18.32; D, no abnormal uptake of 68Ga-PSMA was observed in the hepatic lesion (arrow); E, the uptake of 18F-FDG in the 5th lumbar vertebra was abnormally increased (arrow), and the SUVmax was 18.21; F, no abnormal uptake of 68Ga-PSMA was observed in the 5th lumbar vertebra (arrow).
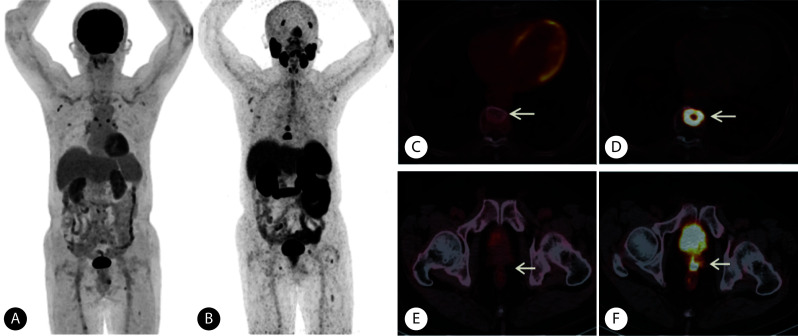
图 3.
PAMS+FDG- lesions on 68Ga-PSMA and 18F-FDG PET/CT in a patient (Gleason score: 4+5=9, adenocarcinoma) with metastatic prostate cancer
一例在68Ga-PSMA和18F-FDG PET/CT表现为PSMA+FDG-的前列腺癌患者(Gleason评分4+5=9分,病理类型为腺癌)
A, The maximum intensity projection of 18F-FDG PET; B, the maximum intensity projection of 68Ga-PSMA PET; C, the high-density lesion in the 9th thoracic vertebra had abnormally increased uptake 68Ga-PSMA (arrow), with a SUVmax of 13.38; D, no abnormal uptake of 18F-FDG was observed in the 9th thoracic vertebra (arrow); E, local recurrent uptake of 68Ga-PSMA in the prostate area was abnormally increased (arrow), with a SUVmax of 11.39; F, no abnormal increase in 18F-FDG uptake was observed in the prostate area (arrow).
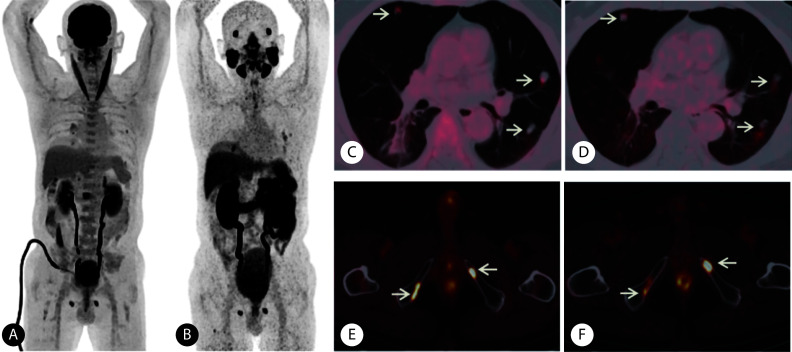
图 4.
PAMS+FDG+ lesions on 68Ga-PSMA and 18F-FDG PET/CT in a patient (Gleason score: 4+5=9, adenocarcinoma) with metastatic prostate cancer
一例在68Ga-PSMA和18F-FDG PET/CT表现为PSMA+FDG+的前列腺癌患者(Gleason评分4+5=9分,病理类型为腺癌)
A, The maximum intensity projection of 18F-FDG PET; B, the maximum intensity projection of 68Ga-PSMA PET; C and D, the uptake of 18F-FDG (C) and 68Ga-PSMA (D) in multiple pulmonary nodules increased abnormally (arrows), and the SUVmax was 3.98 and 8.40, respectively; E and F, the uptake of 18F-FDG (E) and 68Ga-PSMA (F) in lesions of bilateral pubis increased abnormally (arrows), and the SUVmax was 12.90 and 7.11, respectively.
2.2.2. 双阳性组患者病灶部位摄取特点
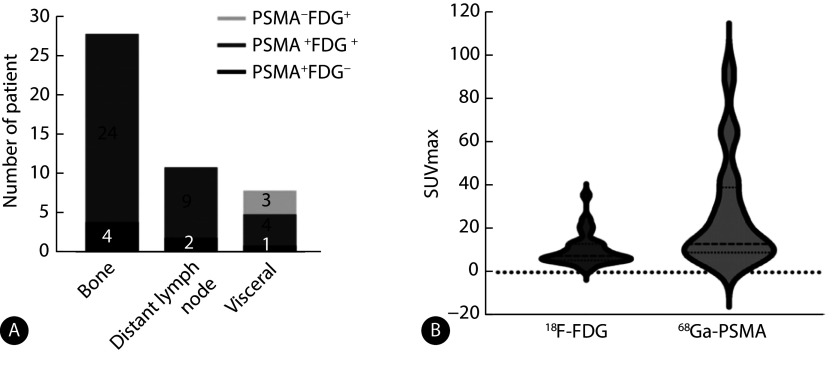
双阳组中骨骼及远处淋巴结转移有85.7%(24/28)及81.8%(9/11)呈现双显像剂共同摄取模式,剩下有14.3%(4/28)及18.2%(2/11)呈现PSMA单阳性模式。而在脏器转移灶中,37.5%(3/8)存在FDG单阳性的摄取模式。比较两种显像剂所有病灶中最大的标准化摄取值(standard uptake maximum upkate, SUVmax),发现病灶PSMA SUVmax中位值高于FDG SUVmax中位值(13.07 vs. 7.62, P<0.001),见图5。
图 5.
Dual-tracer uptake patterns in different metastatic sites (A) and comparison of the maximum SUVmax of the two tracers (B)
转移性前列腺癌68Ga-PSMA和18F-FDG PET/CT双阳性组不同转移部位摄取情况(A)及两种示踪剂最大SUVmax比较(B)
2.2.3. Group A与Group B间临床病理学差异
Group A血清PSA中位值为4.8 ng/mL,Group B血清PSA中位值为0.34 ng/mL,两组间差异有统计学意义(P=0.013)。13例特殊病理类型均位于Group A中,但整体病理类型分布在两组间差异无统计学意义。两组在年龄、Gleason评分及既往治疗方式等之间差异均无统计学意义,具体见表2。
表 2. Relationship between the clinicopathologic characteristics of patients in Group A and Group B.
Group A与Group B组间临床病理学差异
| Parameter | Group A (n=33) | Group B (n=7) | P |
| All abbreviations are explained in the note to Table 1. | |||
| Hormonal status/case | 0.679 | ||
| mCRPC | 22 | 4 | |
| mHSPC | 11 | 3 | |
| Age/yr., median (P25-P75) | 68 (58-77) | 60 (57-71) | 0.249 |
| PSA level/(ng/mL), median (P25-P75) | 4.80 (1.02-100) | 0.34 (0.09-2.50) | 0.013 |
| Gleason score/case | 0.689 | ||
| 8+7 | 15 | 4 | |
| 9 | 18 | 3 | |
| Pathological classification/case | 0.074 | ||
| Typical | 20 | 7 | |
| Specific | 13 | 0 | |
| Previous therapy/case | >0.05 | ||
| Bicalutamide+NHA | 12 | 5 | |
| Others | 11 | 2 | |
2.3. 影响PSA-PFS预后分析
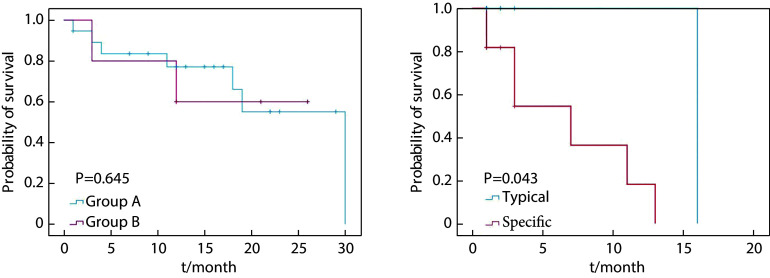
41例患者中,16例患者因为失随访而无法评价其临床结局,因此共 25 例患者进行短期预后评估。最终共 9 例 患者发生PSA进展,PSA中位值为104(4.83~670) ng/mL。16 例患者无PSA进展,PSA中位值为0.34(0.14~1.14) ng/mL,两组间PSA差异有统计学意义(P<0.001)。Group A中8例发生PSA进展,中位PSA-PFS为30个月,Group B中位PSA-PFS尚未达到(log-rank=0.212, P=0.645),即Group B这组人群仍有超过一半的个体尚未发生PSA进展。存在特殊病理类型患者PSA-PFS为7个月,短于经典型病理类型PSA-PFS的16个月,两组间生存曲线差异有统计学意义(log-rank=4.081, P=0.043)。Kaplan-Meier生存分析显示Gleason评分、检查时PSA、治疗方式均与PSA-PFS无关,具体见表3及图6。
表 3. Kaplan-Meier analysis of median PSA-PFS of patients with different clinicopathological features.
不同临床病理学特征PSA进展和未进展组间的Kaplan-Meier生存分析
| Clinicopathological feature | PSA progression (n=9)/case |
Non-PSA progression (n=16)/case |
Log- rank |
P |
| All abbreviations are explained in the note to Table 1. | ||||
| Dual-tracer uptake patterns | 0.212 | 0.645 | ||
| Group A | 8 | 12 | ||
| Group B | 1 | 4 | ||
| Hormonal status | 1.644 | 0.200 | ||
| mCRPC | 9 | 9 | ||
| mHSPC | 0 | 7 | ||
| PSA level/(ng/mL) | 3.305 | 0.069 | ||
| <10 | 4 | 14 | ||
| ≥10 | 5 | 2 | ||
| Gleason score | 0.048 | 0.826 | ||
| 8+7 | 2 | 8 | ||
| 9 | 7 | 8 | ||
| Pathological classification | 4.081 | 0.043 | ||
| Typical | 2 | 12 | ||
| Specific | 7 | 4 | ||
| Previous therapy | 2.003 | 0.157 | ||
| Bicalutamide+NHA | 3 | 13 | ||
| Others | 6 | 3 | ||
图 6.
PSA-PFS survival curves of patients with metastatic prostate cancers in different groups
不同组间的前列腺癌患者PSA-PFS的生存曲线
At the start of follow-up, there were 20 cases in group A, 5 in group B, 14 cases in the typical pathology type group, and 11 cases in the specific pathology type group. PSA-PFS is defined as the time from the start of a PET/CT examination to the PSA progression or death due to PCa. PSA progression is defined as an increase in PSA of at least 25% from baseline.
3. 讨论
部分mPCa由于分化程度差出现PSMA表达下调或消失,其PSMA受抑制程度与葡萄糖转运体1(glucose transporter 1, Glut1)的上调相关,而Glut1与mCRPC患者FDG摄取呈正相关[15-16]。此时18F-FDG PET/CT对于这部分患者可提供增益价值,了解病灶异质性。本研究中通过使用双示踪剂PET/CT(68Ga-PSMA和18F-FDG),回顾性分析评估mPCa病灶摄取模式,发现双阳性(至少一个病灶表现为PSMA+FDG+)摄取模式最为多见,且主要分布在mCRPC患者。骨骼、远处淋巴结和脏器转移灶中均有PSMA单阳性和双阳性摄取模式,但脏器转移中有约1/3的病灶表现为FDG单阳性,提示18F-FDG PET/CT检查对于mPCa脏器转移的优势,与既往研究结果符合[17]。CHEN等[12-13]研究显示对于存在FDG+PSMA-病灶的患者与PSA水平高且Gleason评分高相关,但因为本研究单纯FDG阳性的患者只有1例,所以合并到双显像剂阳性组,研究FDG阳性组和PSMA单阳性组与临床病理参数的关系,发现病灶FDG阳性的mPCa患者PSA水平更高,但未发现与Gleason评分的显著相关性,可能的原因是本研究中绝大部分为Gleason评分8~9分的患者,且样本量少,统计分析结果可能存在一定的选择偏倚。
前列腺导管内癌与神经内分泌分化是PCa的特殊病理类型,研究显示与疾病进展及不良预后相关[18-20]。很大一部分PCa患者经过多线治疗后,肿瘤病理类型也可发生转化,如神经内分泌分化,特别是一些高Gleason评分的患者[21]。相较于68Ga-PSMA PET,18F-FDG PET更易发现存在神经内分泌分化的病灶[22],在本研究中均表现出PSMA和FDG的双摄取,且更易出现PSA进展,治疗反应不佳。
靶向PSMA的RLT已证实有延长mCRPC患者预后的作用[7]。RLT重要治疗指征是肿瘤病灶有PSMA表达[8]。但是由于之前多线治疗和肿瘤生物学的异质性,肿瘤PSMA的表达可能会减少或丢失。有研究显示病灶FDG+PSMA-提示肿瘤更具侵袭性且预后不佳[23-25],且是mCRPC接受RLT治疗总生存期的阴性预测因子,因此有的RLT临床研究排除FDG阳性的患者[26-27]。本研究对患者进行短期预后评估,发现存在病灶FDG阳性的患者发生PSA进展的比例较FDG阴性的患者高,但可能因为随访时间短,样本量不足,尚未表现出差异有统计学意义。
本研究存在一定局限性:首先本研究是一个回顾性的探索性研究。由于样本量较少,FDG单阳性的患者只有1例,因此无法比较该类患者与PSMA阳性患者的临床病理特征。其次在进行PSA-PFS预后分析方面分析的影响因素较少,未加入一些额外因素如分子病理学信息,预后随访时间也较短,仅得到特殊病理类型影响PSA-PFS预后,尚未发现FDG阳性组患者预后与PSMA单阳组区别等其他影响因素,以致无法进行后续多因素分析。此外对于18F-FDG及68Ga-PSMA阳性病灶的判断标准目前无统一标准,结果判读存在偏倚。因此后续需优化评价标准,扩大样本量,搜集更全面的临床及病理信息,进行前瞻性研究。
总之,mPCa多表现为68Ga-PSMA和18F-FDG双示踪剂摄取,血清PSA水平是预测病灶FDG阳性的可靠指标。存在导管内癌及神经内分泌分化的PCa病灶易表现为FDG阳性且易出现PSA进展。
* * *
作者贡献声明 代洪媛负责正式分析、调查研究、研究方法、初稿写作和审读与编辑写作,黄淑辉负责经费获取、调查研究、研究方法和初稿写作,田甜负责调查研究和初稿写作,侯乃峰负责调查研究,曾浩和魏强负责提供资源和监督指导,黄蕤负责论文构思、数据审编、正式分析、研究项目管理、监督指导、初稿写作和审读与编辑写作。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿,并同意对工作的所有方面负责。
Author Contribution DAI Hongyuan is responsible for formal analysis, investigation, methodology, writing--original draft, and writing--review and editing. HUANG Shuhui is responsible for funding acquisition, investigation, methodology, and writing--original draft. TIAN Tian is responsible for investigation and writing--original draft. HOU Naifeng is responsible for investigation. ZENG Hao and WEI Qiang are responsible for resources and supervision. HUANG Rui is responsible for conceptualization, data curation, formal analysis, project administration, supervision, writing--original draft, and writing--review and editing.All authors consented to the submission of the article to the Journal. All authors approved the final version to be published and agreed to take responsibility for all aspects of the work.
利益冲突 本文作者魏强是本刊编委会编委。该文在编辑评审过程中所有流程严格按照期刊政策进行,且未经其本人经手处理。除此之外,所有作者声明不存在利益冲突。
Declaration of Conflicting Interests WEI Qiang is a member of the Editorial Board of the journal. All processes involved in the editing and reviewing of this article were carried out in strict compliance with the journal's policies and there was no inappropriate personal involvement by the author. Other than this, all authors declare no competing interests.
Funding Statement
四川省科学技术厅项目(No. 24ZDYF0154)资助
Contributor Information
洪媛 代 (Hongyuan DAI), Email: 1975596497@qq.com.
蕤 黄 (Rui HUANG), Email: huang_rui@scu.edu.cn.
References
- 1.郑荣寿, 陈茹, 韩冰峰, 等 2022年中国恶性肿瘤流行情况分析. 中华肿瘤杂志. 2024;46(3):221–231. doi: 10.3760/cma.j.cn112152-20240119-00035. [DOI] [Google Scholar]; ZHENG R S, CHEN R, HAN B F, et al Cancer incidence and mortality in China, 2022. Chin J Oncol. 2024;46(3):221–231. doi: 10.3760/cma.j.cn112152-20240119-00035. [DOI] [PubMed] [Google Scholar]
- 2.BOORJIAN S A, EASTHAM J A, GRAEFEN M, et al A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol. 2012;61(4):664–675. doi: 10.1016/j.eururo.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 3.FAROLFI A, CALDERONI L, MATTANA F, et al Current and emerging clinical applications of PSMA PET diagnostic imaging for prostate cancer. J Nucl Med. 2021;62(5):596–604. doi: 10.2967/jnumed.120.257238. [DOI] [PubMed] [Google Scholar]
- 4.HAN S, WOO S, KIM Y J, et al Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74(2):179–190. doi: 10.1016/j.eururo.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 5.FENDLER W P, CALAIS J, EIBER M, et al Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856–863. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WEBER M, FENDLER W P, RAVI KUMAR A S, et al Prostate-specific membrane antigen positron emission tomography-detected disease extent and overall survival of patients with high-risk nonmetastatic castration-resistant prostate cancer: an international multicenter retrospective study. Eur Urol. 2024;85(6):511–516. doi: 10.1016/j.eururo.2024.01.019. [DOI] [PubMed] [Google Scholar]
- 7.KIM Y J, KIM Y I Therapeutic responses and survival effects of 177Lu-PSMA-617 radioligand therapy in metastatic castrate-resistant prostate cancer: a meta-analysis. Clin Nucl Med. 2018;43(10):728–734. doi: 10.1097/rlu.0000000000002210. [DOI] [PubMed] [Google Scholar]
- 8.KRATOCHWIL C, FENDLER W P, EIBER M, et al EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT) Eur J Nucl Med Mol Imaging. 2019;46(12):2536–2544. doi: 10.1007/s00259-019-04485-3. [DOI] [PubMed] [Google Scholar]
- 9.HOFMAN M S, VIOLET J, HICKS R J, et al [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–833. doi: 10.1016/s1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 10.ALIPOUR R, AZAD A, HOFMAN M S. Guiding management of therapy in prostate cancer: time to switch from conventional imaging to PSMA PET? Ther Adv Med Oncol, 2019, 11: 1758835919876828.
- 11.PEREZ P M, HOPE T A, BEHR S C, et al Intertumoral heterogeneity of 18F-FDG and 68Ga-PSMA uptake in prostate cancer pulmonary metastases. Clin Nucl Med. 2019;44(1):e28–e32. doi: 10.1097/rlu.0000000000002367. [DOI] [PubMed] [Google Scholar]
- 12.CHEN R, WANG Y, ZHU Y, et al The added value of 18F-FDG PET/CT compared with 68Ga-PSMA PET/CT in patients with castration-resistant prostate cancer. J Nucl Med. 2022;63(1):69–75. doi: 10.2967/jnumed.120.262250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CHEN R, WANG Y, SHI Y, et al Diagnostic value of 18F-FDG PET/CT in patients with biochemical recurrent prostate cancer and negative 68Ga-PSMA PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(9):2970–2977. doi: 10.1007/s00259-021-05221-6. [DOI] [PubMed] [Google Scholar]
- 14.SWIHA M, PAPA N, SABAHI Z, et al. Development of a visually calculated SUV(mean) (HIT Score) on screening PSMA PET/CT to predict treatment response to 177Lu-PSMA therapy: comparison with quantitative SUV(mean) and patient outcomes. J Nucl Med, 2024, 65(6): 904-908.
- 15.BAKHT M K, LOVNICKI J M, TUBMAN J, et al Differential expression of glucose transporters and hexokinases in prostate cancer with a neuroendocrine gene signature: a mechanistic perspective for 18F-FDG imaging of PSMA-suppressed tumors. J Nucl Med. 2020;61(6):904–910. doi: 10.2967/jnumed.119.231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WEBER M, HADASCHIK B, FERDINANDUS J, et al Prostate-specific membrane antigen-based imaging of castration-resistant prostate cancer. Eur Urol Focus. 2021;7(2):279–287. doi: 10.1016/j.euf.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 17.JADVAR H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging, 2013, 40(Suppl 1): S5-S10.
- 18.MIURA N, MORI K, MOSTAFAEI H, et al The prognostic impact of intraductal carcinoma of the prostate: a systematic review and meta-analysis. J Urol. 2020;204(5):909–917. doi: 10.1097/ju.0000000000001290. [DOI] [PubMed] [Google Scholar]
- 19.HU C D, CHOO R, HUANG J Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol. 2015;5:90. doi: 10.3389/fonc.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WASINGER G, CUSSENOT O, COMPÉRAT E. Clinical management of intraductal carcinoma of the prostate. Cancers (Basel), 2024, 16(9): 1650.
- 21.WANG H T, YAO Y H, LI B G, et al Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol. 2014;32(30):3383–3390. doi: 10.1200/jco.2013.54.3553. [DOI] [PubMed] [Google Scholar]
- 22.PARIDA G K, TRIPATHY S, DATTA GUPTA S, et al Adenocarcinoma prostate with neuroendocrine differentiation: potential utility of 18F-FDG PET/CT and 68Ga-DOTANOC PET/CT over 68Ga-PSMA PET/CT. Clin Nucl Med. 2018;43(4):248–249. doi: 10.1097/rlu.0000000000002013. [DOI] [PubMed] [Google Scholar]
- 23.THANG S P, VIOLET J, SANDHU S, et al Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for 177Lu-labelled PSMA radioligand therapy. Eur Urol Oncol. 2019;2(6):670–676. doi: 10.1016/j.euo.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 24.PAN J, ZHANG T, CHEN S, et al. Nomogram to predict the presence of PSMA-negative but FDG-positive lesion in castration-resistant prostate cancer: a multicenter cohort study. Ther Adv Med Oncol, 2024, 16: 17588359231220506.
- 25.LAVALLÉE E, BERGERON M, BUTEAU F A, et al Increased prostate cancer glucose metabolism detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in localised Gleason 8-10 prostate cancers identifies very high-risk patients for early recurrence and resistance to castration. Eur Urol Focus. 2019;5(6):998–1006. doi: 10.1016/j.euf.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 26.JADVAR H The VISION forward: recognition and implication of PSMA-/18F-FDG+ mCRPC. J Nucl Med. 2022;63(6):812–815. doi: 10.2967/jnumed.121.263274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MICHALSKI K, RUF J, GOETZ C, et al Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy. Eur J Nucl Med Mol Imaging. 2021;48(6):2024–2030. doi: 10.1007/s00259-020-05160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]