Abstract
目的
探究结肠癌患者错配修复蛋白MutS同系物(MutS homolog, MSH)2、MSH6、绒毛蛋白(villin)表达及与病理特征的关系。
方法
选取我院2017年1月–2021年9月经病理确诊的310例结肠癌患者,采用免疫组化法检测MSH2、MSH6和villin表达,分析MSH2、MSH6、villin表达与结肠癌患者临床病理特征的相关性。应用多因素logistic回归分析MSH2、MSH6、villin表达与结肠癌临床病理参数的相关性,用Kaplan-Meier生存曲线比较不同蛋白表达情况的结肠癌患者2年生存率。
结果
患者癌组织中的MSH2、MSH6和villin蛋白阴性表达率分别为8.71%(27/310)、9.35%(29/310)和46.13%(143/310);癌旁组织中的3种蛋白阴性表达率分别为3.23%(10/310)、4.19%(13/310)和9.68%(30/310),3种蛋白在癌组织中的阴性率均高于癌旁组织(P<0.05)。回归分析显示:癌组织中MSH2及MSH6的表达与结肠癌患者年龄、肿瘤病灶位置、肿瘤分化程度和淋巴结转移相关(P<0.05);癌组织中villin表达与结肠癌患者肿瘤浸润深度、淋巴结转移、远处转移、临床分期相关(P<0.05)。MSH2、MSH6阴性表达患者2年生存率分别为51.85%、44.83%,低于MSH2、MSH6阳性表达的患者(79.51%、80.43%,P<0.05);癌组织中villin阴性表达的患者2年生存率为67.83%,低于villin阳性表达患者(85.03%,P<0.05)。癌组织中的MSH2、MSH6、villin均阴性表达(简称“三阴表达”)的患者有13例(4.1%),其2年生存率为30.77%(4/13),低于不符合三阴表达的结肠癌患者〔79.12%(235/297),P<0.05〕。
结论
MSH2、MSH6、villin表达与结肠癌患者临床病理特征相关,检测这3种蛋白表达可能有利于辅助临床进行结肠癌诊治及预后评估。
Keywords: 结肠癌, 错配修复蛋白MutS同系物2, 错配修复蛋白MutS同系物6, 绒毛蛋白, 病理特征
Abstract
Objective
To examine the relationship between the expressions of mismatch repair proteins, MutS homolog 2 (MSH2) and MutS homolog 6 (MSH6), and villin and the pathological features in patients with colon cancer.
Methods
A total of 310 cases of colon cancer patients who were treated at our hospital between January 2017 and September 2021 were selected. The diagnosis of colon cancer of all patients was verified by pathological evaluation. Immunohistochemistry was used to determine the protein expressions of MSH2, MSH6, and villin. The correlation between the expressions of MSH2, MSH6, and villin and the clinicopathological parameters in patients with colon cancer was analyzed accordingly. Multivariate logistic regression was used to analyze the correlation between the expressions of MSH2, MSH6, and villin and the clinicopathological parameters of colon cancer. Kaplan-Meier survival curve was used to compare the 2-year survival rates of colon cancer patients with different expression levels of the proteins.
Results
Among the 310 patients with colon cancer, the negative expression rates of MSH2, MSH6, and villin proteins in cancer tissues were 8.71% (27/310), 9.35% (29/310), and 46.13% (143/310), respectively. The negative expression rates of the three proteins in tissues adjacent to cancer were 3.23% (10/310), 4.19% (13/310), and 9.68% (30/310), respectively. The negative expression rates of the three proteins in cancer tissues were all higher than those in adjacent tissues (P<0.05). Regression analysis showed that the expression of MSH2 and MSH6 in cancer tissues was correlated with the age, the location of tumor lesions, tumor differentiation degree, and lymph node metastasis in colon cancer patients (P<0.05). The expression of villin in the cancer tissue is correlated with the depth of tumor infiltration, lymph node metastasis, distant metastasis, and clinical staging status in colon cancer patients (P<0.05). The 2-year survival rates of patients with negative expressions of MSH2 and MSH6 were 51.85% and 44.83%, respectively, which were lower than those of patients with positive expression of MSH2 and MSH6 (79.51% and 80.43%, P<0.05). Thirteen patients (4.1%) had negative expression of MSH2, MSH6, and villin (referred to as "triple negative expressions") in the cancer tissues, and their 2-year survival rate was 30.77%, which was lower than that of colon cancer patients who did not meet the criteria for triple negative expressions (79.12% [235/297], P<0.05).
Conclusion
The expressions of MSH2, MSH6, and villin are closely correlated with the pathological features of colon cancer patients. Evaluating the expression of the three proteins may assist in the clinical diagnosis, treatment, and prognosis evaluation of colon cancer.
Keywords: Colon cancer, Mismatch repair protein MutS homolog 2, Mismatch repair protein MutS homolog 6, Villin, Pathological characteristics
结肠癌的疾病进展、预后与相关基因及其表达产物密切相关,DNA错配修复缺陷导致的微卫星不稳定性在结肠癌诊治及预后评估方面有重要提示意义,错配修复缺陷蛋白具有维持人基因组稳定性和降低自身突变的作用,MutS同系物2(MutS homolog 2, MSH2)及MutS同系物6(MutS homolog 6, MSH6)是关键错配修复蛋白,MSH2、MSH6可识别并修复DNA在复制中的错配现象,若二者阴性表达会造成肿瘤基因组微卫星不稳定[1-2]。绒毛蛋白(villin)属于钙调节的肌动蛋白结合蛋白,表达于正常成人的小肠黏膜、胰管、肝胆管、胆囊上皮等组织中,其被认为是重要的肠道分化标志物之一[3]。目前,错配修复蛋白和villin表达在结肠癌风险评估、诊断和预后评估中的价值已多有报道,错配修复蛋白与约85%遗传性非息肉病性结直肠癌存在明显联系,证实了villin参与结直肠腺癌的发生发展过程,但MSH2、MSH6、 villin与临床病理特征的相关性仍存在争议[4-6]。基于此,本研究通过检测行外科手术治疗结肠癌患者的MSH2、MSH6和villin表达情况,分析上述蛋白与结肠癌患者病理特征的关系,以期为结肠癌临床诊治提供参考依据。
1. 资料与方法
1.1. 研究对象
选择2017年1月–2021年9月在我院诊治的结肠癌患者310例作为研究对象。纳入标准:①按诊断标准[7],经病理切片确诊为原发性结肠癌;②均接受结肠癌根治术治疗且术后进行免疫组化检测MSH2、MSH6及villin表达;③病例资料详细、病理记录完整;④患者及家属均知情并签署同意书。排除标准:①结肠恶性间质瘤及淋巴癌;②家族性腺瘤型息肉病;③合并其他恶性肿瘤;④术前已行化疗、放疗或其他免疫抑制剂治疗;⑤伴有精神认知障碍;⑥随访资料缺失。本研究经院伦理委员会批准(批准号HNYZLLWYH-2021-013)。收集入组患者年龄、病理分型、组织学类型、病灶大小、部位、分化程度、浸润深度、转移情况及临床分期等。
1.2. 免疫组化染色检测MSH2、MSH6和villin表达
结肠癌根治术后,取入组患者结肠癌组织及距癌组织边缘2 cm的癌旁组织,于−80 ℃冷冻保存待检。将标本固定、脱水、石蜡包埋后切片,厚度约4 μm,经脱蜡、抗原修复后,脱蜡水化,冲洗、浸泡,消除内源性过氧化酶活性,室温孵育。一抗为来源于北京艾柏森公司的兔抗人MSH2、MSH6和villin单克隆抗体,稀释浓度1∶100。4 ℃冰箱保存过夜之后冲洗,滴加二抗并孵育,再次冲洗、显色、苏木精复染、脱水、封片后,以PBS为阴性对照,在400高倍显微镜下观察切片,每张随机选取5个视野。
MSH2、MSH6以细胞核内有棕黄色颗粒为阳性,细胞核内无着色为阴性;villin以细胞浆内有棕黄色着色为阳性,以细胞浆内无着色为阴性。以每个视野下的染色情况和阳性细胞占比进行评价,染色从无色、浅黄色到棕黄/褐色,分别计0分、1分、2分;阳性细胞从1%~25%、26%~50%、51%~75%、>75%,分别计1分、2分、3分、4分。检测结果=染色评分×阳性细胞占比评分,总分0~2分为阴性表达,≥3分为阳性表达[8]。结果均由经验丰富的2名病理科医师行双盲法阅片。计算MSH2、MSH6和villin的阴性表达率。
1.3. 随访患者生存情况
入组患者术后均进行2年门诊或电话形式随访,前6个月每月随访1次,之后为每3个月随访1次,统计患者生存率及总生存期。总生存期为从随机分组开始到患者死亡时间。
1.4. 统计学方法
采用SPSS 24.0整合分析资料数据,计数资料蛋白缺失率、阳性率以例数(%)表示,行χ2检验;应用多因素logistic回归分析MSH2、MSH6、villin表达与结肠癌临床病理参数的相关性,α纳入=0.05,α排除=0.10;用Kaplan-Meier法绘制结肠癌患者生存曲线,行log rank χ2检验比较不同蛋白表达情况的结肠癌患者2年生存率。P<0.05为差异有统计学意义。
2. 结果
2.1. MSH2、MSH6和villin在结肠癌中的表达
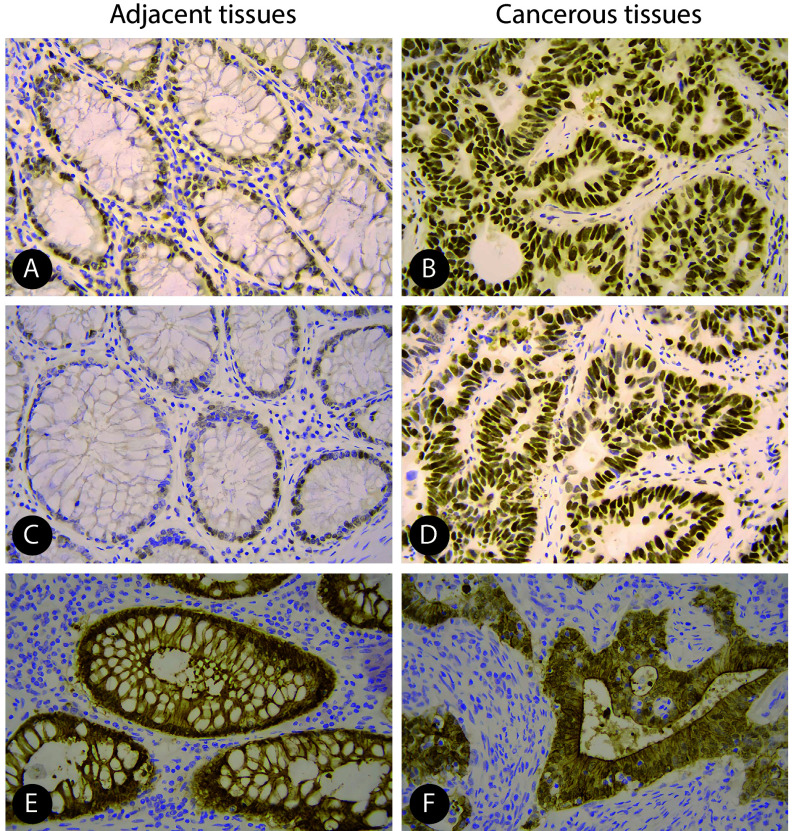
310例结肠癌患者,癌组织中的MSH2、MSH6和villin阴性表达率分别为8.71%(27/310)、9.35%(29/310)和46.13%(143/310);癌旁组织中的MSH2、MSH6和Vllin阴性表达率分别为3.23%(10/310)、4.19%(13/310)和9.68%(30/310)。MSH2、MSH6和villin在癌组织中的阴性率均高于癌旁组织(χ2=8.307、6.538、102.375,P<0.05),见图1。
图 1.
Staining results for MSH2 (A and B), MSH6 (C and D), and villin (E and F) protein expression in the tissues adjacent to a tumor (A, C, and E) and the cancerous tissues (B, D, and F) of colon cancer patients (original magnification ×400)
结肠癌患者癌旁组织(A、C、E)及癌组织(B、D、F)MSH2(A、B)、MSH6(C、D)、villin(E、F)表达的免疫组化染色(×400)
2.2. 癌组织中MSH2、MSH6、villin表达与结肠癌临床病理参数的关系
相较于年龄>60岁、肿瘤病灶位于左半结肠、高分化、无淋巴结转移的结肠癌患者,年龄≤60岁、肿瘤病灶位于右半结肠、中低分化、淋巴结转移患者MSH2及MSH6阴性表达率较高(P<0.05);相较于浸润深度在T1~ T2、淋巴结无转移、远处无转移及临床Ⅰ期患者,浸润深度在T3~ T4、淋巴结转移、远处转移及临床Ⅱ~ Ⅲ期的结肠癌患者villin阴性表达率较高(P<0.05),见表1。
表 1. Relationship between the negative expression of MSH2, MSH6, and villin proteins and clinical pathological parameters of colon cancer.
癌组织中MSH2、MSH6、villin蛋白阴性表达与结肠癌临床病理参数的关系
| Clinical pathological parameter | n | MSH2/case (%) | χ 2 | P | MSH6/case (%) | χ 2 | P | Villin/case (%) | χ 2 | P |
| Age | 5.009 | 0.025 | 4.578 | 0.032 | 2.155 | 0.142 | ||||
| ≤60 yr. | 166 | 20 (12.05) | 21 (12.65) | 83 (50.00) | ||||||
| >60 yr. | 144 | 7 (4.86) | 8 (5.56) | 60 (41.67) | ||||||
| Pathological type | 0.229 | 0.632 | 0.366 | 0.545 | 2.153 | 0.142 | ||||
| Ulceration | 208 | 17 (8.17) | 18 (8.65) | 102 (49.04) | ||||||
| Protuberance | 102 | 10 (9.80) | 11 (10.78) | 41 (40.20) | ||||||
| Histological type | 0.233 | 0.629 | 0.857 | 0.355 | 0.461 | 0.497 | ||||
| Mucinous adenocarcinoma | 273 | 23 (8.42) | 24 (8.79) | 124 (45.42) | ||||||
| Non-mucinous adenocarcinoma | 37 | 4 (10.81) | 5 (13.51) | 19 (51.35) | ||||||
| Diameter of the lesion | 0.816 | 0.366 | 0.335 | 0.563 | 0.548 | 0.459 | ||||
| ≤5 cm | 197 | 15 (7.61) | 17 (8.63) | 94 (47.72) | ||||||
| >5 cm | 113 | 12 (10.62) | 12 (10.62) | 49 (43.36) | ||||||
| Focal site | 5.629 | 0.018 | 5.059 | 0.024 | 0.270 | 0.603 | ||||
| Left hemicolon | 136 | 6 (4.41) | 7 (5.15) | 65 (47.79) | ||||||
| Right hemicolon | 174 | 21 (12.07) | 22 (12.64) | 78 (44.83) | ||||||
| Degree of differentiation | 5.514 | 0.019 | 6.288 | 0.012 | 0.977 | 0.323 | ||||
| Well-differentiated | 82 | 2 (2.44) | 2 (2.44) | 34 (41.46) | ||||||
| Medium and low levels of differentiation | 228 | 25 (10.96) | 27 (11.84) | 109 (47.81) | ||||||
| Infiltration depth | 0.268 | 0.605 | 0.031 | 0.861 | 16.300 | <0.001 | ||||
| T1-T2 | 79 | 8 (10.13) | 7 (8.86) | 21 (26.58) | ||||||
| T3-T4 | 231 | 19 (8.23) | 22 (9.52) | 122 (52.81) | ||||||
| Lymphatic metastasis | 5.025 | 0.025 | 4.970 | 0.026 | 7.786 | 0.005 | ||||
| No | 178 | 10 (5.62) | 11 (6.18) | 70 (39.33) | ||||||
| Yes | 132 | 17 (12.88) | 18 (13.64) | 73 (55.30) | ||||||
| Distant metastasis | 0.379 | 0.528 | 0.155 | 0.694 | 4.421 | 0.035 | ||||
| No | 244 | 20 (8.20) | 22 (9.02) | 105 (43.03) | ||||||
| Yes | 66 | 7 (10.61) | 7 (10.61) | 38 (57.58) | ||||||
| Pathological stage | 3.321 | 0.190 | 3.310 | 0.191 | 18.551 | <0.001 | ||||
| Ⅰ | 36 | 3 (8.33) | 3 (8.33) | 5 (13.89) | ||||||
| Ⅱ | 151 | 9 (5.96) | 10 (6.62) | 71 (47.02) | ||||||
| Ⅲ | 123 | 15 (12.20) | 16 (13.01) | 67 (54.47) |
2.3. 多因素logistic回归分析癌组织中MSH2、MSH6、villin表达与结肠癌临床病理参数的相关性
将单因素分析中有统计学意义的指标引入多因素logistic回归模型,结果显示:MSH2及MSH6的阴性表达与结肠癌患者年龄、肿瘤病灶位置、肿瘤分化程度和淋巴结转移密切相关(P<0.05);villin阴性表达与结肠癌患者肿瘤浸润深度、淋巴结转移、远处转移、临床分期密切相关(P<0.05),见表2。
表 2. Multivariate logistic regression analysis of the correlation between MSH2, MSH6, and villin expression and clinical pathological parameters of colon cancer.
多因素logistic回归分析癌组织中MSH2、MSH6、villin表达与结肠癌临床病理参数的相关性
| Variable | β | SE | Wald χ2 | OR | 95% CI | P |
| β: partial regression coefficient; SE: standard error; OR: odds ratio; CI: confidence interval. | ||||||
| Negative expression of MSH2 | ||||||
| Age≤60 yr. | 1.020 | 0.487 | 4.387 | 2.773 | 1.068-7.203 | 0.037 |
| Tumor lesion located in the right colon | 1.043 | 0.416 | 6.286 | 2.838 | 1.256-6.413 | 0.013 |
| Moderate to low differentiation | 1.486 | 0.518 | 8.230 | 4.419 | 1.601-12.198 | 0.004 |
| Lymph node metastasis | 0.893 | 0.341 | 6.858 | 2.442 | 1.252-4.765 | 0.009 |
| Negative expression of MSH6 | ||||||
| Age≤60 yr. | 0.935 | 0.406 | 5.304 | 2.547 | 1.149-5.645 | 0.022 |
| Tumor lesion located in the right colon | 0.933 | 0.379 | 6.060 | 2.542 | 1.209-5.343 | 0.014 |
| Moderate to low differentiation | 1.563 | 0.475 | 10.828 | 4.773 | 1.881-12.110 | 0.001 |
| Lymph node metastasis | 0.542 | 0.246 | 4.854 | 1.719 | 1.062-2.785 | 0.028 |
| Negative expression of villin | ||||||
| Infiltration depth is between T3-T4 | 0.667 | 0.285 | 5.477 | 1.948 | 1.114-3.406 | 0.019 |
| Lymph node metastasis | 0.641 | 0.234 | 7.504 | 1.898 | 1.200-3.003 | 0.006 |
| Transfer appears in the distance | 0.415 | 0.168 | 6.102 | 1.514 | 1.090-2.105 | 0.014 |
| Clinical stages Ⅱ-Ⅲ | 1.739 | 0.543 | 10.257 | 5.692 | 1.963-16.499 | 0.001 |
2.4. 癌组织中不同MSH2、MSH6、villin表达的结肠癌患者生存情况
整体上,患者2年生存率为77.10%(239/310)。癌组织MSH2阴性表达者的2年生存率为51.85%(14/27),低于MSH2表达阳性者〔79.51%(225/283)〕(log rank χ2=11.769,P<0.05);MSH6阴性表达者的2年生存率为44.83%(13/29),低于MSH6表达阳性者〔80.43%(226/281)〕(log rank χ2=16.976,P<0.05);villin阴性表达的患者2年生存率为67.83%(97/143),低于villin阳性表达者〔85.03%(142/167)〕(log rank χ2=4.718,P<0.05),癌组织MSH2、MSH6、villin均阴性表达患者的2年生存率为30.77%(4/13),低于其他结肠癌患者(不符合癌组织MSH2、MSH6、villin均阴性表达)的79.12%(235/297)(log rank χ2=17.547,P<0.05),见图2。
图 2.
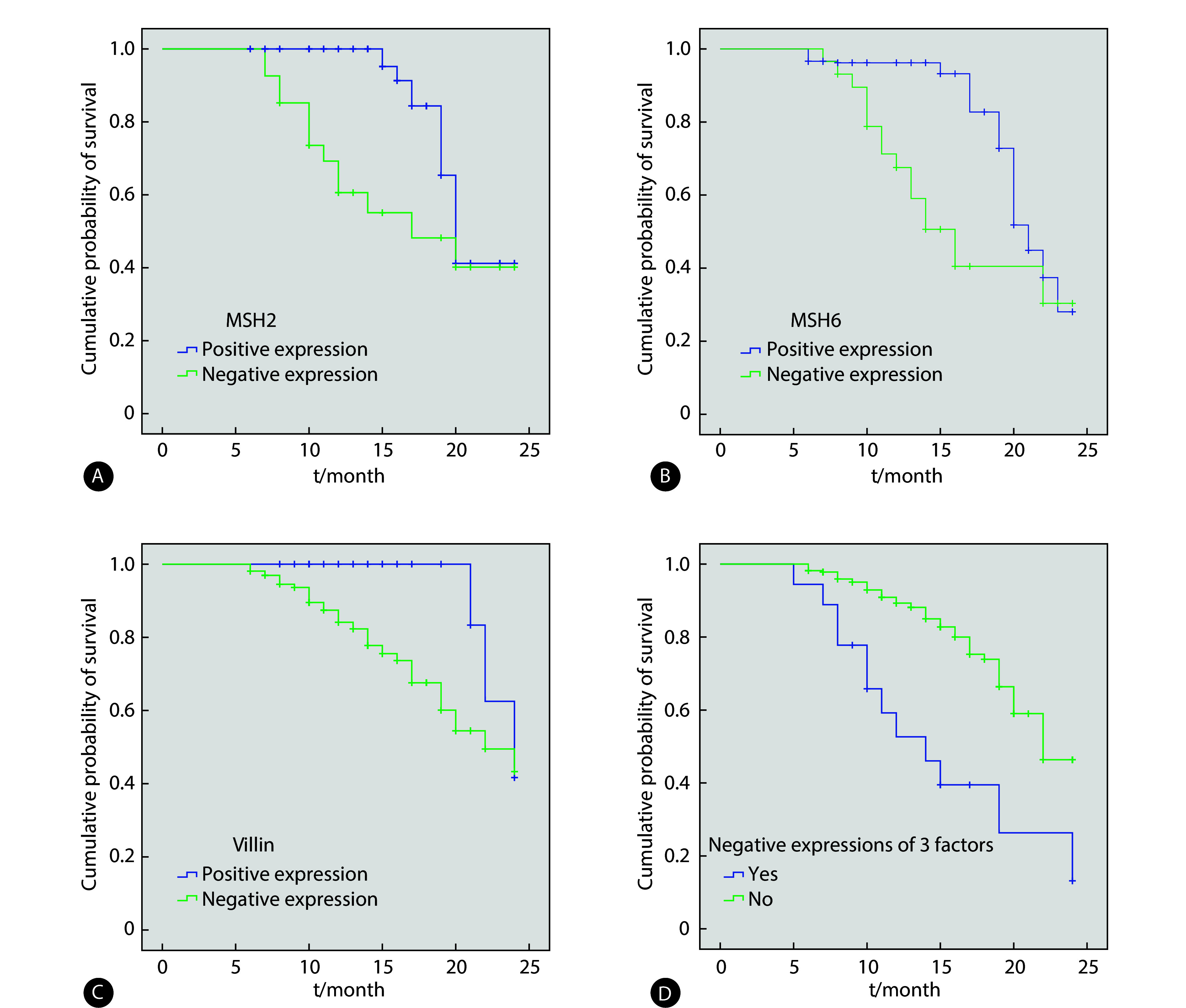
Different expressions of MSH2, MSH6, and villin proteins in cancer tissues affect Kaplan-Meier curve of overall survival of patients with colon cancer
癌组织中不同MSH2、MSH6和villin蛋白表达状态影响结肠癌患者总生存的Kaplan-Meier曲线
A: MSH2 expression; B: MSH6 expression; C: villin expression; D: combination of three factors (MSH2, MSH6, and villin).
3. 讨论
目前我国结肠癌发病率及病死率仍处于较高水平[9-10]。研究表明,年龄、肿瘤分期、淋巴结转移等影响结肠癌术后患者预后[11]。而对分子生物学深层次的研究,让临床认识到分子类型可能与肿瘤病理特征、肿瘤生物学行为有关[12]。故分析不同分子生物学分型的结肠癌患者病理特征,对临床防治结肠癌有重要意义。
目前,临床认为肿瘤微卫星不稳定性是癌症发病的分子生物学机制之一[13-14]。MSH2及MSH6是DNA错配修复过程的必需蛋白,二者失活会导致微卫星不稳定[15-16]。本研究310例结肠癌患者癌组织中的MSH2、MSH6阴性表达率分别为8.71%、9.35%,与马春涛等[17]结果类似。国外研究显示,肿瘤患者癌组织中错配修复蛋白家族成员整体阴性表达率约15%[18],与国内研究有一定差异,这可能与不同地区、种族人群遗传因素和生活方式存在差异有关[19]。本研究中癌组织MSH2、MSH6阴性率均高于癌旁组织,这与癌组织中错配修复蛋白缺失可造成结肠癌肿瘤细胞高度微型不稳定性的病理过程相符。谢仲鹏等[20]发现,癌组织中错配修复蛋白阴性与结肠癌患者年龄、肿瘤部位、肿瘤大小、分化程度、组织类型有关。潘思琼等[21]研究则表明,出现淋巴结转移的结肠癌患者癌组织中的MSH2及MSH6阴性率较高。本研究结论与以往研究类似,原因可能与癌组织中MSH2、MSH6的功能协同性有关,二者结合后形成二聚体复合物发挥识别并参与完成错配修复的功能,也与结肠癌分子生物学发病机制相一致。另外,本研究中,癌组织中MSH2、MSH6阴性患者2年生存率普遍较低,说明癌组织MSH2、MSH6表达与结肠癌患者预后有关,提示癌组织MSH2、MSH6可能为结肠癌预后评估指标。
villin能使肠黏膜上皮管腔表面的肌动蛋白聚集成微丝,具有捆绑、切割、戴帽和成核等功能[22-24]。该蛋白表达仅限于上皮性肿瘤而不表达于肉瘤、黑色素瘤、淋巴瘤等,具备组织特异性[25-27]。也有研究发现,villin蛋白在腺样分化的胃肠道癌、胆囊癌等癌组织呈异常表达[27-29],本研究结果与之类似,且显示浸润深度在T3~T4、淋巴结转移、远处转移及临床Ⅱ~Ⅲ期的结肠癌患者癌组织中villin阴性率较高,这说明癌组织villin阳性表达减少可能参与了结肠腺癌的浸润转移。本研究中,癌组织villin阴性者2年生存率低于villin阳性者,说明癌组织villin表达也与结肠癌患者生存期有关,提示临床可将癌组织中的villin作为结肠癌预后评估指标。另外,本研究癌组织中3种蛋白均阴性表达的患者2年生存率远低于非三者均阴性患者,说明在结肠组织从正常-腺瘤-腺癌的演变过程中,癌组织中MSH2、MSH6和villin的表达均呈逐渐下降趋势,虽然MSH2、MSH6与villin作用机制不同,但作用结果类似,联合检测三者表达可提高结肠癌预后评估准确性。
综上所述,结肠癌组织MSH2、MSH6、villin表达与部分病理特征具有相关性,检测癌组织中的这3种蛋白表达可能有利于辅助临床进行结肠癌诊治及预后评估。但由于本研究结论来自单一中心的小样本,未来仍需多中心大样本进行进一步验证。
* * *
作者贡献声明 常方方负责论文构思、正式分析和初稿写作,胡晓舒负责经费获取和提供资源,温一阳负责研究方法和提供资源,李平负责监督指导,皇甫赟、张凤娟、 谭静和曹雪霞负责调查研究。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿,并同意对工作的所有方面负责。
Author Contribution CHANG Fangfang is responsible for conceptualization, formal analysis, and writing--original draft. HU Xiaoshu is responsible for funding acquisition and resources. WEN Yiyang is responsible for methodology and resources. LI Ping is responsible for supervision. HUANGFU Yun, ZHANG Fengjuan, TAN Jing, and CAO Xuexia are responsible for investigation.All authors consented to the submission of the article to the Journal. All authors approved the final version to be published and agreed to take responsibility for all aspects of the work.
利益冲突 所有作者均声明不存在利益冲突
Declaration of Conflicting Interests All authors declare no competing interests.
Funding Statement
河南省医学科技攻关计划项目(No. LHGJ20220003)、河南省医学科技攻关计划联合共建项目(No. LHGJ20230717)和河南省重点研发与推广专项(科技攻关)研究项目(No. 212102310794)资助
References
- 1.姜钟翔, 黄丹阳, 姜小叶, 等 结肠癌中SLC11A1基因的表达及其临床意义. 重庆医科大学学报. 2022;47(1):105–112. doi: 10.13406/j.cnki.cyxb.002962. [DOI] [Google Scholar]; JIANG Z X, HUANG D Y, JIANG X Y, et al The expression and clinical significance of SLC11A1 in colon cancer. J Chongqing Med Univ. 2022;47(1):105–112. doi: 10.13406/j.cnki.cyxb.002962. [DOI] [Google Scholar]
- 2.刘申香, 徐永成, 严雪冰, 等 老年左右半结肠癌患者临床病理特征对预后的影响. 实用临床医药杂志. 2023;27(4):113–116. doi: 10.7619/jcmp.20223286. [DOI] [Google Scholar]; LIU S X, XU Y C, YAN X B, et al Influence of clinicopathological characteristics of elderly patients with left and right colon cancer on prognosis. J Clin Med Pract. 2023;27(4):113–116. doi: 10.7619/jcmp.20223286. [DOI] [Google Scholar]
- 3.BORHAN A, NOZARIAN Z, ABDOLLAHI A, et al Evaluation of the relationship between expression of villin and gelsolin genes and axillary lymph node metastasis in patients with breast cancer. Iran J Pathol. 2021;16(1):27–32. doi: 10.30699/ijp.2020.121532.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SALEM M E, BODOR J N, PUCCINI A, et al Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int J Cancer. 2020;147(10):2948–2956. doi: 10.1002/ijc.33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SALARI S, GHADYANI M, KARIMI M, et al Immunohistochemical expression pattern of MLH1, MSH2, MSH6, and PMS2 in tumor specimen of iranian gastric carcinoma patients. J Gastrointest Cancer. 2022;53(1):192–196. doi: 10.1007/s12029-020-00566-x. [DOI] [PubMed] [Google Scholar]
- 6.DETLEFSEN S, JAKOBSEN M, NIELSEN M F B, et al Expression of CD117, CK17, CK20, MUC4, villin and mismatch repair deficiency in pancreatic intraductal papillary mucinous neoplasm. Pathol Res Pract. 2021;217(1):153312. doi: 10.1016/j.prp.2020.153312. [DOI] [PubMed] [Google Scholar]
- 7.国家卫生计生委医政医管局, 中华医学会肿瘤学分会 中国结直肠癌诊疗规范(2017年版) 中华胃肠外科杂志. 2018;21(1):92–106. doi: 10.3760/cma.j.issn.0529-5815.2018.04.001. [DOI] [Google Scholar]; National Health and Family Planning Commission Medical Administration Bureau, Oncology Branch of the Chinese Medical Association Chinese diagnosis and treatment guidelines for colorectal cancer (2017 Edition) Chin J Gastrointest Surg. 2018;21(1):92–106. doi: 10.3760/cma.j.issn.0529-5815.2018.04.001. [DOI] [Google Scholar]
- 8.尚红, 王毓三, 申子瑜. 全国临床检验操作规程(第4版). 北京: 人民卫生出版社, 2015: 629-790.; SHANG H, WANG Y S, SHEN Z Y. National Clinical Laboratory Operating Procedures (4th edition). Beijing: People's Health Publishing House, 2015: 629-790.
- 9.聂慧芳, 孙百军, 吕艺 2011—2017年沈阳市城区居民结直肠癌发病和死亡的趋势分析. 肿瘤. 2022;42(7):499–507. doi: 10.3781/j.issn.1000-7431.2022.2106-0402. [DOI] [Google Scholar]; NIE H F, SUN B J, LYU Y Trend analysis of incidence and mortality of colorectal cancer in residents of Shenyang urban area from 2011 to 2017. Tumor. 2022;42(7):499–507. doi: 10.3781/j.issn.1000-7431.2022.2106-0402. [DOI] [Google Scholar]
- 10.杨欣, 卢癸凤, 胡文慧, 等. MiR-671-3p通过CKAP4抑制结肠癌细胞的增殖和迁移. 遵义医科大学学报, 2021, 44(5): 600−606.; YANG X, LU K F, HU W H, et al. MicroRNA-671-3p inhibits the proliferation and migration of colon cancerthrogh CKAP4. J Zunyi Med Univ, 2021, 44(5): 600−606.
- 11.黄庆, 邹旻红, 李旺林, 等 左右半结肠黏液腺癌术后患者生存特征分析: 一项基于SEER数据库的研究. 实用医学杂志. 2021;37(10):1351–1356. doi: 10.3969/j.issn.1006-5725.2021.10.024. [DOI] [Google Scholar]; HUANG Q, ZOU M H, LI W L, et al Survival differences of postoperative patients with left-side and right-side colonic mucinous adenocarcinoma: a study based on SEER database. J Clin Med Pract. 2021;37(10):1351–1356. doi: 10.3969/j.issn.1006-5725.2021.10.024. [DOI] [Google Scholar]
- 12.秦晓丹, 孙慧玲, 潘蓓, 等 miR-1229-3p抑制结直肠癌疾病进展及作为潜在生物标志物的研究. 诊断学理论与实践. 2023;22(5):429–440. doi: 10.16150/j.1671-2870.2023.05.003. [DOI] [Google Scholar]; QIN X D, SUN H L, PAN B, et al miR-1229-3p inhibits the malignant progression of colorectal cancer and serves as a potential biomarker. J Diagn Concepts Pract. 2023;22(5):429–440. doi: 10.16150/j.1671-2870.2023.05.003. [DOI] [Google Scholar]
- 13.蒋松松, 陈刚 微卫星不稳定性检测在结直肠外科的应用及其临床意义. 中国肿瘤外科杂志. 2022;14(5):498–503. doi: 10.3969/j.issn.1674-4136.2022.05.017. [DOI] [Google Scholar]; JIANG S S, CHEN G Application and clinical significance of microsatellite instability detection in colorectal surgery. Chin J Surg Oncol. 2022;14(5):498–503. doi: 10.3969/j.issn.1674-4136.2022.05.017. [DOI] [Google Scholar]
- 14.MAIO M, ASCIERTO P A, MANZYUK L, et al Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase Ⅱ KEYNOTE-158 study. Ann Oncol. 2022;33(9):929–938. doi: 10.1016/j.annonc.2022.05.519. [DOI] [PubMed] [Google Scholar]
- 15.HONG S, ZHANG J, LIU S, et al Protein profiles reveal MSH6/MSH2 as a potential biomarker for hepatocellular carcinoma with microvascular invasion. Hepatol Res. 2024;54(2):189–200. doi: 10.1111/hepr.13971. [DOI] [PubMed] [Google Scholar]
- 16.OAKNIN A, GILBERT L, TINKER A V, et al Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase Ⅰ, single-arm study. J Immunother Cancer. 2022;10(1):e003777. doi: 10.1136/jitc-2021-003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.马春涛, 许春芳, 张海玲 错配修复蛋白和Ki-67在结直肠癌中的表达及其临床意义. 胃肠病学. 2019;24(1):39–42. doi: 10.3969/j.issn.1008-7125.2019.01.009. [DOI] [Google Scholar]; MA C T, XU C F, ZHANG H L Expression and clinical significance of mismatch repair protein and Ki-67 in colorectal cancer. Chin J Gastroenterol. 2019;24(1):39–42. doi: 10.3969/j.issn.1008-7125.2019.01.009. [DOI] [Google Scholar]
- 18.CHALABI M, FANCHI L F, DIJKSTRA K K, et al Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 19.何杨, 周珏, 孙翔云, 等 结直肠癌组织错配修复蛋白表达与临床病理特征关系的随机森林分析. 中华肿瘤防治杂志. 2022;29(6):408–413. doi: 10.16073/j.cnki.cjcpt.2022.06.05. [DOI] [Google Scholar]; HE Y, ZHOU J, SUN X Y, et al Analysis of mismatch repair protein expression and clinicopathological characteristics in radical resection specimens of colorectal cancer based on random forest algorithm. Chin J Cancer Prev Treat. 2022;29(6):408–413. doi: 10.16073/j.cnki.cjcpt.2022.06.05. [DOI] [Google Scholar]
- 20.谢仲鹏, 李海荣, 吴余, 等 海南地区结直肠癌中错配修复蛋白及多药耐药蛋白的表达及意义. 临床与实验病理学杂志. 2020;36(10):1205–1208. doi: 10.13315/j.cnki.cjcep.2020.10.016. [DOI] [Google Scholar]; XIE Z P, LI H R, WU Y, et al Expression and significance of mismatch repair proteins and multidrug resistance proteins in colorectal cancer in Hainan region. JClin Exp Pathol. 2020;36(10):1205–1208. doi: 10.13315/j.cnki.cjcep.2020.10.016. [DOI] [Google Scholar]
- 21.潘思琼, 陆奉科, 李山 错配修复蛋白MLH1、MSH2、PMS2、MSH6及p53在结直肠癌中的表达及意义. 海南医学. 2021;32(1):4–7. doi: 10.3969/j.issn.1003-6350.2021.01.002. [DOI] [Google Scholar]; PAN S Q, LU F K, LI S Expression and significance of mismatch repair proteins MLH1, MSH2, MSH6, PMS2, and p53 in colorectalcancer. Hainan Med J. 2021;32(1):4–7. doi: 10.3969/j.issn.1003-6350.2021.01.002. [DOI] [Google Scholar]
- 22.张海梅, 张嘉刚, 易敏, 等 NF-κB、villin蛋白的表达与胃癌临床病理特征及患者预后的相关性. 贵州医药. 2022;46(1):13–16. doi: 10.3969/j.issn.1000-744X.2022.01.004. [DOI] [Google Scholar]; ZHANG H M, ZHANG J G, YI M, et al Correlation between expression of NF-κB and villin protein and clinicopathological characteristics of gastric cancer and prognosis of patients. Guizhou Med J. 2022;46(1):13–16. doi: 10.3969/j.issn.1000-744X.2022.01.004. [DOI] [Google Scholar]
- 23.STENVALL C A, TAYYAB M, GRÖNROOS T J, et al Targeted deletion of keratin 8 in intestinal epithelial cells disrupts tissue integrity and predisposes to tumorigenesis in the colon. Cell Mol Life Sci. 2021;79(1):10–13. doi: 10.1007/s00018-021-04081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HASANI S, YOUNG L, VAN N W, et al Inhibition of mitochondrial fission activates glycogen synthesis to support cell survival in colon cancer. Cell Death Dis. 2023;14(10):664–666. doi: 10.1038/s41419-023-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LI Z, ROCK J B, ROTH R, et al Dual stain with satb2 and ck20/villin is useful to distinguish colorectal carcinomas from other tumors. Am J Clin Pathol. 2018;149(3):241–246. doi: 10.1093/ajcp/aqx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PARK J W, SEO M J, CHO K S, et al Smad4 and p53 synergize in suppressing autochthonous intestinal cancer. Cancer Med. 2022;11(9):1925–1936. doi: 10.1002/cam4.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.李方, 刘赛娜, 齐长海, 等 CK7、CK20、Villin、CDX-2、MUC-2、PAX8在卵巢原发性黏液性肿瘤和继发性腹膜假黏液瘤中的表达及鉴别诊断意义. 诊断病理学杂志. 2023;30(7):643–647. doi: 10.3969/j.issn.1007-8096.2023.07.005. [DOI] [Google Scholar]; LI F, LIU S N, QI C H, et al Expression of CK7, CK20, Vilin, CDX-2, MUC-2 and PAX8 in primary ovarian mucinousneoplasms and secondary pseudomyxoma peritonei and their implications in differential diagnosis. J Diag Pathol. 2023;30(7):643–647. doi: 10.3969/j.issn.1007-8096.2023.07.005. [DOI] [Google Scholar]
- 28.DUM D, LENNARTZ M, MENZ A, et al Villin expression in human tumors: a tissue microarray study on 14, 398 tumors. Expert Rev Mol Diagn. 2022;22(6):665–675. doi: 10.1080/14737159.2022.2104122. [DOI] [PubMed] [Google Scholar]
- 29.ALTINTAS S, BAYRAK M, ALTINTAS Y Prognostic value of CDX2 and Villin expression in advanced stage colorectal carcinoma. J Coll Physicians Surg Pak. 2019;29(11):1057–1061. doi: 10.29271/jcpsp.2019.11.1057. [DOI] [PubMed] [Google Scholar]