Abstract
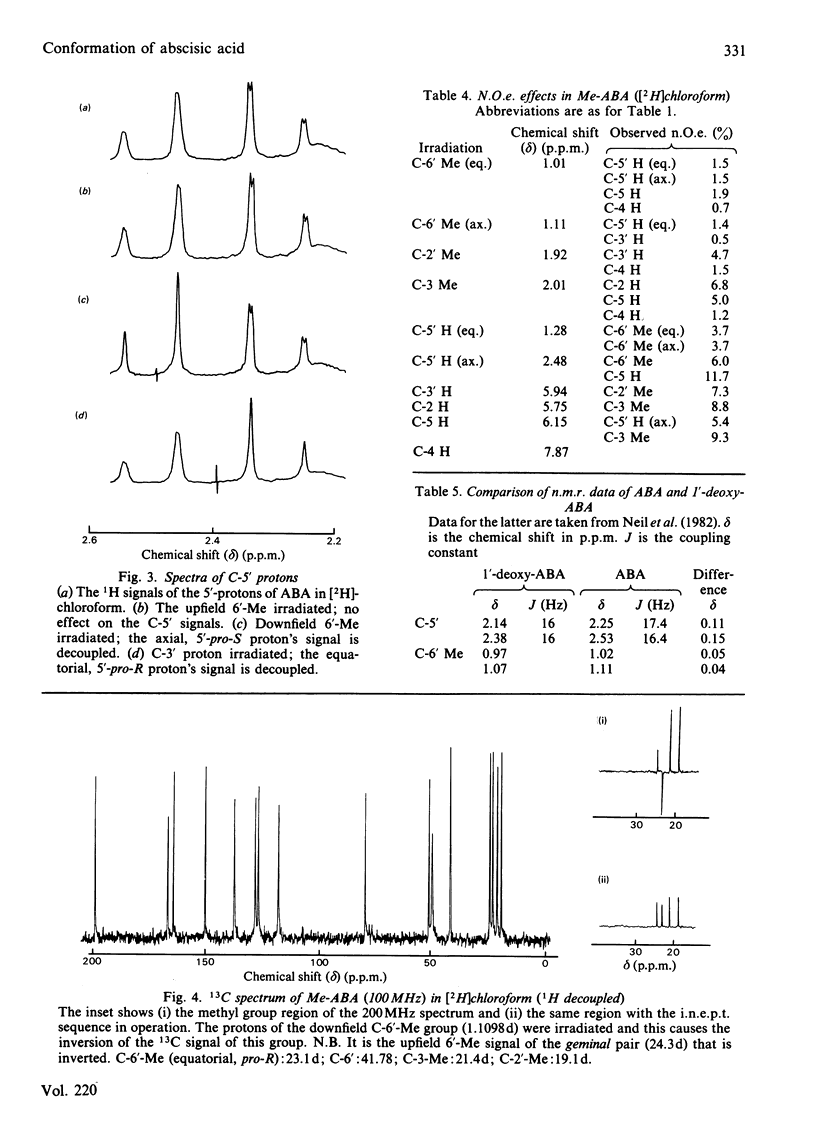
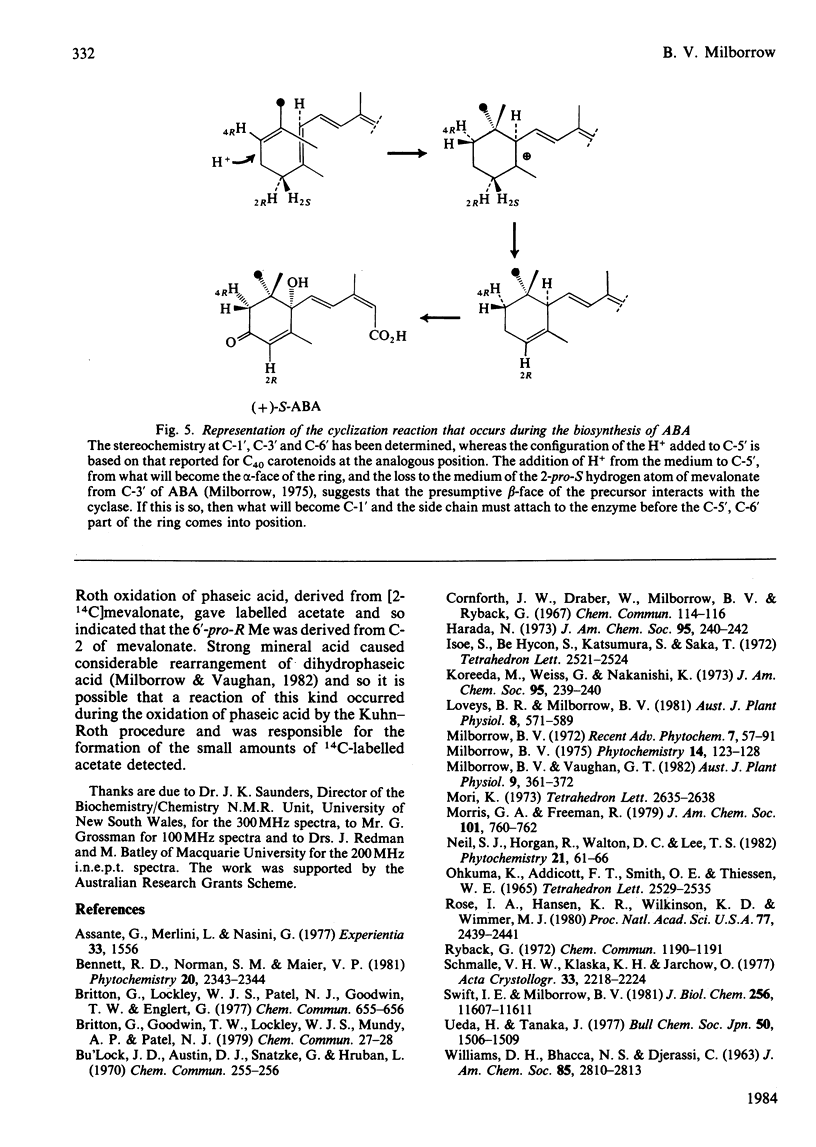
The n.m.r. spectrum of abscisic acid (ABA) formed from [1,2-13C2]acetate by the fungus Cercospora rosicola shows 13C-13C coupling between C-6' (41.7 p.p.m.; 36 Hz) and the downfield 6'-methyl group (6'-Me) (24.3 p.p.m, 36 Hz). This 6'-Me, therefore, is derived from C-3' of mevalonate [Bennett, Norman & Maier (1981) Phytochemistry 20, 2343-2344]. An i.n.e.p.t. (insensitive nuclei enhanced by polarization transfer) pulse sequence demonstrated that the downfield 13C signal is produced by the 6'-Me that gives rise to the upfield 1H 6'-Me signal (23.1 d). The absolute configuration of this, the equatorial 6'-Me group, was determined as 6'-pro-R by decoupling and n.O.e. (nuclear-Overhauser-enhancement) experiments at 300 MHz using ABA, ABA in which the axial 6'-pro-S 5'-hydrogen atom had been exchanged with 2H in NaO2H and the 1',4'-cis- and 1',4'-trans-diols formed from these samples. The configuration at C-1' and at C-6' are now compatible with a chair-folded intermediate during cyclization, as proposed for beta- and epsilon-rings of carotenoids. ABA in solution exists, as in the crystalline form, with the ring in a pseudo-chair conformation. The side chain is axial and the C-3 Me and the C-5 hydrogen atoms are predominantly cis(Z).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Rose I. A., Hanson K. R., Wilkinson K. D., Wimmer M. J. A suggestion for naming faces of ring compounds. Proc Natl Acad Sci U S A. 1980 May;77(5):2439–2441. doi: 10.1073/pnas.77.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift I. E., Milborrow B. V. Stereochemistry of beta-, gamma-, and epsilon-ring formation in bacterial C50. J Biol Chem. 1981 Nov 25;256(22):11607–11611. [PubMed] [Google Scholar]


