Abstract
Electronic exchange of health care data demands code/terminology systems. In the Scandinavian countries, the IFCC-IUPAC’s Nomenclature for Properties and Units (NPU) terminology is used for results in biochemistry, pharmacology, and immunology. Implementation, use and administration of NPU has differed between the countries despite similar health care and lab sectors. In Norway and in one Swedish region NPU – with supplementary SNOMED CT codes is also used for reporting results in microbiology. In Denmark and to some extent in Norway and Sweden NPU is also used for ordering tests. In Norway NPU (as part of NLK) has since 2018 been mandatory in requesting governmental reimbursement for laboratory tests. The numbers of national codes vary considerably (DAN: 303, NOR: 1612, SWE: 415). Furthermore, in Denmark >3500 local codes are used for requisition and to communicate more details with the analytical result than the NPU terminology allows. Also, in Norway the NPU codes are by many lab professionals considered insufficient for communicating all relevant information with results. However, the Norwegian reimbursement system has been a strong motivator for implementing international NPU codes. We find it necessary to add information about “how” a measurement is done to the information about “what” is measured in the laboratory report. Until this is settled otherwise, we suggest an increased pragmatism towards producing national codes including method specific information. Furthermore, we recommend that organisations responsible for classifications have heavy professional participation and decision-making competencies in order to lead and guide implementation and optimal use of the classifications.
Keywords: Classifications, data exchange, information technology, interoperability, LOINC, metrology, NPU, SNOMED CT, terminology, vocabulary
Introduction
Electronic exchange of health care data between organisations, regions and countries demands common transmission protocols and agreed code and terminology systems. Exchange of medical prescriptions has recently been implemented within EU using Health Level Seven (HL7), Fast Health Interoperability Resources (FHIR), which is the current standard transmission protocol for electronic communication of health care data in Europe. Currently it is planned to extend exchange of health care data to cover other types of structured health data, including laboratory test results [1]. Transmission of laboratory test results requires an unambiguous standardization defining what is measured, how it is done and what the test results are.
The Scandinavian countries are among the most digitalized countries in the world [2] including their health care systems which for several years have been virtually fully digitalised with electronic health records (EHR) in hospitals [3,4] and at general practitioners (GP) [5,6] and with electronic laboratory information management systems (LIMS) in all hospital laboratories [5]. Furthermore, as is described in this paper, national health care databases to a varying extent collect health information on every citizen in each country, presenting the data in a secure way to the individual patient and to health care professionals with legal permissions.
This paper describes the implementation and current use of the IFCC-IUPAC’s Nomenclature for Properties and Units (NPU) terminology in the Scandinavian countries for electronic exchange of laboratory test results and to some extent also for requisition and reimbursement of laboratory tests. The Scandinavian countries have similar health care systems, laboratory organisations and IT architecture in their health care sectors. However, the implementation, use and administration of the NPU terminology have differed between the countries. The different approaches in the three countries together with common experiences highlight strengths and opportunities as well as weaknesses and shortcomings of the NPU terminology in real life. This forms the basis of some recommendations on how to proceed to reach further harmonization. The descriptions and recommendations are based on the authors’ experience and opinions.
The national release centres of the Scandinavian countries were given the possibility to review the paper before publication. A few comments were received from the Swedish release centre and they have been incorporated in the paper.
Historical Background
Clinical chemical tests were introduced in health care 100 years ago [7]. For many years results varied between laboratories due to use of non-standardised methods, which was noted in a paper from 1947 [8]. Since then, the number of different tests and test procedures has grown exponentially, and it became obvious that some form of logical and systematic description of how test results should be reported was necessary. During the 1950s, the Système International d’Unités (SI) was developed containing six base units and a list of coherent units by Bureau International des Poids et Mesures. In the same period, the Danish medical doctors René Dybkær and Kjeld Jørgensen strived to find a systematic and consistent way to describe what was measured in the various clinical laboratory tests and used the SI as a basis for the units. Their work was published 1966 in a small book [9], later referred to as “the Silver book” of the International Union of Pure and Applied Chemistry (IUPAC) [10]. In this book the principles of the Nomenclature for Properties and Units (NPU) were given, and the syntax “System—Component; kind of quantity” - for example “Plasma—Glucose; substance concentration” - was introduced to describe what was measured in a laboratory test.
With the advancements of IT technology from the early 1990s and world wide web, electronic communication between health care organizations became possible. Medical laboratories were among the first to embrace IT technology advances that are now vital in automation of laboratory production. This development emphasized the earlier acknowledged need of a standardized terminology for communication of laboratory tests. Due to the close and similar laboratory traditions, cultures, and ties between the laboratory communities in the Scandinavian countries, the choice of the NPU terminology as a national terminology for communication of some laboratory results in Denmark, Norway, and Sweden, supporting more than 20 million citizens (Figure 1), may not come as a surprise.
Figure 1:
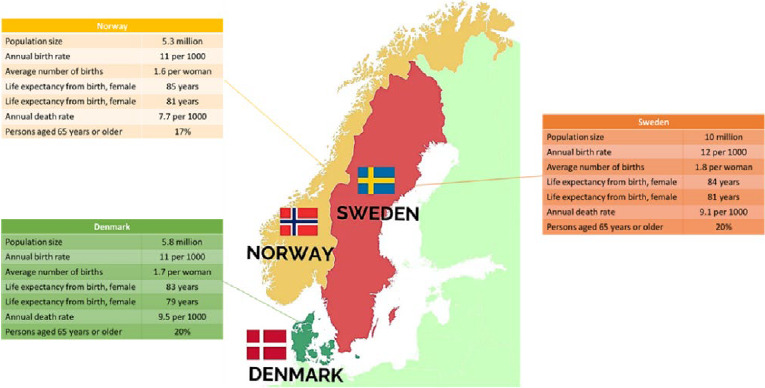
Health demographics (modified figure from [4], approved by the authors).
NPU and the Release Centres
The NPU terminology was endorsed by the International Federation of Clinical Chemistry (IFCC) [11] and IUPAC in 1995. In the following years, the IFCC-IUPAC Committee-Subcommittee on Nomenclature and Properties and Units (C-SC-NPU) headed by the Danish medical doctor Henrik Olesen, published several recommendations, technical reports, and a user’s guide [12]. NPU terminology is an international laboratory terminology with the purpose of providing descriptions (NPU codes) of measurands to present what is being measured in the patient and its values. The NPU codes are established according to following principles [9]:
Each code has a unique meaning of what is measured representing an in vivo patient property. Methods or description of sample material (e.g., EDTA plasma, serum, lithium-heparin plasma) cannot be included in the codes.
The terms in the descriptions are internationally defined and traceable to international vocabularies from relevant fields.
The NPU codes are unique and well defined. These characteristics are established by the many stringent rules that exist in the terminology. Each concept used in the NPU code is traceable to an international nomenclature/terminology/classification. Stability of NPU codes over time is another characteristic emphasized by the NPU organisation. However, the importance of this characteristic relative to other characteristics as granularity and coverage is discussed later in this paper.
The administration of the NPU terminology is at present situated at the Danish Health Data Authority. This administration takes on the roles as both the International NPU Release Centre and the Danish National NPU Release Centre [13]. The terminology is free of use, but establishment of National NPU Release Centres is encouraged for the support of national implementation, translation of English terms into national languages, assignment of National Short Names (trivial name) to NPU codes and to establish national codes (DNK, NOR and SWE codes), when needed to support local laboratories. National codes have the same structure as NPU codes but might not follow the strict rules of the NPU terminology. No coordination of local codes is attempted.
Health Care Laboratory Organisation
Basic demographic data of the Scandinavian countries are given in Figure 1. In Denmark and Sweden, five and 21 regions respectively take responsibility for hospitals, general practitioners (GPs), and medical specialists in private practice. In all three countries, the municipalities have responsibilities for social services, nursing homes, rehabilitation services, etc. The only difference between the countries with respect to health care organisation is that GPs refers to the municipalities in Norway.
Most hospitals in the three countries have biochemistry laboratories, while immunology, microbiology, pharmacology, pathology, and genetics are centralized to varying extent and typically organized into separate departments at larger hospitals. At smaller hospitals, these disciplines may have certain functions within the frames of the biochemistry labs, often supported professionally by specialists from larger hospitals.
In Denmark and Norway, departments of clinical biochemistry are responsible for most phlebotomies in hospitals, while in Sweden the laboratories are not involved in in-house phlebotomies. In all three countries, biochemistry hospital laboratories run outpatient clinics for phlebotomies, as do GPs. In Denmark and Sweden, many GPs also do phlebotomies for tests ordered by hospital departments.
Private laboratories (ex. Fürst and Unilabs) operate on contract with the regions and are in Denmark mostly used for rarely requested tests, while in Norway they perform a broader repertoire of tests ordered from primary health care.
In Sweden Unilabs and Synlab operate as private laboratory organisations in agreement with the regions. In Denmark and Norway, a certain - primarily microbiological - test repertoire has been centralized to national laboratories (Statens Serum Institute (SSI)) and Folkehelseinstitutet, respectively), while in Sweden specific microbiology laboratories are appointed as “National reference laboratories” for given infectious agents within the “Swedish laboratory network for microbiology” (Svensk laboratorienätverk inom mikrobiologi) [14]. Esoteric tests within microbiology are centralized to the Public Health Agency of Sweden.
NPU in Denmark
Health care IT architecture and communication
Electronic communication of health care data in Denmark was introduced by MedCom [15], which was established in 1994 as a public organization with the mission of facilitating the digital cooperation between authorities, public organizations, private entities, and companies who are all linked to the Danish healthcare sector. MedCom is financed and owned by the Ministry of Health, Danish Regions and The Danish municipalities. With the support of relevant national laboratory societies, MedCom established three national protocols for communicating laboratory results for pathology, microbiology, and a joint standard for clinical biochemistry and clinical immunology [15].
NPU terminology is applied both for requesting tests and for reporting the results in the joint communication standard (biochemistry and immunology). In the two other communication standards, national derived nomenclatures for reporting results are used: MDS (microbiology) and PatoSnomed (pathology), respectively (Table 1).
Table 1:
NPU and other terminologies in the Scandinavian countries.
| Disciplines | Denmark | Norway | Sweden |
| Immunology/transfusion | NPU | NPU | NPU |
| Medical Biochemistry | NPU | NPU | NPU |
| Medical Genetics | - | - | - |
| Microbiology | MDS | NPU | NPU |
| Pathology | SNOMED1) | SNOMED1) | SNOMED1) |
| Pharmacology | NPU | NPU | NPU |
| NPU classification | Denmark | Norway | Sweden |
| Total number of active national and NPU-codes | 247632) | 109323) | 90274) |
| Number of national codes | 3032) | 16123) | 4154) |
| Use of NPU classification | |||
| Test requesting | Yes | Yes | Yes |
| Reporting results | Yes | Yes | Yes |
| Economic reimbursement | No | Yes | No |
Nomenclature for Properties and Units (NPU), Codes for Microbiology in Denmark (MDS), Systemized Nomenclature of Medicine (SNOMED)
1) Different versions of SNOMED are used
2) As of December 2023. (LabTerm updated 01.12.2023)
3) As of December 2023 (NLK version 78280.70)
4) As of November 2023
From 2003, Danish citizens have had access to their laboratory results through a national e-health portal named Sundhed.dk [15]. The initiative to establish Sundhed.dk came from the Association of County Councils in Denmark, the Ministry of Health and others in 2001. The goals of Sundhed.dk are much broader than giving access to laboratory results, and it includes descriptions of radiological examinations, clinical information and medication. Today, the compiled health care data are considered an E-health record. However, the original part of Sundhed.dk is the system presenting laboratory results. These are divided into pathology, microbiology, and clinical biochemistry/immunology sections, to which the respective laboratories transfer the patient results.
Besides Sundhed.dk, Denmark has since 2006 had a national database for biochemical and immunological requisitions, which following download form the basis for phlebotomies performed at hospitals or at GPs – MedCom request hotel [15]. This allows patients being treated by specialized services at major hospitals, sometimes in other parts of the country, to have samples for biochemical tests drawn by the GP or a local hospital. This calls for a common classification supporting requesting across the country. The NPU terminology is used for this purpose in the absence of an alternative. Due to shortcomings in the NPU terminology for describing concepts used for request like panels, reflex tests, or specified groups of tests, several local (actually regional) codes have been introduced for requesting purposes, and being regional, they are translated, when requests are produced in one region and samples drawn in another.
Implementation and use of NPU terminology
In 2001, the Danish Health Authority recommended the NPU terminology as the national laboratory terminology for reporting laboratory results. This was supported and initially recommended by the Danish Society of Clinical Biochemistry (DSKB). Despite the recommendation of the society, implementation was accompanied by discussions and frustrations among laboratory professionals. As illustrated in Table 2, some quantities have numerous local codes to cover needs of requesting and for separating results of a given quantity, which may not be comparable e.g. Potassium measured in venous plasma, venous serum, venous blood, arterial or capillary blood or by various methods of differing technical quality.
Table 2:
Number of codes for 10 common measurands.
| Measurand | Denmark | Norway | Sweden | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I | N | L | I | N | L* | 0 | N | L | |
| P-Potassium | 1 | 0 | 19 | 1 | 0 | - | 1 | 1 | ? |
| B-Hemoglobin | 1 | 0 | 22 | 0 | 1 | - | 1 | 1 | ? |
| P-Sodium | 1 | 0 | 18 | 1 | 0 | - | 1 | 0 | ? |
| B-Leukocytes | 2 | 0 | 3 | 1 | 0 | - | 1 | 0 | ? |
| P-Alanine aminotransferases | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | ? |
| P-C-Reactive protein | 1 | 0 | 7 | 1 | 1 | - | -** | 0 | ? |
| B-Thrombocytes/Platelets | 1 | 0 | 8 | 1 | 0 | - | 1 | 0 | ? |
| P-Creatinine | 3 | 0 | 14 | 3 | 0 | - | 1 | 0 | ? |
| P-Alkaline Phosphatases | 1 | 0 | 0 | 1 | 0 | - | 1 | 0 | ? |
| P-Albumin | 2 | 0 | 2 | 1 | 1 | - | 1 | 1 | ? |
I = International code, N= National code, L = Local code
P=Plasma, B=Blood, I = International code, N= National code, L = Local code
* Laboratories were asked if they used local codes for reporting of results to external systems
** For P—CRP, no data regarding NPU-code usage in Sweden was collected
MedCom publish lists of all NPU-codes including national and local codes by the five regions in Denmark and by health care organisations e.g., SSI on their home page [15]. From the lists it can be estimated that just for clinical biochemistry, almost 3600 local codes and 112 DNK-codes were in use during winter 2022/23. Thus, only approximately 1/3 of the exciting national codes (Table 1) were actually used by the regions. These figures do not include local codes used for scientific projects or for reporting results of external quality controls. It can’t be excluded that to some extent the vast number of local codes in Denmark might be caused by lack of knowledge of official or national codes or that it just was considered faster to use a local code instead of searching for the correct official or national code.
Local codes are communicated nationwide without any limitations, and from the regional code lists it appears that some local codes from one region are also used in one, or even two, neighbouring regions, thus approaching - unofficial - national codes.
Administration of NPU terminology
The first official NPU administration in Denmark was established at Rigshospitalet, Copenhagen in 1996. The administration was relocated into the Danish Health Authority in 2002 and is at present sited in the Danish Health Data Authority, which creates new NPU on requests from Danish laboratories or national codes when a national scientific laboratory society makes a recommendation of a definition of a term, e.g. an algorithm which is not covered by NPU codes. National codes have the same structure as NPU codes, but they might use terms that have no internationally accepted definitions.
NPU In Norway
Health care IT architecture and communication
Several IT systems are used both in primary care and in hospitals. For three of the four health regions IT systems in hospitals are separate from systems in primary care. The Central Norway Regional Health Authority (RHA) is in the process of implementing a common IT system for hospitals and primary care centres in this geographical region. To facilitate exchange of information between hospitals and primary care, the government launched the national Summary Care Record (SCR) in 2014. The SCR contains information such as selected journal documents and medication. The Norwegian government also has a national e-health portal [17] where citizens can read their hospital electronic records. As of March 2023, the only included laboratory information presented in this portal and SCR are SARS-Cov2- test results. A new national portal for laboratory and radiology results is under development (“Pasientens prøvesvar”), and this process will also include the introduction of laboratory results into SCR.
Implementation of NPU terminology
Work on a national terminology for reporting of laboratory tests was first initiated by the government in 2004 based on the need for unified definitions for governmental reimbursement [18]. The Norwegian directorate of health decided that the NPU terminology should be used. The first edition of a Norwegian version, “Norsk laboratoriekodeverk” (NLK) was published in January 2012. As demonstrated by the parliamentary proposition “One citizen – One patient journal” (2012), there was in this time also an increased focus on improving the interchange and accessibility of patient information [19]. Thus, as stated by the Directory of eHealth, the primary aim of NLK was to obtain unambiguous communication of both requests and results of laboratory tests [18]. The secondary aim was to obtain a national coding system that could be used for statistical and financial purposes. Somewhat ironically, the mandatory use of NLK in Norway is for the secondary purpose, and not the primary: use of NLK for communication has been nationally recommended since October 2014, whereas use of NLK for making governmental reimbursement claims has been nationally required since 2018. The standardized use of NLK codes in the XML result message consists of the NLK code itself + the “norsk bruksnavn” (“Norwegian usage name”). The laboratories may also replace NLK codes with local code + local names, whenever the NLK codes do not properly cover the communication needs (for instance due to lack of granularity on method used). Since anatomical collection site or more detailed information about the sample material cannot be described by the NPU terminology, the Directorate of e-health has published supplementary tables for specimen type (specimen material) and specimen source (anatomical location), for use by for instance medical microbiology, to annotate what was collected and from where, respectively. However, the use of these tables are not obligatory and standardized between laboratories, as their proper use is not adequately clarified. Furthermore, free text is accepted. In 2023, the directorate of e-health also introduced a supplementary table for measurement methods.
The Directorate of health has driven the development and implementation of NLK with input from laboratory professions at the Directorate’s request. Generally, the laboratory professionals supported the idea of a unified coding system. However, there was also widespread concern directed both at the process, the administration of NLK and more specific issues [20]. Initially, the plan was to include all laboratory disciplines in NLK. However, anatomic pathology was already using a Norwegian version of SNOMED and it was soon decided that pathology should not be included in NLK. Medical genetics did not find the terminology suitable for the field and also declined to be included. However, some genetic analyses that are performed by medical biochemistry, pharmacology and immunology laboratories were included in NLK.
Use of NPU terminology
Even though use of NLK is mandatory in the messages used for governmental reimbursement of laboratory tests, local codes can still be used for requesting and reporting, and later mapped to NLK for reimbursement. NLK currently has 10,932 codes (version 7280.70, January 1, 2024). NPU includes a section for National codes that follow NPU terminology but are not included in the international NPU system. There are currently 1612 NOR codes (Table 1).
Use of NLK for governmental reimbursement
In Norway, laboratory testing in an outpatient setting (testing of patients at GP offices, at hospital outpatient clinics, or under municipal care) is covered financially by national reimbursement from the The Norwegian Health Economics Administration (Helfo). Claims from the laboratories to Helfo are made based on NLK codes [21]. Laboratory testing in an inpatient setting is not covered by Helfo, but by the institutions themselves. However, NLK reimbursement codes and price categories are often used as a basis for billing here also.
Each code in NLK was placed into a price category based on reports of costs from laboratories in Norway (e.g., 13 categories for medical biochemistry). The pricing of several thousand measurands based on cost reports from many laboratories is complicated, and while the overall pricing might be accurate, it is likely that many individual measurands are priced too low or too high. However, this problem probably would exist no matter which classification was used for reimbursement.
Laboratories bill for tests with information specified with patient identification and each performed analysis. This data is collected into a database hosted by the Directorate of health (KUHR database), and anonymized data can be made available upon request. Thus, it is now possible to examine regional variation in the use of laboratory tests [22].
Use of NLK for test requesting and for reporting of results
NLK is recommended for communications of both requesting and reporting results of laboratory tests. No national data on the use of NLK for reporting of results exist. Most results are probably reported with NLK codes, and the compliance with NLK is most likely highest for medical biochemistry.
We contacted representatives of the 21 public health trusts and two private laboratories by e-mail on March 17th 2023, to enquire about the use of NLK and local codes for reporting of the ten most commonly used tests in medical biochemistry. Thirteen laboratories responded. As illustrated in table 2, all laboratories use NLK codes for these measurands. However, some commented that local codes were still in use for communication with some primary care centres with IT systems that were not compatible with NLK codes. In the Central Norway RHA, local codes have been implemented for reporting of results for both hospitals and primary care centres included in the regional IT system, while NLK is used for electronic result reports for primary care centres not using the regional IT system. Laboratories sometimes find the NPU terminology unfit for their clinical needs. In some cases, the national NPU centre has shown some flexibility in instituting national codes (NOR codes), examples being NOR05091 P-CRP high sensitivity (P—C-reactive protein; mass c.(high sensitivity; proc.) =? mg/L) as an alternative to NPU19748 P—C-reactive protein; mass c. =? mg/L, and a national code with the unit g/dL for Haemoglobin.
Administration of NPU terminology
NLK is administered by the Directorate of e-health which is the Norwegian national release centre for NPU codes. The directorate cooperates with the international release centre in Copenhagen. The directorate is also supported by a council of laboratory professionals and councils for each laboratory discipline. Requests for new NLK codes are handled by the national release centre which will check if a relevant code already exists in the NLK or the International NPU system and discuss with the laboratory professionals in the relevant council (-s). New versions of NLK are published 5 times a year and all laboratories must update their IT systems for relevant changes in each version.
NPU in Sweden
Health care IT architecture and communication
There are at least four different LIMS in use for clinical chemistry in Sweden, e.g., Analytix (CGM), Flexlab (Tieto), LabVantage (Software Point), and Labka II (CSC). To date there are nine different EHR in Sweden. In the next coming years there will be two major EHR, i.e., 17 regions will use Cosmic (Cambio) and two regions Millennium (Cerner). Two regions are about to start a procurement for new EHRs. For primary health care, several smaller systems are in use. Electronic laboratory requests and result reports are often organized specifically for the major systems in the 21 regions, often using local coding schemes for properties measured. The company InfoSolutions provides solutions for electronic communication in other cases.
The national patient overview (NPÖ) initiated in 2014 is a service where health care professionals, with necessary permissions, can view e.g., electronic health care records and laboratory results from different regions and municipalities via the national service platform [23]. The national service platform serves as a switch to enable a safe and efficient information exchange between the health care IT-systems and different national services, e.g., NPÖ. Inera, co-owned by Swedish Association of Local Authorities and Regions (SALAR) and the regions and municipalities, develop and manage the national infrastructure. 1177 journal is the counterpart for citizens to access the information in their health care records from different health care providers, as well as their laboratory results. With these national e-health solutions, it became obvious that a national coding system was necessary for proper identification of laboratory tests, and that the NPU system was an established standard without alternatives for use in Sweden. Since then, Equalis, a company co-owned by the SALAR, the Swedish Medical Society and Swedish Institute of Biomedical Laboratory Science (IBL), with responsibility for External Quality Assessment and also the national release centre for NPU codes, has been engaged together with Inera in the further development of the national information specification for laboratory medicine. The information specification describes how a laboratory result should be structured and coded for exchange of information between the different health care IT systems and the different services (e.g., NPÖ and 1177 journal) via the national service platform. In 2020, the national information specification for exchange of laboratory results was extended to enable the display of results from microbiology, including cultures and susceptibility tests (Figure 2).
Figure 2:
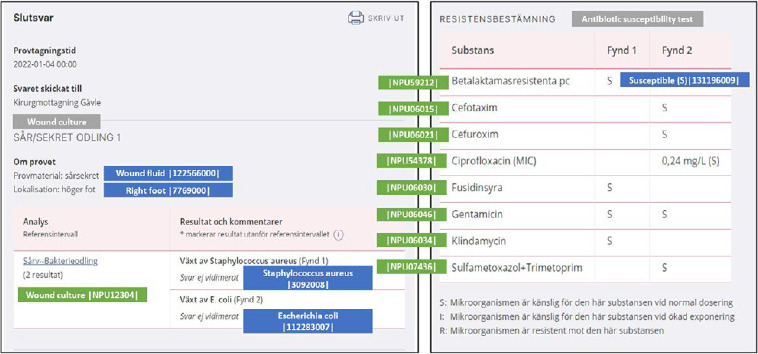
Example of a microbiology culture result and susceptibility test in the Swedish National Patient overview (NPÖ).
There are many patient groups that need to have laboratory tests on a regular basis, e.g., diabetes, rheumatic diseases, and certain types of cancer. Instead of contacting the health care unit or having a meeting with the physician each time, the patient can use the service 1177 “Egen provhantering” and decide when to take the tests and at which health care unit [24]. The physician has then already decided which test that should be taken and how often. To this end, there will be less administration for the health care staff not having to write a referral for tests each time. The patient will also get the results in 1177 journal. NPU-codes are in this case used for both the requests and results. The same service is also used for certain tests that can be ordered by the citizen at any time. A test kit is then sent to the citizen and the test can be taken at home, e.g., chlamydia and gonorrhoea. The test is subsequently sent to the lab and analysed. The results are provided to the citizen in 1177 journal. In this case there is no contact with a health care professional unless the test result is positive. This “testing at home” workflow was also used by many regions during the Covid-19 pandemic for testing of SARS-CoV-2 virus.
Implementation of NPU terminology
The SI was officially introduced in the Swedish health care December 1st, 1975, after a decision by National Board of Health [25] and thereby was also the basic structure of NPU introduced, although without mentioning the term “NPU” which was not coined until later.
No Swedish authority has made any decisions or recommendation about use of a specific terminology. It has therefore been difficult to raise funding for the national management of the NPU terminology. In practice, the cost for national management has been covered by non-paid work and to some extent by the institutions to which these persons happened to be connected. Several investigations into a “National information structure” or framework for the health care system in Sweden have discussed information structure on a general level, without going into details such as which coding system should be used in a common structure and how the management of these coding systems should be funded.
Despite the SFKK recommendation of the NPU coding system, local codes for laboratory tests were still used by the laboratories for a long time, as laboratory results were only locally communicated. There was no central repository of result, for which a common coding system would be required.
The implementation of NPU-codes has been examined within several External Quality Assessment (EQA) programs, where the participants have been asked to report what NPU or SWE-code they are using together with EQA results. Table 1 shows the result for the 10 most common analyses, where 32-48 % reported using a NPU or SWE-code; however, we can’t differentiate if not reporting a code means that a local code is used or that a code simply was not reported. This needs to be addressed in a follow-up.
Use of the NPU terminology
NPU codes are primarily used by the laboratories for reporting of results within clinical chemistry, clinical immunology, clinical microbiology, and transfusion medicine. Equivalent to Denmark and Norway, the Swedish NPU database also contains a recommended report name (up to 25 characters) and a short name (up to 20 characters), which are defined by Equalis to make it easier for health care professionals to understand the measurand. The reason for having a short name in addition to the recommended report name, is that some LIMS and EHR systems have a character limitation for the display of names.
The Swedish NPU database currently has 9027 active codes (November 27, 2023) and 415 national codes. There are different reasons why national codes are created, the most frequent being the need a code with the unit percent (%), which represent 148 (36%) of the Swedish national codes.
The use of local codes is only recommended within an organization, however when reporting results externally to e.g., national e-health services or quality registers, NPU-codes should be used. Before the development of the national information specification to enable exchange of microbiology results, it was challenging for the microbiology laboratories to use NPU-codes. The major disadvantage was that the NPU-codes don’t describe the specimen type, specimen source, method, and findings. To address this problem, SNOMED CT codes were used as a complement to the NPU-codes, and reference sets were created in SNOMED CT to describe these properties. The combined use of NPU and SNOMED CT codes enabled the microbiology laboratories to code the results in a structured manner, including microbiological cultures and susceptibility tests, and present them in national e-health services (NPÖ and 1177 journal) using the national information specification (Figure 2). The implementation of NPU codes within microbiology has raised new issues by the laboratories in Sweden, e.g., the recommended report names are too short for the microbiology analyses (up to 40 or 50 characters would be needed), and that national guidelines would be necessary to facilitate coding of laboratory orders and results with NPU and SNOMED CT codes.
Administration of NPU terminology
During the period 1980-2000, the Swedish Society for Clinical Chemistry (SFKK) appointed a “nomenclature group” which produced recommendations on how to implement the terminology in various areas. e.g., for excretion of substances in urine and faeces [26]. The group was subsequently joined by Urban Forsum, professor of microbiology, who found the basic principles of NPU well applicable also in the field of microbiology. The nomenclature group made the first translation of the NPU terminology into Swedish, and its use was recommended by SFKK.
In 2000, it was decided to move the management of the NPU terminology to Equalis. The organisation of Equalis, with expert groups in various field of laboratory medicine, was considered well suited for the necessary professional development and management of the NPU terminology. It was, however, not clear how the work should be funded. A proposal that costs should be shared by the laboratories in relation to their test volume did not work, because the added value of the NPU terminology was hard to see for laboratories that did not yet use the NPU codes for communication of their results. Equalis therefore decided to end the management of the NPU terminology in 2013. After two years of discussions, 21 separate agreements were reached between Equalis and the regions of Sweden that Equalis should resume the management of the NPU terminology, and that the costs would be shared by the regions according to their number of inhabitants. Equalis is now the National Release Centre for the NPU terminology in Sweden.
Discussion
The need for standardization of terminology within health care, including laboratory activities, is beyond discussion and heavily reinforced by the developments in electronic communication, both nationally and - soon - internationally.
In the Scandinavian countries, the NPU terminology was introduced approximately 20 years ago for this purpose, primarily in the fields of medical biochemistry and immunology, though not without problems. The problems can partly be explained by the fact that requesting – although mentioned in The Silver book of IUPAC [9] - and reimbursement is outside the intended scope of the NPU terminology. However, also in the field of reporting results NPU seems to be considered insufficient by many lab professionals.
In our experience many labs want to inform the users of lab results when methodologically different measurements of the same quantity give different results or are of different quality (uncertainty as a consequence of different analytical and pre-analytical variations), via the classification system used for electronic communication of lab results. Thus, many of the Danish local codes are probably caused by the lab professionals wishing to communicate more details of the analytical result to the recipients than the NPU terminology allows for. NPU was developed with the ambition, that when discrepancies between results due to methodological or calibration differences were demonstrated, this would drive development towards standardization and better compliance. This optimistic goal is - despite more than 25 years have elapsed - still not fulfilled, and unfortunately nothing indicates that the goal will be reached in near future. Furthermore, use of local codes for long time periods should be minimized.
In order to achieve this goal, it must be considered whether the possibility of communicating methodological differences of results are optimally met by extending of the NPU terminology as such, by applying supplemental classifications as in Sweden and partly in Norway, or by switching entirely to other laboratory coding systems.NPU is also used for requesting in all three countries.
Since many laboratories receive orders from several different IT systems, a clear definition and uniform use of codes within and between countries would be a great advantage. The extensive use of local codes in Denmark weakens the use of the MedCom request “hotel” (a national database of biochemical requests) across regional borders. Use of NPU codes for requisition of most biochemical quantities function without problems. But anatomical collection site or more detailed information about the sample material cannot be described by the NPU terminology, and NPU cannot express requisition of some more complicated investigations, as e.g. ordering algorithms, microbiologic tests (where specific microorganisms rarely are asked for, rather which pathogenic microorganisms might be present in the actual sample) or make use of synonyms possible, (e.g. commercial names of drugs rather than the pharmacologic names as an ordering option for the clinician). Thus, there is a need for development of the set of codes for ordering, either in national codes or preferably as an international supplement to NPU.
In Sweden, NPU codes are already supplemented by codes from SNOMED CT in order to describe specimen type and source and measurement method. The combined use of NPU and SNOMED CT codes enable the microbiology laboratories to code the results in a structured manner, including microbiological cultures and susceptibility tests. In Norway the Directorate of e-health also has published supplementary tables for specimen type (specimen material), specimen source (anatomical location) and recently also measurement method, and laboratories are encouraged to use the terms as described in the tables. However, it’s our experience that free text descriptions are commonly used, and detailed instructions on how to use the tables is lacking (e.g. how granular should the anatomical reporting be). Furthermore, not all Norwegian laboratories have implemented the use of supplementary tables.
The introduction of funding based on NPU (NLK) in Norway was controversial among many laboratory professionals. However, the previous system was considered outdated and had unclear definitions for the pricing of many tests. According to the Norwegian experience, the dual purpose of using NLK for reimbursement and requesting/reporting has led to a minimal use of local and national codes but also to some unfortunate consequences. Due to technical limitations in the LIMS some laboratories have to report results on all codes for which they claim reimbursement. This leads to “spam” and uninformative test results, simply to trigger charges for the laboratories (e.g., for microbial susceptibility testing, it may be clinically beneficial to report only a few of the tested antibiotics to a clinician, but in order to have the full reimbursement the laboratory may need to report all of them). Second, if laboratories “hunt” for the most profitable NLK codes, this may also impact on their use for communication purposes. If there are two almost similar NLK codes for the same component (e.g., the same component with two different units of measure) the laboratories may choose to report and send reimbursement claims for whatever code has the highest reimbursement category. However, this is not unique for NLK/NPU, and the problem probably would be the same with alternative coding systems, or perhaps even greater with a more granular coding system than NPU.
Thus, the purpose and/or method of implementation of the NPU terminology in a country seem to have profound effects on the way the terminology is used. In Norway, where NPU serves the purpose of partly funding the laboratories, this has probably contributed to a more strict application to the official NPU-codes also for requesting and reporting. However, local codes are still used in some situations. In Denmark the early introduction of universal electronic communication of both laboratory requests and test results, combined with an administrative habit of using local codes as the solution to many real or experienced shortcomings of the NPU terminology, has led to an extensive use of local codes resulting in a very complicated situation in nationwide IT systems, such as quality databases and Sundhed.dk.
It is a common experience in the Scandinavian countries that introduction of the NPU terminology received criticism by many representatives from laboratories, despite their principal support for the idea of a standardized and international terminology for communicating data. In Norway, the discussion was summarized by Westin et al [20]. In Denmark, the discussions never became as loud and explicit as in Norway, perhaps caused by veneration towards the Danish masterminds of the NPU classification combined with a greater ownership to the terminology compared to Norway, where the implementation of NLK was initiated and driven by central authorities. However, especially when NPU was introduced also for requesting in Denmark, the shortcomings of the terminology became obvious. In the period from 2005 to 2010, a couple of initiatives towards choosing or developing an alternative classification supporting requisition was launched by the Danish health authorities [27], though without success. Could the shortcomings with the implementation of the NPU terminology have been avoided with the LOINC terminology [28], which is used in many countries? The LOINC terminology have many similarities with NPU, from which it originally stems, but also some principal differences [29]. One is that LOINC does not prescribe a specific unit to be used. By using the system Unified Code for Units of Measure (UCUM) to describe the unit, it should be clear for the requester, which unit has been used to express the result from the local laboratory. Another feature of the LOINC system is that the term “System” sometimes is used to denote specimen type instead of the metrological system as in the NPU system. Thus, a measurement of the concentration of free calcium ions in a full blood sample with an ion selective electrode in a blood gas instrument can not be distinguished from measurement of calcium ions in a specimen of serum or heparinplasma. The NPU terminology provide only a single code, as the quantity intended to be measured is the same regardless of specimen type or measurement technique. On request from users’ information about measurement method and specimen can be included in unique LOINC codes.
This more pragmatic approach within the LOINC system, which allows the user to use multiple units, and to some extent specify sample material and method types within unique LOINC codes, is no doubt appreciated by some users because, at first glance, it can be easier to find a LOINC code, which maps to a local concept. On the other hand, the rapidly increasing number of LOINC codes driven by the need to include increasing non-structured information on specimen and method types, we think will make the system less and less comprehensible. It seems therefore necessary, both for the LOINC and NPU system, to model the necessary information about “how” a measurement is done (such as used specimen type and measurement method) in fields separated from the information about “what” is measured. This is also the way forward suggested within the X-eHealth project [1] for how health care data should be shared in the European Health Data Space. The suggested model will be described in a FHIR profile for the exchange of laboratory data [30].
The main strengths of the NPU terminology are that the NPU codes are unique and unambiguous. These characteristics are important to avoid confusion and miscommunication about laboratory results and, thereby increase patient safety. According to the NPU organisation, codes should also be stable over time. This is emphasized as the main reason for not including method principle/procedure in the codes, as this constantly evolves due to the technological method development. Codes including methodology, calibrators and/or supplier will be used only temporarily, and eventually they will be replaced with other codes. It is argued that this will have impact on the continuity of presentation of laboratory results, and that it will increase the administrative burden for the laboratories.
However, NPU codes have already to some extend been supplemented by national codes (table 1), by supplemental codes in Sweden and by almost 3600 local (actually regional) codes in Denmark, in order to overcome this shortcoming. Thus, the Scandinavian labs are already working with a number of supplemental codes or by high numbers of local NPU codes, suggesting that the argument about stability of codes over time is not accepted by laboratory professionals, who seem to give higher priority to needs of complete information when reporting results or for ensuring correct requesting of tests. However, the cost of this variation of implementation of the code system is lack of comparability of test results over time and between regions.
In summary, it seems the stringent rules that forms the basis of the NPU terminology, and strict adherence to the rules when managing the terminology, defines both the strengths and the deficiencies of the classification system. According to the critics of the NPU classification, it is insufficient in terms of holding the entire amount of data, which laboratories need for requests, and about which they wish to inform the users of test results. Paradoxically, this is caused by deliberate restrictions in the NPU terminology in order to keep the codes stable over time. Thus, in our view, there is a need to identify and implement a pragmatic and functional compromise concerning the extent of the standardization.
Recommendations
The shortcomings of the NPU terminology can theoretically be met either by A) expanding the NPU terminology per se, B) supplementation of the NPU terminology by further classifications holding the information needed for requesting and on analytical methodology, or C) by abandoning NPU and switching to other coding systems (i.e., LOINC), which, however, also needs supplementations. If option B is chosen, we recommend using international classifications, e.g., SNOMED CT for supplementation of the NPU terminology as has been done in Sweden. One of these alternatives must be realized in order to expand electronic communication of health care data from the Scandinavian countries to the international level.
Implementation history of NPU terminology in Scandinavia illustrates the drawbacks of a very rigorous adherence to terminological restrictions. As we find national codes preferable to (numerous) local codes, we recommend pragmatism towards producing national codes in the National Release Centres, even if it sometimes deviates from the principles of the NPU terminology.
Furthermore, the history of implementation of NPU in the Scandinavian countries emphasizes the importance of good terminology governance including heavy lab-professional participation with decision-making competencies to lead and guide implementation of the communication classifications in order to ensure optimal use of the classifications.
Abbreviations
- EDIFACT
Electronic Data Interchange for Administration, Commerce and Transport
- EHR
Electronic health record
- EU
European Union
- FHIR
Fast Health Interoperability Resources (an HL7 specification for Health care)
- GP
General Practitioner
- Helfo
The Norwegian Health Economics Administration
- HL7
Health Level Seven
- IFCC
International Federation of Clinical Chemistry and Laboratory Medicine
- IU
International Unit
- IUPAC
International Union of Pure and Applied Chemistry
- LIMS
Laboratory Information Management System
- LOINC
Logical Observation Identifiers Names and Codes
- NLK
Norsk laboratoriekodeverk
- NPU
Nomenclature for Properties and Units
- SCR
Summary Care Record (Norway)
- SI
the Système International d’Unités
- SNOMED CT
Systemized Nomenclature of Medicine – Clinical Terms
- SSI
Statens Serum Institut (Denmark)
- UCUM
Unified Code for Units of Measure
- XML
Extensible Markup Language
Footnotes
Declaration of Conflict of interests
The authors of this article declare that there is no conflict of interest with regard to the content of this manuscript.
R. Ceder, K. Toska,, Y.B. Hansen and G. Nordin are, or have been, members of the IFCC committee on Nomenclature, Properties and Units (C-NPU) in collaboration with IUPAC.
References
- 1.X-eHealth Deliverable D5.3 - Laboratory Requests and Reports guideline and functional specifications. (available from www.x-ehealth.eu/wp-content/uploads/2022/09/D5.3-Laboratory-Requests-and-Reports-guideline-and-functional-specifications.pdf)
- 2.2023. OECD Digital Government Index: Results and key findings, OECD Public Governance Policy Papers, No. 44, OECD Publishing, Paris, 10.1787/1a89ed5een. [DOI] [Google Scholar]
- 3.Fragidis LL, Chatzoglou PD. Implementation of a nationwide electronic health record (EHR). Int J Health Care Qual Assur. 2018;31(2):116-130. DOI: 10.1108/IJHCQA-09-2016-0136. PMID: 29504871. [DOI] [PubMed] [Google Scholar]
- 4.Laugesen K, Ludvigsson JF, Schmidt M, Gissler M, Valdimarsdottir UA, Lunde A, Sørensen HT. Nordic Health Registry-Based Research: A Review of Health Care Systems and Key Registries. Clin Epidemiol. 2021;13:533-554. DOI: 10.2147/CLEP.S314959. PMID: 34321928; PMCID:PMC8302231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen I. Digitalisering af laboratoriekommunikationen. Et overblik over udviklingen de seneste 40 år. 2019: 1-20 https://medcom.dk/wp-content/uploads/2023/03/digitalisering-af-laboratoriekommunikationen_2019.pdf (Accessed:08/06/2024)
- 6.Duval Jensen J, Ledderer L, Beedholm K. How digital health documentation transforms professional practices in primary healthcare in Denmark: A WPR document analysis. Nurs Inq. 2023;30(1):e12499. DOI: 10.1111/nin.12499.Epub 2022 May 10. PMID: 35538598; PMCID: PMC10078429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folin O, Wu H. A system of blood analysis. JBC 1919;38:81-110 [Google Scholar]
- 8.Belk WP, Sunderma FW. A survey of the accuracy of chemical analyser in clinical laboratories Am J Clin Pathol 1947;17:853-861 [DOI] [PubMed] [Google Scholar]
- 9.Dybkær R, Jørgensen K. Quantities and Units in Clinical Chemistry -Including Recommendation 1966 of the Comission on Clinical Chemistry of the International Union of Pure and Applied Chemistry and of the International Federation for Clinical Chemistry. Munksgaard, Copenhagen: (1967) [Google Scholar]
- 10. https://iupac.org/what-we-do/books/(Accessed:06/03/2024)
- 11. https://ifcc.org/search/NPU%20terminology/(Accessed: 06/03/2024)
- 12.Petersen UM, Dybkær R, Olesen H. Properties and units in the clinical laboratory sciences Part XXIII. The NPU terminology, principles and implementation: A user’s guide (IUPAC Technical Report). Pure Appl Chem. 2012;84:137-165 [DOI] [PubMed] [Google Scholar]
- 13.Dybkær R. Nomenclature for Properties and Units (NPU) Fødsel- Udvikling – Fremtid https://www.nfkk.org/wpcontent/uploads/kbn-2015-1.pdf
- 14. https://www.folkhalsomyndigheten.se/slim/nationella-referenslaboratorier/ (Accessed: 06/03/2024)
- 15. https://medcom.dk/medcom-in-english/ (Accessed: 06/03/2024)
- 16. https://www.sundhed.dk (Accessed: 06/03/2024)
- 17. https://www.helsenorge.no/ (Accessed: 06/03/2024)
- 18.Stortingsmelding 5 (2003-2004). https://www.regjeringen.no/no/dokumenter/stmeld-nr-5-2003-2004-/id197375/ (Accessed: 06/03/2024)
- 19.Stortingsmelding 9 (2012-2013). https://www.regjeringen.no/no/dokumenter/meld-st-9-20122013/id708609/ (Accessed: 06/03/2024)
- 20.Westin AA, Weste AL, Stray-Pedersen A, Lohne K, Chen Y, Olaussen RW. The Norwegian Laboratry Coding System – what became of the visions? Tidsskr Nor Laegeforen 2016;136:1370-1372 [DOI] [PubMed] [Google Scholar]
- 21. https://www.ehelse.no/standardisering/standarder/bruk-av-laboratoriekodeverk-i-rekvirering-og-svarraportering-av-medisinske-tjenester (Accessed: 06/03/2024)
- 22. Medisinsk biokjemi: Oversikt og variasjon i bestilling av analyser i primærhelsetjenesten og på poliklinikker i spesialisthelsetjenesten i Norge i 2018 (uio.no) [Google Scholar]
- 23. https://www.inera.se (Accessed:06/03/2024)
- 24. https://www.inera.se/tjanster/alla-tjanster-a-o/1177-egenprovhantering (Accessed: 06/03/2024)
- 25.Socialstyrelsen. In Medicinalstyrelsens Författningssamling (1975). [Google Scholar]
- 26.Andersson J, Kallner A, Lindstedt S, de Verdier C-H. Nomenklatur för urin-och faeceskomponenter. SFKKs Medlemsblad. 1993:160-163. SFKKs Medlemsblad 1993: 160-163. [Google Scholar]
- 27.Hilsted L, Olesen H, Bruunshuus I, Magdal U. NPU-terminologien og specialet Klinisk Biokemi. DSKB-Nyt 2007; (3):9-11 [Google Scholar]
- 28. https://loinc.org/ (Accessed: 06/03/2024)
- 29.Bietenbeck A, Boeker M, Schulz S. NPU, LOINC, and SNOMED CT: a comparison of terminologies for laboratory results reveals individual advantages and a lack of possibilities to encode interpretive comments. J Lab Med 2018;42(6):267-275 [Google Scholar]
- 30. https://hl7.org/fhir/ (Accessed: 06/03/2024)


