Abstract
Background
Clinical biochemistry analyzers are essential for diagnosing and monitoring various diseases and conditions. However, the procurement of these analyzers is often based on the initial purchase cost, which does not reflect the total cost of ownership.
Methods
We applied a novel approach to include all hidden costs to run parameters (consumables and accessories) on a cost-per-reportable test (CPRT) basis. Fixed expenses like water purification plant, HIS connectivity, and electricity backup were assumed to be included in the cost per test itself, while the calibration cost was distributed uniformly in the calculation of CPRT itself. This CPRT was taken to compare the financial results of different bids.
Results
The cost per reportable test received after applying our novel approach in maintenance-free reagent rental basis bid was 47.4% lower than the previous cost per test for the purchased equipment.
Conclusion
This substantial decrease in cost with our novel approach reduced laboratory expenses possible with accurate comparison among analyzers with uniform specifications after eliminating the hidden expenses.
Keywords: Clinical biochemistry analyzers, cost per test, Reagent rental basis, Rate contract basis, Hidden costs
Introduction
Procuring clinical biochemistry analyzers in hospitals is a complex process, influenced by various factors, including workload, competition, and the need for a comprehensive comparison model [1]. The decision between purchasing an instrument outright or opting for a reagent rental agreement carries significant implications for laboratory requirements, budgeting and long-term needs [2,3]. This decision necessitates thoroughly evaluating several key factors, such as financial considerations, maintenance requirements, technological advancements, scalability, instrument quality and reliability and the specifics of contractual terms [4].
Some parameters to consider are listed in Table 1.
Table 1:
Purchase basis and maintenance-free rental basis for laboratory equipment.
| S.No. | Parameters | Purchase basis | Maintenance-free rental basis |
|---|---|---|---|
| 1. | Initial investments | High | None |
| 2. | Approval process | Complex; subject to budget | Simplified |
| 3. | AMC/CMC | Mandatory | Not required |
| 4. | Closed system | Possible | Guaranteed |
| 5. | Service quality | Variable | Consistently good |
| 6. | Reagent price | Less competitive | Competitive |
| 7. | Condemnation process | Challenging | Not required |
| 8. | Technology | Risk of obsolescence | Upgradable per tender terms |
| 9. | Overall pricing | Potentially lower | Potentially higher |
The best decision may be different for individual labs. The tender process may provide competition in pricing in both cases. However, in the purchase process, the focus remains concentrated on instrument pricing (and reagent purchase as per uncompetitive rate contract), while on a rental basis, cost per test is the sole focus in many cases [2]. Because of hidden costs, the concept of fair competition is easily compromised without even a sense of losing it. A calibrator set may cost around five times the reagent kit for the same parameter [5]. Also, a few underutilized tests may cost less, while some high throughput tests may be a little costlier. One may easily be cheated if factors like the number of tests, cost of calibration, cost of wash and clean solutions are not included in decision-making algorithms. Despite the critical nature of this decision, there is a noticeable research gap in the field. Comprehensive studies considering all the hidden costs associated with both procurement strategies are lacking. Traditional models often overlook costs such as maintenance, calibration, and wash and clean solutions. Furthermore, the impact of these costs on the overall pricing strategy is often underestimated, leading to potential financial inefficiencies.
We hypothesized that a more comprehensive comparison that includes all hidden costs would provide a more accurate cost representation for justifiable comparison. Thus, a significant cost reduction may be achieved with actual competition. Therefore, this study was planned with our novel calculative approach to test the above hypothesis.
Methodology
The study/tender process was conducted at the Dr Ram Manohar Lohia Institute of Medical Sciences in Lucknow. The tender was published online in accordance with government guidelines. The duration of the contract was set to be 60 months.
The bid was divided into two components: Technical and Financial.
Technical bid: A comprehensive package was planned, which included all necessary items such as a water plant, computer, printer, and HIS connectivity. Preferably, a minimum of 2 or 3 bids must be accommodated per the state’s tender guidelines to ensure competitive pricing. Both dry and wet chemistry-based bids were permitted, with a disclaimer that certain items not necessary as per technology (like a water plant for dry chemistry platforms) were exempted.
Financial bid: This component involved a price comparison for 30 commonly used biochemical parameters. The monthly tests were calculated by averaging the throughput of the last six months, as taken from the Hospital Information System (HIS). Similarly, the cost of accessories/consumables was also calculated by dividing the cost per pack by the number of tests that could be run with the pack. The Cost per Test (CPT) was determined as follows:
Cost per test calculation for parameters
Table 2 presents the cost per test (CPT) calculation for 30 parameters. The CPT was calculated using the formula CPT = c/T, where c is the kit cost and T is the number of tests.
Table 2:
Cost analysis of diagnostic kits for various tests.
| S.No. | *Parameters | Cost of kit [c] | No of tests (T) | CPT (=c/T) |
|---|---|---|---|---|
| 1 | Albumin | |||
| … | ||||
| 30 | Uric Acid |
* All parameters’ details in Table 4.
Other unit costs
The cost of accessories/consumables to run each parameter was also calculated as described for CPT calculation and added as a separate column for each (Annexure for CPT, Table 4).
Table 4:
Cost analysis and reduction in reagent pricing.
| S.N. | Parameters | No. of test per month (n) | CPT (Old) (%) | CPRT (New) (%) |
|---|---|---|---|---|
| 1 | Albumin | 2000 | 100 | 78 |
| 2 | ALP | 5000 | 100 | 34 |
| 3 | ALT | 5000 | 100 | 63 |
| 4 | Amylase | 800 | 100 | 21 |
| 5 | AST | 5000 | 100 | 60 |
| 6 | Calcium | 200 | 100 | 93 |
| 7 | Cholesterol | 1500 | 100 | 91 |
| 8 | CK | 300 | 100 | 90 |
| 9 | CKMB | 100 | 100 | 9 |
| 10 | Creatinine Jaffe | 7000 | 100 | 100 |
| 11 | Direct Bilirubin | 5000 | 100 | 94 |
| 12 | Ferritin | 200 | 100 | 9 |
| 13 | GOT | 400 | 100 | 71 |
| 14 | Glucose HK | 5000 | 100 | 77 |
| 15 | HDL | 1500 | 100 | 68 |
| 16 | hs-CRP | 100 | 100 | 86 |
| 17 | Iron | 450 | 100 | 92 |
| 18 | ISE (Na, K, CI) | 5000 | 100 | 39 |
| 19 | LDH | 100 | 100 | 87 |
| 20 | LDL | 1500 | 100 | 7 |
| 21 | Lipase | 700 | 100 | 100 |
| 22 | Magnesium | 400 | 100 | 90 |
| 23 | Phosphorous | 500 | 100 | 92 |
| 24 | Total Bilirubin | 5000 | 100 | 45 |
| 25 | Transferrin | 100 | 100 | 49 |
| 26 | Triglyceride | 1500 | 100 | 54 |
| 27 | Total Protein | 1500 | 100 | 96 |
| 28 | UIBC | 300 | 100 | 88 |
| 29 | Urea | 6500 | 100 | 81 |
| 30 | Uric Acid | 1800 | 100 | 90 |
| Reduction (%) | 52.6 |
| Net reduction (%) | 47.4 |
ALP: Alkaline phosphatase, ALT: Alanine transaminase, AST: Aspartate transaminase, CK: Creatinine kinase: CK-MB: Creatine kinase-MB, GGT: gammaglutamyl transferase, HDL: High density lipoprotein, hs-CRP: High sensitive creactive protein, ISE: Ion selective electrode, LDH: Lactate dehydrogenase, LDL: Low density lipoprotein, UIBC: Unsaturated iron binding capacity.
Calibration requirement data
The calibration requirement data was directly obtained from our instruments and was found to be 0.97 per 100 tests. It was averaged to be once per hundred tests in general. The ultimate aim was to include the cost of calibration in decision-making. Caution was taken to ensure that the cost per test remained primary while other costs were added (in total/fraction). It was not individualized for each test, as the same calibrator set could be utilized for many tests.
Cost of calibration per test (CPCT)
The cost of calibration per test (CPCT) was calculated using the following formula: CPCT = r X [(Vc X n)+ Vd]100
Where:
r is the rate per μL, calculated as the cost of the calibrator set divided by the total volume of the calibrator set,
Vc is the volume required to calibrate once,
n is the number of times the calibrator has to be run (if in duplicate, n=2; in triplicate, n=3),
Vd is the dead volume (the volume that can’t be picked up by the sample probe from a cuvette).
All these data were taken on the company’s letterhead and verified from kit/calibrator inserts (submitted in PDF along with the tender document as a mandatory condition).
Cost per reportable test (CPRT):
It is the total cost of the reagent to run an individual parameter. It includes CPT, cost per accessories/ consumables, and CPCT.
| S.No. | Parameters* | No. of test per month(n) | Name of the calibrator | Cost of Calibrator | Vol of calibrator per set(μL) | Rate/μL (r) | Calibrator vol to be used per cycle of calibration(in μ,L)(a) | Times of calibration run (in (numerical)-(b) | Dead volume- (c) | Calibrator vol per calibration [d=(aXb)+c] | Cost per calibration CPC (e = r X d) |
Cost of calibration per test CPCT [=e/100] ** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Albumin | |||||||||||
| .. | … …. | |||||||||||
| 30 | Uric Acid | |||||||||||
*A11 parameters’ details in Table 4.
** Taking one calibration per 100 tests (as per interpretation from previous six-month data from our lab). The parameter sequence should be the same as mentioned in the bid (col no 1-30 remains the same as in ‘Format for Submitting the Financial Bid.
Final financial bid
The final financial bid charges included an annual maintenance contract (AMC), comprehensive maintenance contract (CMC), consumables, uninterruptible power supplies (UPS), water purification system, battery, and HIS connectivity. The maintenance charge (AMC/CMC, consumables like halogen lamps) cost of any other requirements like water purification system, UPS, batteries, and hospital information system (HIS) connectivity for data transfer was also assumed to be zero. The vendor was supposed to include it in the pricing of reagents/calibrators. Anything not mentioned in the final financial bid chart was considered a hidden charge and assumed to be zero as an essential tender condition. The cost of controls was intentionally not included in the tender as third-party controls are desirable for better quality per the national accreditation board for testing and calibration laboratories (NABL) norms.
The cost per test is the key factor among initial verification/validation, calibration, QC run, repeat run, and dilution. Since we cannot predict it exactly, it would be better to accept it as essential maintenance/ expanse to manage hassle-free services. If fixing it is the vendor’s responsibility, it would be natural for him to keep the cost per test on the higher side to avoid losses.
Cost per reportable test (CPRT; column 9) was multiplied by the test volume (column 3) of the same parameter and put in column 10 as total cost (predicted per month on given test volume.
The sum of all rows in column 10 (total offered value of the above parameters) was compared among different vendors to decide the lowest bidder. Any breakdown was fixed to be charged with a daily fine unless the backup was not provided to ensure smooth running without affecting turnaround time (TAT), which is often considered a key marker of the lab’s efficiency. The condition of providing upgradation in terms of technology and throughput on the departmental committee’s demand was implemented to address any such need during the contract period.
Formulas used for final financial bid calculation
Table 3 presents the formulas used for the final financial bid calculation for 31 parameters. The Cost per Run Test (CPRT) was calculated using the formula CPRT = CPT + cost of accessories to run each parameter + Cost of consumables + Others + CPCT, where CPT is the cost per test and CPCT is the cost of calibration per test (Annexure for CPRT, Table 4).
Table 3:
Cost Analysis of Diagnostic Tests.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| S.No. | Parameter | No. of test per month (n) | Cost per test (CPT) | Cost of accessories to run each parameter | Cost of consumables | Others | Cost of calibration per test (CPCT) | CPRT | Total cost (=nXCPRT) |
| 1 | Albumin | 2000 | |||||||
| .. | … …. | … …. | … …. | … …. | … …. | … …. | … …. | … …. | … …. |
| 30 | Uric Acid | 1800 | |||||||
| Total offered value | |||||||||
Results
The comparison of the cost per test (CPT) rates supplied by the same company (rate contract basis for the purchased equipment) with the reagent rental basis tender procedure resulted in a significant reduction in reagent pricing for the given 30 parameters. The overall reduction in cost was found to be 47.4%.
Parameter-wise cost reduction
A detailed analysis of each parameter revealed varying degrees of cost reduction. For instance, the cost of albumin testing was reduced by 22%, while the reduction for amylase was as high as 79%.
Total cost reduction
As there was no manufacturer certified/ provable way available to calculated CPRT for older prices. We have compared cost per reportable test (CPRT) for post tender prices to cost per test (CPT) of older prices. The cost per test with old prices (CPT, old) was higher than the cost per repeatable test with new prices (CPRT, new) for all parameters except lipase, which was equal after negotiation. Thus, CPT (old) has been taken as 100% and CPRT (new) has been represented as a percentage fraction of CPT (old). The data has been represented as percentages to avoid revealing the exact figures. When considering the total cost for all parameters, the new CPRT resulted in a net reduction of 47.4% in cost compared to the old CPT. This substantial decrease in cost demonstrates the effectiveness of the reagent rental basis tender procedure in reducing laboratory expenses.
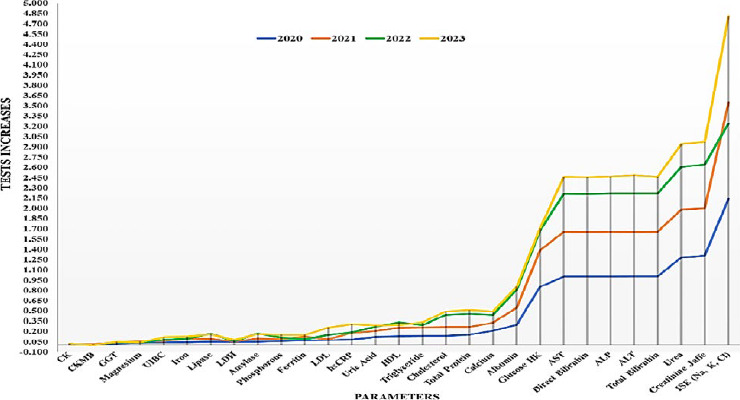
On analysis of the last 4 years annual workload, we found that the mean (standard deviation) increase in the number of tests per consecutive year was 166 % (SD 24%), 126% (SD 34%), and 116% (SD 24%) in years 2021, 2022 and 2023 respectively.
Discussion
The study aimed to develop a novel approach for procuring clinical biochemistry analyzers that considered all hidden costs, including maintenance. The hypothesis was that this approach would provide a more accurate representation of the actual cost of clinical biochemistry analyzers, leading to more cost-effective decisions and improved hospital budgeting. The study compared the cost per test (CPT) rates supplied by the same company (rate contract basis for the purchased equipment) with the reagent rental basis tender procedure. This substantial decrease (47.4%) in cost demonstrates the effectiveness of the novel approach in reducing laboratory expenses and supports the hypothesis of the study. High workload, fair competition, precise calculations and inclusion of sufficient bidders may be attributed to the same. The maximum cost saving was in ISE electrolytes, as electrodes were quoted as free by the lowest bidder in our case. However, it was not the same with other participant bidders. Although the cost reduction per individual parameter was maximum for CKMB, ferritin and LDL, the overall cost saving was minimal due to a much lesser test volume of these parameters. This can be interpreted as a strategy of the bidder to provide low test volume kits on throw away prices to get the contract with minimum quoted price. The LDL is done by calculation at many centres (not at our institute), the leadership of the vendor company might have misunderstood it this way and hence underquoted for the same. However, the intrest of the institute was not compromised (rather amplified) as we invited the bid at the real workload. Though the reagent contracts may vary widely between countries, the spirit of organizing a fair competition shall always remain. Elimination of hidden costs is one such way to provide an equal platform. We have excluded the taxes while calculating as it may vary per state-specific regulations. The study also provides a detailed analysis of the cost reduction for each parameter, which can help identify the most cost-efficient parameters and optimize the laboratory workflow. The previous studies have focused on the cost of reagents and calibrators alone, without considering the other costs that are involved in running the analyzers. Smith et al. (2018) compared the cost of reagents and calibrators for different types of analyzers but did not include the cost of maintenance, labor, electricity, or waste disposal [6]. Similarly, a study by Jones et al. (2020) compared the cost of reagents and calibrators for different brands of analyzers but did not account for the cost of accessories, consumables, or calibration [7]. These studies may have underestimated the cost of clinical biochemistry analyzers and led to biased or inaccurate decisions. Furthermore, the previous studies have used a rate contract basis for the purchased equipment, which is a conventional approach involving the purchase of the analyzers, reagents, and calibrators separately. This approach places the burden of maintaining the analyzers and providing the accessories and consumables on the users. This approach also leads to the overconsumption of reagents and calibrators and underutilization of the analyzers; Lee et al. (2018) used a rate contract basis for the purchased equipment and found that the cost of reagents and calibrators was higher than the cost of the analyzers [8]. Similarly, a study by Chen et al. (2017) used a rate contract basis for the purchased equipment and found that the utilization rate of the analyzers was lower than the optimal level [9]. During the contract period, we assumed that the workload increase would be random and proportional. To validate this, we analyzed the pattern of workload increase over the past four years. The workload increase was consistent for most parameters, as shown by the low standard deviation each year. However, there were notable exceptions for specific parameters, specifically CKMB, CK-total, and GGT. These findings lend support to our initial hypothesis. It’s crucial to highlight that all parameters saw a proportionally more significant increase between 2020 and 2021. This was mainly due to the decrease in workload during the COVID-19 pandemic. Through multiple discussion sessions with multiple vendors, we ensured that our specifications were at par with at least four of them; to the best of our understanding, all four were approved by the United States Food and Drugs Administration (USFDA). Only three participated, while the one with dry chemistry-based technology refrained, possibly due to pricing issues. The study has some limitations that should be acknowledged. First, the study used a convenience sample size from one laboratory, which may limit the generalizability of the results to other laboratories. Thus, each lab must quote the true data (tests per month) in its bid. Any mismatch may severely compromise the purpose; only the tests done in the lab must be included. A slight deviation in a high throughput test may create a significant difference. If some new or low yield parameter is to be included, its workload should be taken as 100 (minimum size of kit on most of the biochemistry analyzers) so that it can be finished before expiry (preferably at least 6 months in our case). Smaller labs may prefer cost-effectiveness, but if a lab has a sufficient workload, procuring an approved autoanalyzer may be necessary to restrict low-quality/technology bidders. Secondly, the study only considered the cost of reagents and calibrators, which may not capture the total cost of running the clinical biochemistry analyzers. Future studies should include other costs, such as human resources, electricity, and waste disposal, to assess the total cost of ownership of the clinical biochemistry analyzers. However, human resources recruitment is complicated due to expertise and state regulations. We have practiced at least two staff training sessions in our biochemistry lab for a long time in compliance with NABH (National Accreditation Board of Hospital and Health Care Providers) guidelines for good laboratory practices. The vendors also comply as staff training may indirectly affect business outcomes. Though we did not include staff training as an essential condition in our procurement process, it would be better to include it for better compliance. However, we must clearly define training specifications like staff strength, number of sessions, standardization like inviting specific external professional expert(s), different expanses and the outcome expectations must be clearly defined to ensure the quality as well as helping the vendors in planning the budget. As our proposed model is maintenance-free reagent rental basis installation, this cost for the same may be included either in reagent charges or as a separate entity in the final financial bid. With the later approach, payments may be cleared after the expected outcomes are achieved satisfactorily.
Conclusion
This study introduces a comprehensive method for procuring clinical biochemistry analyzers that considers all hidden costs, including maintenance, consumables, accessories, and calibration. By incorporating these costs into the Cost Per Reportable Test (CPRT), we observed a significant reduction of 47.4% in costs when using a maintenance-free reagent rental basis bid, compared to the previous cost per test for purchased equipment. This approach allows for a more accurate comparison among analyzers with similar specifications by effectively accounting for hidden expenses. As a result, this method can potentially revolutionize the procurement process of clinical biochemistry analyzers, leading to more cost-effective diagnostic services. Future research should focus on validating this approach across diverse laboratory settings to confirm its effectiveness and applicability. Furthermore, future studies should consider including additional factors such as human resources, staff training, and other operational expenses to provide a more holistic view of the total cost of ownership.
Figure 1:
Year-wise test load versus parameters.
Footnotes
Declaration
This original article has not been published before and is not currently being considered for publication elsewhere. All authors have read and approved the study. The authors declare no conflict of interest.
Ethical Statement
This study does not involve any human or animal samples. It is a cost comparison study conducted using machinery.
References
- 1.Sakr S, Elgammal A. Towards a comprehensive data analytics framework for smart healthcare services. Big Data Research. 2016;4:44-58. [Google Scholar]
- 2.Matricardi D. The Credit Card and the Reagent Rental. Laboratory Medicine. 2005;36(8):462-464. [Google Scholar]
- 3.Abayomi EA, Landis RC. Flow cytometry as the spearhead for delivering sustainable and versatile laboratory services to HIV-burdened health care systems of the developing world: A Caribbean model. Cytometry Part B: Clinical Cytometry: The Journal of the International Society for Analytical Cytology. 2008;74(S1):S80-S89. [DOI] [PubMed] [Google Scholar]
- 4.Thakker P, Japee G. Emerging technologies in accountancy and finance: A comprehensive review. European Economic Letters (EEL). 2023;13(3):993-1011. [Google Scholar]
- 5.Locke D, Hoyt CC. Companion diagnostic requirements for spatial biology using multiplex immunofluorescence and multispectral imaging. Frontiers in Molecular Biosciences. 2023;10:1051491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith L, Kohli M, Smith AM. Expanding the dynamic range of fluorescence assays through single-molecule counting and intensity calibration. Journal of the American Chemical Society. 2018;140(42):13904-13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones S, McGowan C, Boyle S, Ke Y, Chan CH, Hwang H. An overview of sample preparation in forensic toxicology. Wiley Interdisciplinary Reviews: Forensic Science. 2022;4(2):e1436. [Google Scholar]
- 8.Lei Y, Li N, Guo L, Li N, Yan T, Lin J. Machinery health prognostics: A systematic review from data acquisition to RUL prediction. Mechanical systems and signal processing. 2018;104:799-834. [Google Scholar]
- 9.Chen S, Shi R, Ren Z, Yan J, Shi Y, Zhang J. A blockchainbased supply chain quality management framework. In2017 IEEE 14th international conference on e-business engineering (ICEBE) 2017;172-176. [Google Scholar]


