Abstract
Adequate vitamin D status during pregnancy is important for developing fetal bone strength and densityand may play a role in preventing a range of skeletal and non-skeletal diseases in both mothers and children. We previously identified Mongolian women of reproductive age to have the lowest vitamin D levels yet observed in any population globally, which renders this population uniquely important in vitamin D research. In this study, we measured the seasonal distribution of 25-hydroxyvitamin D (25(OH)D) concentration in 390 healthy third trimester pregnant women living in urban and rural Mongolia using DiaSorin LIAISON and compared this distribution to that of 206 third trimester women living in Boston, USA. Also, we analyzed seasonally-independent associations between (25(OH)D) levels and selected predictors in both groups using quantile regression. Mean 25(OH)D levels were significantly higher and less seasonal in Boston (seasonal range: 27.1±7.0 to 31.5±7.7 ng/ml) than in Mongolia (seasonal range: 11.2±3.9 to 19.2±6.7 ng/ml). Adjusting for month of blood draw, higher 25(OH)D levels were significantly associated with older age, lower gravidity, lower BMI, and lack of a college or university degree among Boston participants, however, only gravidity was robust to multivariable adjustment. No assessed characteristics were independently predictive in Mongolia, likely due to universally low 25(OH)D levels and a resulting lack of between-person variation. In conclusion, vitamin D status among pregnant Mongolians is severely depressed throughout the year and should be addressed through fortification and supplementation, while in the U.S.), deficiency is associated with specific characteristics targetable through supplementation.
Keywords: vitamin D deficiency, 25-hydroxyvitamin D, pregnancy, rickets, nutritional epidemiology, Mongolia
Introduction
During pregnancy, adequate maternal 25-hydroxyvitamin D (25(OH)D) is necessary to support the mother’s requirement and to provide sufficient substrate for fetal 1,25(OH)2D production, which is needed in turn to support ossification of the fetal skeleton (the rate of which doubles in the third trimester [1–4]). Maternal vitamin D deficiency during pregnancy is therefore plausibly associated with impaired fetal growth and development. As most of an infant’s vitamin D in its first months of life are not obtained from breast milk or the sun, but rather from trans-placental transfer during pregnancy, infants born to vitamin D deficient pregnant mothers are at risk of deficiency both at and after birth. Recent syntheses of randomized controlled trials have varyingly implicated vitamin D supplementation during pregnancy in reduced risk in offspring of low birth weight [5–7], small for gestational age [6,7], preterm birth [5], reduced risk of fetal or neonatal mortality [7], asthma recurrent wheeze at 0–3 years [6,8], and possibly maternal risk of pre-eclampsia [5]. Offspring weight also has been shown to be increased at 3, 6, 9, and 12 months of age with vitamin D supplementation in pregnancy [7].
Mongolia is an important location for studying vitamin D deficiency due to the country’s high latitude and cold climate (which reduce endogenous vitamin D synthesis by decreasing incident ultraviolet B intensity and sun exposure, respectively) and the scarcity of vitamin D-rich foods or supplements Mongolia [9]. We previously identified 25(OH)D levels in Ulaanbaatar women of reproductive age (WRA) in March-April to be the lowest of any study population globally (79.3% were <10 ng/ml) [10], while in a nationwide survey, we found 85.1% and 80.1% of urban and rural non-pregnant WRA to have levels <10 ng/ml in January-March and 100% to consume inadequate dietary vitamin D in winter (median intake: 29.1 and 18.2 IU/day, respectively, compared 144 IU/day in the U.S.) [11,12]. The Fifth National Nutrition survey found that nationwide, 75.4% of pregnant Mongolians have levels below <20 ng/ml and 20.2% between 20 and 30 ng/ml in September-November 2016 [13], we found a mean status of 7.6 ng/ml among 360 rural pregnant Mongolians from February 2015-December 2016 [14], while data from an earlier 2000–2001 study of 62 Ulaanbaatar pregnant women found mean levels of 12.7, 11.7, 9.7, and 7.7 ng/ml in summer, fall, winter, and spring, respectively [15].
Neither the seasonal distribution of vitamin D status outside Ulaanbaatar, nor the statistical associations between status and individual characteristics have been studied in Mongolian pregnant women. We therefore investigated the seasonal distributions and seasonally-independent predictors of vitamin D status in Ulaanbaatar and two rural areas of Mongolia (Bulgan and Selenge) among third trimester pregnant women. We also compared these distributions and predictors to those of third trimester women in Boston, USA.
Methods
Study population
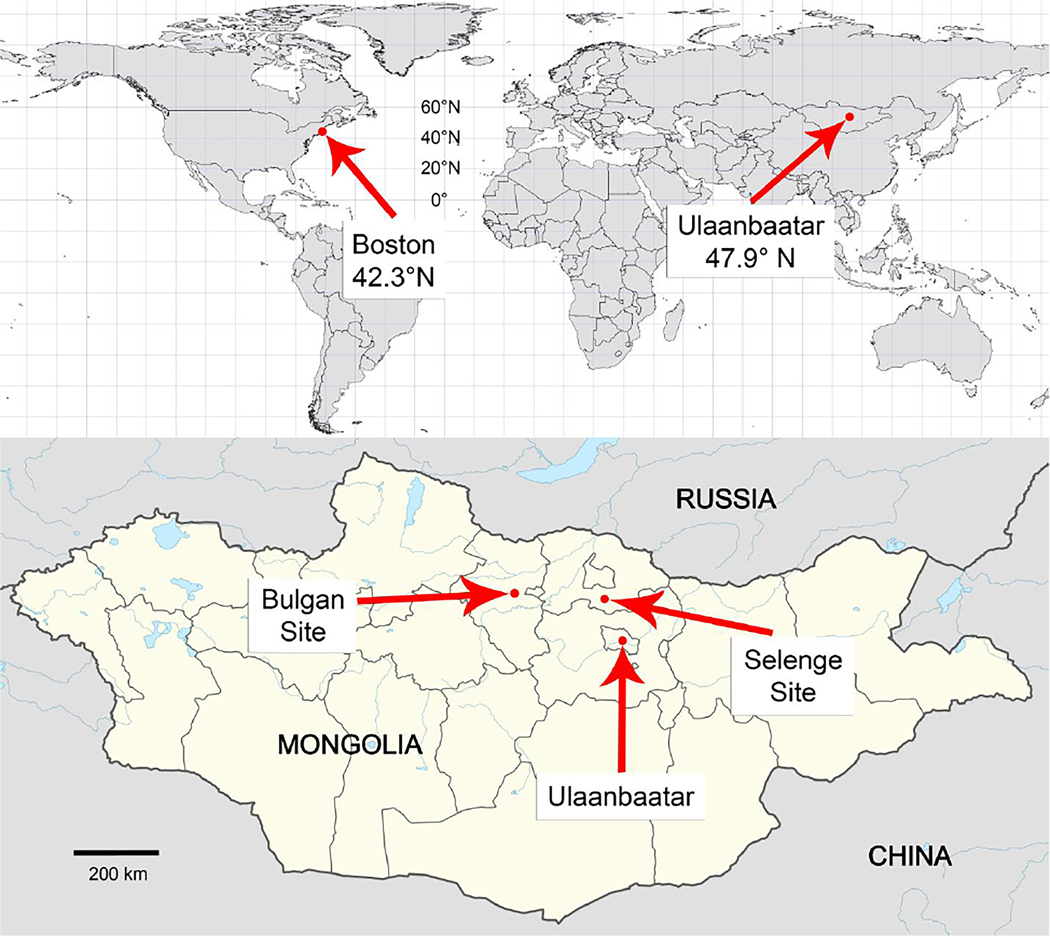
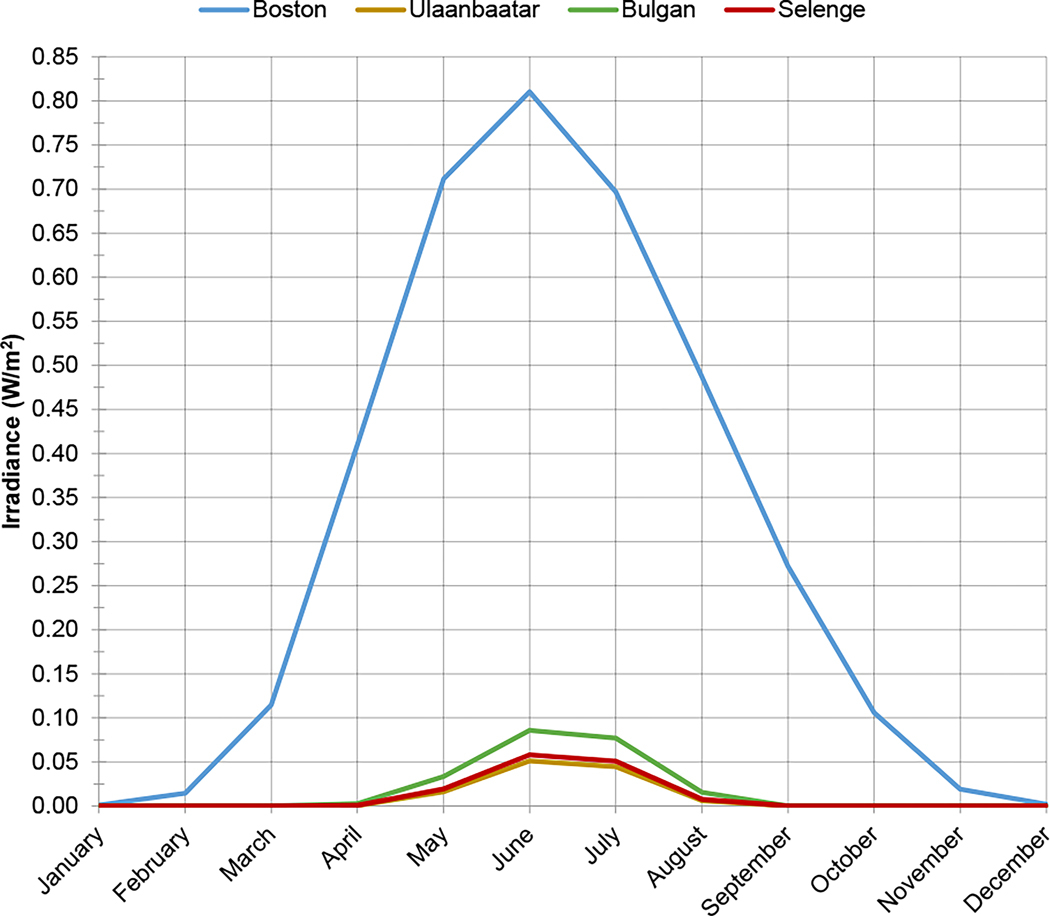
Eligible participants included late second or third trimester (22–33 weeks) pregnant women ≥18 years of age with naturally conceived, singleton, presumed to be viable pregnancies who were receiving prenatal care at Brigham and Women’s Hospital, Massachusetts, or one of three hospitals in Mongolia: the National Center for Maternal and Child Health in the capital municipality of Ulaanbaatar, or either the general hospital of Bulgan province or the Mandal sub-district hospital in Selenge province (both located in northern Mongolia). Boston and Ulaanbaatar are densely populated urban areas, whereas Bulgan is a town whose hospital serves its own and surrounding rural residents, and Mandal is a rural area. Figure 1 shows a map of study locations. For each location, Figure 2 compares the intensity of solar radiation in the ultraviolet B wavelength range in which previtamin D is produced (280–320 nm) at 12:00pm on the 15th of each month, estimated based on each location’s geographic coordinates and elevation using the Tropospheric Ultraviolet-Visible (TUV) Model v.5.3 [16].
Figure 1. Boston and Mongolia lie in temperate northern latitudes.
Map of study locations. Surface altitudes: Boston (Brigham and Women’s Hospital): 11m; Ulaanbaatar (National Center for Maternal and Child Health): 1340m; Bulgan (Bulgan General Hospital): 1213m; Selenge (Mandal Soum Hospital): 865m.
Figure 2. Ultraviolet B irradiance in Boston exceeds that in Mongolia from February to November.
Estimated ultraviolet B irradiance is presented for each study location at 12:00pm on the 15th of each month.
Participants from Boston were identified as part of an ongoing longitudinal study of women with multiple samples collected during pregnancy, of whom 206 women eligible for inclusion in the present study were opportunistically sampled to have their third trimester serum samples (collected from 10/30/2012 to 1/7/2015) analyzed for vitamin D. These 206 women were 64.7% white, 11.0% African-American, 15.5% Hispanic, 6.8% Asian, and 2.4% other races/ethnicities. In Mongolia, 412 participants were enrolled in the present study, 390 of whom were confirmed eligible, retained in follow-up, and from whom third trimester serum samples (collected from 9/6/11 to 6/5/2013) were analyzed for 25(OH)D. The sample of size of 596 was selected to allow adequate statistical power for a separate analysis comparing concentrations of other analyses also measured in collected third trimester serum samples (estradiol, estriol, prolactin, progesterone) between Boston and Mongolia. All participants provided written informed consent prior to enrollment in the present study. This study was approved by the ethical review boards of the Mongolian Ministry of Health, National University of Mongolia, U.S. National Cancer Institute, and Brigham and Women’s Hospital.
Data collection
Information on each participant and her index pregnancy was abstracted from medical charts, including age, gravidity, sex of index pregnancy, pre-pregnancy and 3rd trimester BMI measured by stadiometer and clinical scale, while race/ethnicity, supplement use, smoking, alcohol use, education, and marriage status were obtained during an interview. Ten ml of blood were collected from each participant at a recorded gestational duration, delivered immediately to the hospital laboratory, allowed to clot at room temperature, and centrifuged. Serum was aliquoted into labeled cryotubes and frozen in storage until being shipped to Boston Children’s Hospital. 25-hydroxyvitamin D (25(OH)D) was measured using an enzyme-linked immunosorbent assay (ELISA) from Immunodiagnostic Systems Inc. (Fountain Hills, AZ). This assay employs a competitive binding technique: 25(OH)D labeled with biotin is added to the samples, this sample mixture is added to a microtitre plate coated with a highly specific antibody to 25(OH)D, and the sample 25(OH)D competes with the biotin-labeled 25OHD to bind sites on the plate. Hence, with increasing concentrations of sample 25(OH)D, the amount of biotin-labeled 25OHD bound to the ELISA plate is reduced. After a washing step to remove unbound components, avidin labeled with an enzyme (horseradish peroxidase) is added, the avidin binds to the biotin and after another incubation, the plate is washed to remove unbound enzyme. Substrate is added, and a color is generated that is indirectly proportional to the concentration of 25(OH)D in the sample. The assay is sensitive down to concentrations of 2.0 ng/ml. Day-to-day variability of the assay at concentrations of 16.1, 28.8 and 52.9 ngl/ml are 4.6, 6.4 and 8.7%, respectively. The assay is standardized to the NIH Vitamin D Standardization Program ID-LC-MS/MS Reference Method Procedure, FDA-approved, and validated against a mass spectrometric analysis platform in the DEQAS-certified clinical laboratory of Boston Children’s Hospital.
Statistical analysis
Descriptive statistics were tabulated for the Boston and Mongolia study populations and compared across locations using chi-square tests. For Boston, Mongolia, and study locations within Mongolia (Ulaanbaatar, Bulgan, and Selenge) in each season (spring: March-May; summer: June-August; autumn: September-November; winter: December-February), the mean, median, and proportion of each group falling within vitamin D sufficiency categories was described [17], and means were compared across locations and seasons using independent sample t-tests. Seasonally-weighted annual statistics (i.e. annual statistics that would have been obtained had exactly 25% of the participants from each location been sampled in each season) were also estimated. The association between assessed predictors and median 25(OH)D concentration was assessed using quantile regression in order to account for potential influential effects of outlying observations, as well as skew in model residuals in Mongolia analyses. Separate models were run for Boston and Mongolia. For both countries, two sets of models were run in which predictors included (1) month of assessment (represented by a 12-category variable) and each predictor entered individually in its own model, and (2) month of assessment and all predictors entered simultaneously in one model. Models 1 and 2 also evaluated the effect of square terms for each continuous predictor, while Model 2 additionally evaluated all pair-wise interactions. The proportion of deviance explained was measured for Model 2; a model only including month of assessment; and the multivariable model excluding month of assessment [18]. Statistical analyses were conducted using R v. 3.5.3.
Results
Population characteristics are shown in Table 1 for Boston and Mongolian participants. The distribution of season of assessment did not differ significantly between the two locations, nor did gravidity or child’s sex in index pregnancy. In comparison with Boston participants, Mongolian participants were significantly younger (mean age: 28 vs. 34 years), assessed at earlier gestation lengths (mean length: 219 vs. 246 days), had significantly lower pre-pregnancy, 3rd trimester, and pre-pregnancy to 3rd trimester change in BMI), were less likely to have a college or university degree (35% vs. 74%), to be single (2% vs. 18%), and to have ever drank alcohol (5.1% vs. 9.7%) or smoked during pregnancy (0.3% vs. 5.4%). In Mongolia, 75.9% of participants used a multivitamin during pregnancy, 0.3% (1 participant) used vitamin D, and 10.3% used both.
Table 1:
Characteristics of study population by location
| Characteristic | Boston (N=206) | Mongolia (N=390) | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Season of assessment | Spring | 50 | 24.3 | 94 | 24.1 | 0.61 |
| Summer | 53 | 25.7 | 116 | 29.7 | ||
| Autumn | 42 | 20.4 | 82 | 21.0 | ||
| Winter | 61 | 29.6 | 98 | 25.1 | ||
| Study location | Ulaanbaatar | n/a | n/a | 196 | 50.3 | n/a |
| Bulgan | 107 | 27.4 | ||||
| Selenge | 87 | 22.3 | ||||
| Maternal age (years) | <25 | 10 | 4.9 | 116 | 29.7 | <0.0001 |
| 25 to 29 | 32 | 15.5 | 132 | 33.8 | ||
| 30 to 34 | 72 | 35.0 | 78 | 20.0 | ||
| 35+ | 92 | 44.7 | 64 | 16.4 | ||
| Race/ethnicity | White | 132 | 64.1 | n/a | n/a | |
| African-American | 23 | 11.2 | ||||
| Asian | 14 | 6.8 | ||||
| Hispanic | 32 | 15.5 | ||||
| Other | 5 | 2.4 | ||||
| Gestation length at 3rd trimester assessment (months) | <7 | 0 | 0.0 | 109 | 27.9 | <0.0001 |
| 7 to 8 | 29 | 14.1 | 246 | 63.1 | ||
| 8+ | 177 | 85.9 | 35 | 9.0 | ||
| Gravidity (including current pregnancy) | 0 | 38 | 18.4 | 92 | 23.6 | 0.34 |
| 1 | 58 | 28.2 | 101 | 25.9 | ||
| 2 | 53 | 25.7 | 82 | 21.0 | ||
| 3+ | 57 | 27.7 | 115 | 29.5 | ||
| Sex of child in current pregnancy | Male | 101 | 49.3 | 206 | 53.0 | 0.39 |
| Female | 104 | 50.7 | 183 | 47.0 | ||
| Pre-pregnancy BMI | <25 | 110 | 54.7 | 289 | 75.5 | <0.0001 |
| 25 to 30 | 51 | 25.4 | 75 | 19.6 | ||
| 30+ | 40 | 19.9 | 19 | 5.0 | ||
| 3rd trimester BMI | <25 | 32 | 15.8 | 103 | 26.5 | <0.0001 |
| 25 to 30 | 71 | 35.0 | 196 | 50.4 | ||
| 30+ | 100 | 49.3 | 90 | 23.1 | ||
| Pre-pregnancy to 3rd trimester change in BMI | <3 | 34 | 17.2 | 82 | 21.4 | 0.00 |
| 3 to 6 | 106 | 53.5 | 236 | 61.6 | ||
| 6+ | 58 | 29.3 | 65 | 17.0 | ||
| College or university degree | No | 54 | 26.2 | 254 | 65.1 | <0.0001 |
| Yes | 152 | 73.8 | 136 | 34.9 | ||
| Currently married | No | 36 | 17.5 | 8 | 2.1 | <0.0001 |
| Yes | 170 | 82.5 | 382 | 97.9 | ||
| Supplement use during pregnancy | None | n/a | 53 | 13.6 | n/a | |
| Multivitamin | 296 | 75.9 | ||||
| Vitamin D | 1 | 0.3 | ||||
| Multivitamin + vitamin D | 40 | 10.3 | ||||
| Ever drank alcohol during pregnancy | No | 186 | 90.3 | 370 | 94.9 | 0.03 |
| Yes | 20 | 9.7 | 20 | 5.1 | ||
| Ever smoked during pregnancy | No | 194 | 94.6 | 388 | 99.8 | <0.0001 |
| Yes | 11 | 5.4 | 1 | 0.3 | ||
Footnote: BMI: body mass index. Statistics are tabulated after excluding missing values. Extent of missingness (n): pre-pregnancy BMI: 12, 3rd trimester BMI: 4; sex of child in current pregnancy: 2, pre-pregnancy to 3rd trimester change in BMI: 15, ever smoked during pregnancy: 2. Race/ethnicity was not assessed in Mongolia and supplement use was not assessed in Boston. p-values are derived from chi-squared tests. Shading: p<0.05.
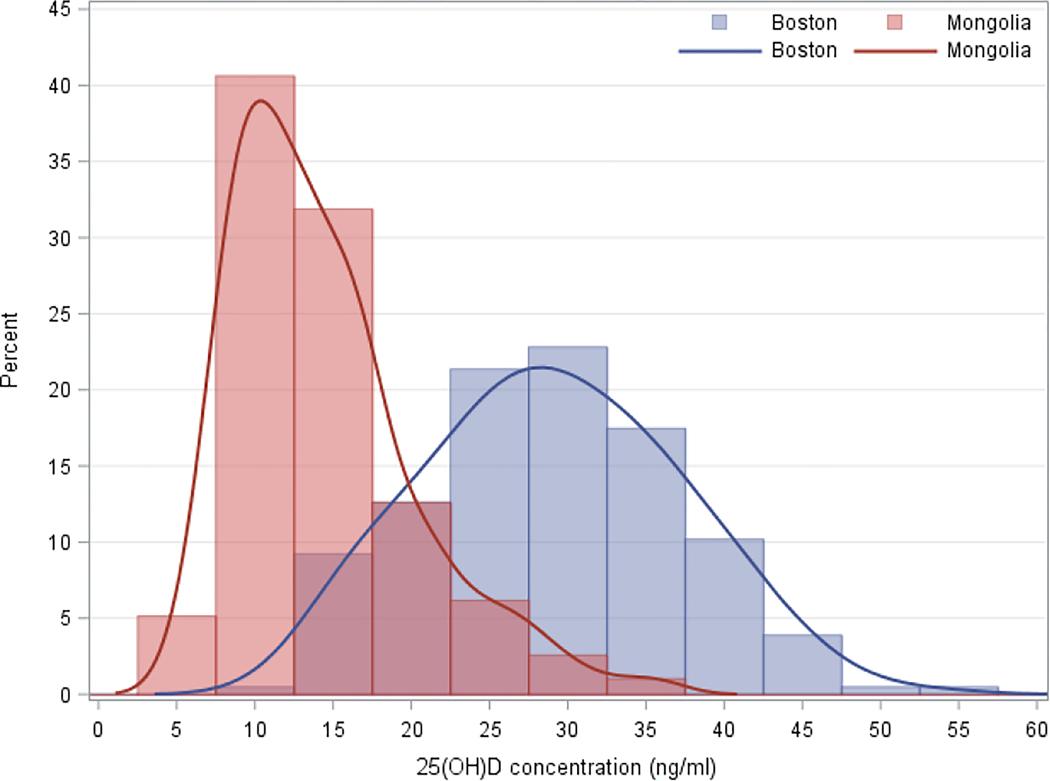
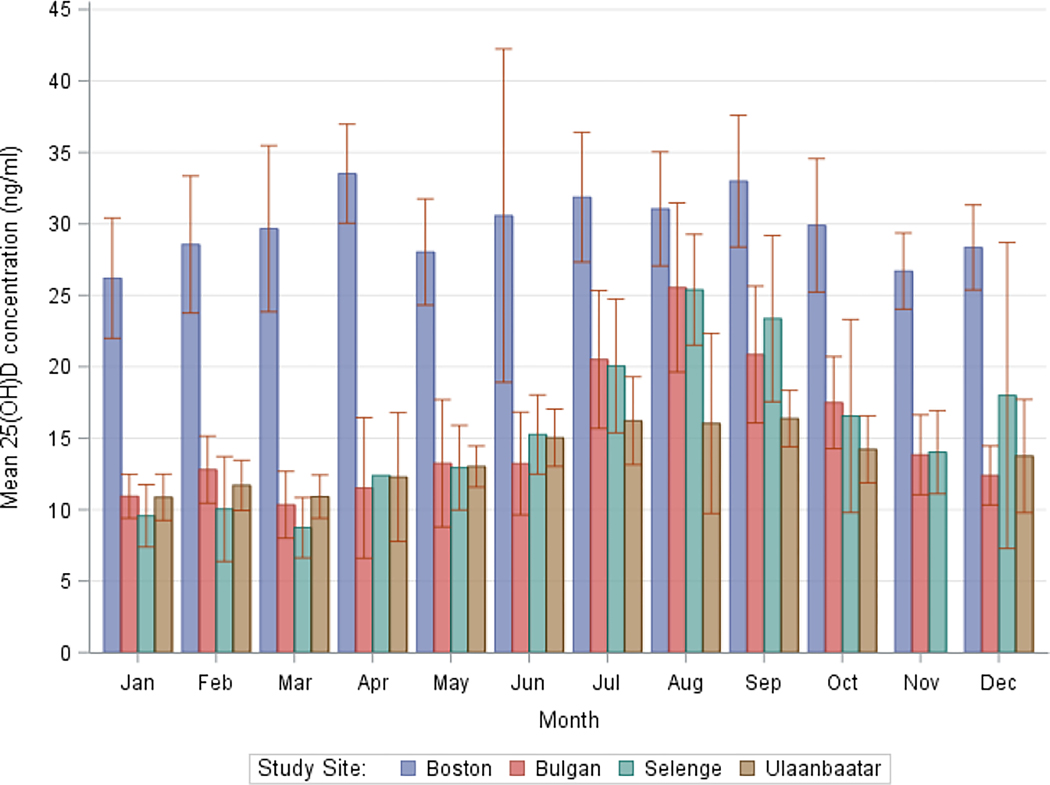
The seasonally-weighted annual mean serum 25(OH)D concentration in Boston and Mongolia was 29.7±9.3 and 14.4±6.0 ng/ml, respectively (Table 2), with participants in the capital Ulaanbaatar (13.2 ng/ml) having significantly lower concentrations than in both Bulgan and Selenge (15.2 and 15.3 ng/ml, respectively). The annual distribution of concentrations was normally-distributed in Boston and left-skewed in Mongolia (Figure 3). Mean concentrations displayed relatively little seasonality in Boston (seasonal range: 27.1 to 31.5 ng/ml) (Table 2, Figure 4), where at least 84% of the population remained above 20 ng/ml in every season. By contrast, mean concentrations were highly seasonal in Mongolia (seasonal range: 11.2 to 19.2 ng/ml), where at least 61% of the population remained below 20 ng/ml in every season. Seasonality of mean monthly measurements was the least pronounced in Ulaanbaatar: while the August mean in Bulgan and Selenge was 1.7 times those locations’ annual means, Ulaanbaatar saw an increase of only 1.2 times its annual mean during the peak in July.
Table 2:
Third trimester serum 25(OH)D concentration category by season and location
| Season | Location | N | 0 to 10 ng/ml | 10 to 20 ng/ml | 20 to 30 ng/ml | 30+ ng/ml | <20 ng/ml | <30 ng/ml | Mean | SD | Median | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||||||
| Seasonally-weighted * | Boston | 206 | 0 | 0.0 | 31 | 14.9 | 79 | 38.3 | 96 | 46.8 | 31 | 14.9 | 110 | 53.2 | 29.7 | 9.3 | 29.0 |
| Mongolia | 389 | 101 | 26.0 | 224 | 57.6 | 56 | 14.3 | 8 | 2.1 | 325 | 83.6 | 381 | 97.9 | 14.4 | 6.0 | 13.2 | |
| Ulaanbaatar | 195 | 58 | 29.9 | 117 | 60.0 | 17 | 8.7 | 3 | 1.4 | 175 | 89.9 | 192 | 98.6 | 13.2 | 4.9 | 12.5 | |
| Bulgan | 107 | 22 | 20.4 | 63 | 58.7 | 19 | 18.1 | 3 | 2.8 | 85 | 79.1 | 104 | 97.2 | 15.2 | 6.2 | 13.7 | |
| Selenge | 87 | 23 | 26.5 | 46 | 52.3 | 17 | 19.1 | 2 | 2.2 | 69 | 78.8 | 86 | 97.9 | 15.3 | 7.1 | 13.4 | |
| Spring | Boston | 50 | 0 | 0.0 | 8 | 16.0 | 17 | 34.0 | 25 | 50.0 | 8 | 16.0 | 25 | 50.0 | 30.3 | 10.0 | 30.0 |
| Mongolia | 94 | 42 | 44.7 | 50 | 53.2 | 2 | 2.1 | 0 | 0.0 | 92 | 97.9 | 94 | 100 | 11.2 | 3.9 | 10.2 | |
| Ulaanbaatar | 54 | 23 | 42.6 | 30 | 55.6 | 1 | 1.9 | 0 | 0.0 | 53 | 98.2 | 54 | 100 | 11.4 | 4.0 | 10.2 | |
| Bulgan | 25 | 10 | 40.0 | 14 | 56.0 | 1 | 4.0 | 0 | 0.0 | 24 | 96.0 | 25 | 100 | 11.5 | 4.1 | 10.1 | |
| Selenge | 15 | 9 | 60.0 | 6 | 40.0 | 0 | 0.0 | 0 | 0.0 | 15 | 100 | 15 | 100 | 9.6 | 3.2 | 8.6 | |
| Summer | Boston | 53 | 0 | 0.0 | 9 | 17.0 | 21 | 39.6 | 23 | 43.4 | 9 | 17.0 | 30 | 56.6 | 30.0 | 11.6 | 27.5 |
| Mongolia | 116 | 20 | 17.2 | 76 | 65.5 | 20 | 17.2 | 0 | 0.0 | 96 | 82.7 | 116 | 99.9 | 15.0 | 5.0 | 14.3 | |
| Ulaanbaatar | 70 | 14 | 20.0 | 45 | 64.3 | 11 | 15.7 | 0 | 0.0 | 59 | 84.3 | 70 | 100 | 14.4 | 4.7 | 13.9 | |
| Bulgan | 20 | 2 | 10.0 | 13 | 65.0 | 5 | 25.0 | 0 | 0.0 | 15 | 75.0 | 20 | 100 | 16.1 | 5.8 | 14.9 | |
| Selenge | 26 | 4 | 15.4 | 18 | 69.2 | 4 | 15.4 | 0 | 0.0 | 22 | 84.6 | 26 | 100 | 15.9 | 5.2 | 16.0 | |
| Autumn | Boston | 42 | 0 | 0.0 | 5 | 11.9 | 10 | 23.8 | 27 | 64.3 | 5 | 11.9 | 15 | 35.7 | 31.5 | 7.7 | 31.9 |
| Mongolia | 82 | 1 | 1.2 | 49 | 59.8 | 26 | 31.7 | 6 | 7.3 | 50 | 61.0 | 76 | 92.7 | 19.2 | 6.7 | 17.3 | |
| Ulaanbaatar | 32 | 1 | 3.1 | 27 | 84.4 | 3 | 9.4 | 1 | 3.1 | 28 | 87.5 | 31 | 96.9 | 15.3 | 4.9 | 14.9 | |
| Bulgan | 27 | 0 | 0.0 | 13 | 48.1 | 11 | 40.7 | 3 | 11.1 | 13 | 48.1 | 24 | 88.8 | 20.8 | 6.5 | 20.4 | |
| Selenge | 23 | 0 | 0.0 | 9 | 39.1 | 12 | 52.2 | 2 | 8.7 | 9 | 39.1 | 21 | 91.3 | 22.8 | 6.7 | 24.9 | |
| Winter | Boston | 61 | 0 | 0.0 | 9 | 14.8 | 34 | 55.7 | 18 | 29.5 | 9 | 14.8 | 43 | 70.5 | 27.1 | 7.0 | 26.7 |
| Mongolia | 98 | 40 | 40.8 | 51 | 52.0 | 6 | 6.1 | 1 | 1.0 | 91 | 92.8 | 97 | 98.9 | 12.3 | 4.5 | 10.9 | |
| Ulaanbaatar | 39 | 21 | 53.8 | 14 | 35.9 | 3 | 7.7 | 1 | 2.6 | 35 | 89.7 | 38 | 97.4 | 11.8 | 5.1 | 9.8 | |
| Bulgan | 35 | 11 | 31.4 | 23 | 65.7 | 1 | 2.9 | 0 | 0.0 | 34 | 97.1 | 35 | 100 | 12.3 | 3.4 | 11.8 | |
| Selenge | 23 | 7 | 30.4 | 14 | 60.9 | 2 | 8.7 | 0 | 0.0 | 21 | 91.3 | 23 | 100 | 13.0 | 5.1 | 11.5 | |
Footnote: Statistics are tabulated after excluding 1 missing vitamin D measurement.
Statistics for the “Seasonally-weighted” rows estimate annual statistics that would have been obtained had exactly 25% of the participants from each location been sampled in each season. Strength of shading is proportional to the percentage of each concentration category within each location. The following pairwise mean comparisons are statistically significant (t-test, α = 0.05): (1) Between locations within seasons: within each season, Boston > Ulaanbaatar, > Bulgan, > Selenge, and > Mongolia as a whole, and within Autumn, Ulaanbaatar < Bulgan and < Selenge; (2) Between seasons within locations: within Boston, Autumn > Winter; within Bulgan, Selenge, and Mongolia separately, all pairwise seasonal comparisons are significant except Spring vs. Winter; and within Ulaanbaatar, all pairwise seasonal comparisons are significant except Spring vs. Winter and Autumn vs. Summer.
Figure 3. Mean third trimester serum 25-hydroxyvitamin D concentration in Boston exceeds that in Mongolia.
Histogram of 25-hydroxyvitamin D by study location. Sample size: Boston=206, Mongolia=390.
Figure 4. Third trimester serum 25-hydroxyvitamin concentrations display less seasonality in Boston than in Mongolia.
Graph of mean and 95% confidence limits of 25-hydroxyvitamin D by month and study location. Sample size: Boston=206, Ulaanbaatar=196, Bulgan=107, Selenge=87. No Ulaanbaatar participants had blood drawn in November.
Adjusting for month of blood drawn, median serum 25(OH)D concentration among Boston participants was significantly associated with maternal age (+0.3 ng/ml per year), gravidity (−5.8 ng/ml among 3+ gravid women in comparison with 0 gravid women), pre-pregnancy and 3rd trimester BMI (−0.3 ng/ml per kg/m3), and education (+4.5 ng/ml among those without a college or university degree). After additionally adjusting for all other evaluated predictors, only gravidity remained independently predictive in Boston (−7.5 ng/ml among 3+ gravid women), particularly among those not currently married (interaction analysis estimated unmarried 3+ gravid women to have a 16.1 ng/ml lower median than their married counterparts). In Mongolia, multivitamin use during pregnancy significantly predicted 25(OH)D in seasonally-adjusted models only (+2.0 ng/ml), and only study location was predictive after multivariable adjustment (+4.1 ng/ml among participants in Selenge compared to those in Ulaanbaatar).
In Boston models, month of assessment, evaluated characteristics, and both month of assessment and evaluated characteristics explained 7.3%, 15.7, and 21.4% of model deviance, respectively, while in Mongolia models, these domains explained 19.8%, 7.3%, and 25.0% of model deviance.
Discussion
In this comparison of 389 Mongolian and 206 Bostonian third trimester pregnant women, we found a very high prevalence of vitamin D deficiency in urban and rural areas of Mongolia in comparison with Boston, USA. To our knowledge, this is the first multi-site study comparing vitamin D levels inside and outside of Mongolia. Vitamin D levels in Mongolia were highly seasonal and minimally explained by participant characteristics, given a lack of between-person variation at the extreme low end of the 25(OH)D distribution. By contrast, vitamin D levels in Boston were found to be relatively steady throughout the year, and we were able to identify discrete seasonally-independent participant characteristics associated with vitamin D status among Bostonian pregnant women.
In comparison with a prior 2000–2001 study of 62 Ulaanbaatar pregnant women which also used an ELISA platform, we found higher mean 25(OH)D levels in Ulaanbaatar in the present study (12.7 vs. 14.4, 11.7 vs. 15.3, 9.7 vs. 11.8, and 7.7 vs. 11.4 ng/ml in summer, autumn, winter, and spring, respectively) [15]. This may in part reflect increased access to vitamin D containing foods following recent globalization of Ulaanbaatar’s food market over the past two decades, and increased attention to vitamin D intake following experimental studies in Mongolia [19]. Nonetheless, in line with previous findings [9], Ulaanbaatar women had the lowest levels in this study, even in July-September during which residents of Selenge and Bulgan enjoyed a brief spike. In Boston, adjusted for age, season, and other factors, increased (3+) gravidity was the only independent predictor of vitamin D status, and may be a proxy for socioeconomic, behavioral, and biological variables which predispose to lower vitamin D levels. Other variables (age, BMI, and education), while not independently predictive and therefore less indicative of a causal effect, deserve attention as targets of screening in the U.S. and are among risk factors reported in prior studies of pregnant and postpartum women in high-income countries [20–24]. As compared with vitamin D levels in Mongolia, a recent assessment of 2004 lactating women from eight areas of neighboring China in different seasons also found an extremely high prevalence of deficiency (85% <12 ng/ml) with significant associations observed between [25(OH)D] and season, ethnicity, education, and income [25]; national assessments have also found a high prevalence of deficiency among almost all age ranges and geographic regions [26–28]. In Russia, although nationally-representative statistics are unavailable, a high prevalence of vitamin D deficiency has been found among 1st and 2nd trimester pregnant women [29], Moscow children and adolescents [30], and residents across west, north, northwest, and northeast Russia [31–33].
Comparatively depressed vitamin D status in Mongolia can likely be explained by a combination of unfavorable environmental factors (minimal sun exposure and intensity), darker skin pigmentation, minimal contribution of dietary vitamin D, and lack of industrial fortification [9]. In Mongolia, dietary inadequacy of nutrients other than vitamin D may also increase the risk of biochemical deficiency. For example, magnesium is required by all of the enzymes that metabolize vitamin D, and magnesium supplementation is associated with 25(OH)D status [34]; in a nationwide analysis of 320 healthy Mongolian adults aged 22–55, we estimated the nationally-weighted prevalence of dietary magnesium inadequacy to be 64% in summer and 66% in winter among urban women, and 79% in summer and 62% in winter among rural women [11]. Only 10.6% of Mongolian participants in this study took a vitamin D supplement. By contrast, 73% of pregnant women in the United States take vitamin D supplements [35] and have access to vitamin D rich foods including vitamin D fortified foods such as milk and orange juice [36]. We have previously projected the effect of vitamin D fortification in Mongolia and suggest it as a suitably effective and safe means of increasing vitamin D status for the adult population nationwide, on top of which more targeted strategies should be applied, such as supplementation [11]; our recent trial found high-dose (4000 IU/day) supplementation alone among 360 pregnant women in rural Mongolia to be safe and effective in increasing vitamin D levels [14]. By contrast, while vitamin D levels in Boston are not optimal year-round, they can be explained by specific screening characteristics which would suggest increased supplementation alone to be an effective approach.
A strength of this study is the measurement of vitamin D concentrations over all seasons collected from third trimester women in both locations. This allowed us to validly compare these distributions between locations and understand and control for the influence of season on vitamin D levels. Although gestation length at assessment differed slightly between locations, this was not identified as a significant predictor in seasonally-adjusted or multivariable-adjusted analyses, making it an unlikely confounder. We lacked information about sun time spent outdoors and clothing worn in each location (which are known to be important predictors), however, variation in sun exposure is captured by month of assessment (which we did control for). We also lacked information on diet. In prior assessments of non-pregnant Mongolian adults, we found vitamin D intake to be extremely low [11] and neither supplement use nor dietary intake to be significantly predictive of serum 25(OH)D concentrations, while in the U.S., both are important contributors to vitamin D status [12,27].
Given the striking difference in serum vitamin D levels between pregnant women in Boston and Mongolia, and the importance of adequate maternal serum vitamin D levels during pregnancy, the authors urge the Mongolian government to implement vitamin D fortification and targeted supplementation for the health of this critical segment of the population [11,14]. While Mongolia represents a particularly extreme case of population vitamin D deficiency, other vitamin D deficiency-endemic countries would also benefit from a multi-pronged approach such as this.
Table 3:
Seasonally-independent factors associated with third trimester serum 25(OH)D concentration
| Seasonally-adjusted | Seasonally+multivariable-adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Location | β | SE | p | β | SE | p | |
| Study location (ref: Ulaanbaatar) | Bulgan | Mongolia | 1.0 | 0.8 | 0.20 | 0.4 | 1.0 | 0.72 |
| Selenge | 1.4 | 0.9 | 0.14 | 4.1 | 1.4 | 0.00 | ||
| Maternal age (years) | Boston | 0.3 | 0.2 | 0.04 | 0.2 | 0.2 | 0.41 | |
| Mongolia | 0.0 | 0.1 | 0.76 | −0.0 | 0.1 | 0.89 | ||
| Not white | Boston | −3.1 | 1.9 | 0.09 | −0.6 | 2.0 | 0.77 | |
| Gestation length at 3rd trimester assessment (months) | Boston | 0.0 | 4.6 | 0.99 | −3.6 | 4.6 | 0.44 | |
| Mongolia | −0.4 | 0.6 | 0.45 | −0.6 | 0.6 | 0.29 | ||
| Gravidity (including current pregnancy) (ref: 0) | 1 | Boston | −0.4 | 2.6 | 0.88 | −1.6 | 2.5 | 0.54 |
| Mongolia | 0.3 | 0.9 | 0.77 | 0.0 | 1.0 | 0.99 | ||
| 2 | Boston | −0.5 | 2.6 | 0.85 | −1.9 | 2.5 | 0.45 | |
| Mongolia | 0.4 | 1.0 | 0.71 | −0.2 | 1.1 | 0.83 | ||
| 3+ | Boston | −5.8 | 2.7 | 0.03 | −7.5 | 2.6 | 0.00 | |
| Mongolia | 0.4 | 0.9 | 0.67 | 0.1 | 1.1 | 0.90 | ||
| Pre-pregnancy BMI | Boston | −0.3 | 0.2 | 0.05 | −0.2 | 0.2 | 0.23 | |
| Mongolia | 0.1 | 0.1 | 0.54 | 0.0 | 0.1 | 0.64 | ||
| 3rd trimester BMI | Boston | −0.3 | 0.1 | 0.04 | ||||
| Mongolia | 0.1 | 0.1 | 0.48 | |||||
| Pre-pregnancy to 3rd trimester change in BMI | Boston | 0.2 | 0.4 | 0.60 | −0.3 | 0.4 | 0.48 | |
| Mongolia | 0.0 | 0.2 | 0.81 | 0.01 | 0.17 | 0.94 | ||
| No college or university degree | Boston | 4.5 | 1.9 | 0.02 | 2.0 | 2.7 | 0.45 | |
| Mongolia | 0.0 | 0.7 | 0.95 | 0.5 | 0.8 | 0.53 | ||
| Not currently married | Boston | 2.7 | 2.3 | 0.23 | −1.2 | 3.0 | 0.68 | |
| Mongolia | −1.9 | 2.1 | 0.35 | −2.1 | 2.5 | 0.41 | ||
| Supplement use during pregnancy (ref: None) | Multivitamin only | Mongolia | 2.0 | 0.9 | 0.03 | 1.7 | 1.0 | 0.08 |
| Vitamin D (with or without multivitamin) | 1.2 | 1.2 | 0.33 | −2.0 | 1.7 | 0.26 | ||
| Drank alcohol or smoked during pregnancy | Boston | −1.3 | 2.6 | 0.62 | −0.1 | 2.5 | 0.97 | |
| Mongolia | 1.6 | 1.3 | 0.20 | 2.0 | 1.3 | 0.14 | ||
Footnote: N, Boston=206; N, Mongolia=390. BMI: body mass index. Statistics are derived from quantile regression estimating median serum 25(OH)D concentration in separate models for Boston and Mongolia, adjusting for either month of assessment alone using a single 12-category variable, or both month of assessment and all other factors in the table (the variable study location is not applicable to and is excluded from all Boston analyses; 3rd trimester BMI is excluded from all multivariable-adjusted analyses due to multicollinearity). Shading: p<0.05. Not shown: in Boston analyses, the square of 3rd trimester BMI is marginally significant in seasonally-adjusted analysis (β=−0.02, SE=0.01, p=0.07) and interaction between 3+ gravidity and “not currently married” is significant in multivariable-adjusted analysis (β=−16.1, SE=7.0, p=0.02).
Highlights.
Vitamin D levels in Mongolian third trimester pregnant women are severely depressed
Levels in pregnant women in Boston are comparatively elevated and less seasonal
Levels in Boston, but not Mongolia, are associated with targetable risk factors
Mongolia should consider a combined approach of fortification and supplementation
This joint approach is warranted in vitamin D deficiency-endemic regions globally
Acknowledgements:
We would like to thank our Scientific and Community Advisory Board at the Maternal and Child Health Center (MMCC), Ulaanbaatar; the OB-GYNs and midwives from the MMCC, Bulgan and Mandal Soum hospitals who assisted this study; Gyals Laboratory (Ulaanbaatar); research assistants Narkhajid and Solongo (Mongolian National University of Medical Sciences); Tanisha D. Evans (PPD Vaccines and Biologics Lab); and Tom Helde (Information Management Services) for their support, expertise, and hard work. Most of all, we would like to thank the women who very kindly and faithfully offered their time in this study.
Funding:
This research was supported by the National Cancer Institute (National Institutes of Health).
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- IU
international unit
- ELISA
enzyme-linked immunosorbent assay
- BMI
body mass index
Footnotes
Declarations of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr. 2008. Aug;88(2):520S–528S. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010. May;202(5):429.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollis BW, Wagner CL. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017. Aug 29;5:17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larqué E, Morales E, Leis R, Blanco-Carnero JE. Maternal and Foetal Health Implications of Vitamin D Status during Pregnancy. Ann Nutr Metab. 2018;72(3):179–192. [DOI] [PubMed] [Google Scholar]
- 5.De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016. Jan 14;(1):CD008873. [DOI] [PubMed] [Google Scholar]
- 6.Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ. 2017. Nov 29;359:j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018. Jul 1;172(7):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolsk HM, Chawes BL, Litonjua AA, Hollis BW, Waage J, Stokholm J, Bønnelykke K, Bisgaard H, Weiss ST. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS One. 2017. Oct 27;12(10):e0186657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromage S, Rich-Edwards JW, Tselmen D, Baylin A, Houghton LA, Baasanjav N, Ganmaa D. Seasonal Epidemiology of Serum 25-Hydroxyvitamin D Concentrations among Healthy Adults Living in Rural and Urban Areas in Mongolia. Nutrients. 2016. Sep 23;8(10). pii: E592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganmaa D, Holick MF, Rich-Edwards JW, Frazier LA, Davaalkham D, Ninjin B, Janes C, Hoover RN, Troisi R. Vitamin D deficiency in reproductive age Mongolian women: a cross sectional study. J Steroid Biochem Mol Biol. 2014. Jan;139:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromage S, Ganmaa D, Rich-Edwards JW, Rosner B, Bater J, Fawzi WW. Projected effectiveness of mandatory industrial fortification of wheat flour, milk, and edible oil with multiple micronutrients among Mongolian adults. PLoS One. 2018. Aug 2;13(8):e0201230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietary Guidelines Advisory Committee 2015. Supplementary Documentation to the 2015 DGAC Report. Washington: 2015 Dietary Guidelines Advisory Committee; 2015. [Google Scholar]
- 13.Public Health Institute of Mongolia (PHI). Nutrition Status of the Population of Mongolia - 5th National Nutrition Survey Report. Ulaanbaatar: Public Health Institute of Mongolia; 2017. [Google Scholar]
- 14.Enkhmaa D, Tanz L, Ganmaa D, Enkhtur S, Oyun-Erdene B, Stuart J, Chen G, Carr A, Seely EW, Fitzmaurice G, Buyandelger Y, Sarantsetseg B, Gantsetseg G, Rich-Edwards J. Randomized trial of three doses of vitamin D to reduce deficiency in pregnant Mongolian women. EBioMedicine. 2018. Dec 11. pii: S2352–3964(18)30564–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uush T, Fraser DR. Situation and Factors Associated with Rickets among Children in Mongolia; Public Health Institute Nutrition Research Center: Ulaanbaatar, Mongolia, 2003. [Google Scholar]
- 16.Madronich S, Flocke S. The role of solar radiation in atmospheric chemistry, in: Boule P (Ed.), Handbook of Environmental Chemistry, Springer Verlag, Heidelberg, 1998, pp. 1–26. [Google Scholar]
- 17.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010. Jan 11;340:b5664. [DOI] [PubMed] [Google Scholar]
- 18.Koenker R, Machado JAF. Goodness of fit and related inference processes for quantile regression. J Am Stat Assoc. 1999. Dec;94(448):1296–1310. [Google Scholar]
- 19.Ganmaa D, Stuart JJ, Sumberzul N, Ninjin B, Giovannucci E, Kleinman K, Holick MF, Willett WC, Frazier LA, Rich-Edwards JW. Vitamin D supplementation and growth in urban Mongol school children: Results from two randomized clinical trials. PLoS One. 2017. May 8;12(5):e0175237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen LB, Abrahamsen B, Dalgård C, Kyhl HB, Beck-Nielsen SS, Frost-Nielsen M, Jørgensen JS, Barington T, Christesen HT. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: a population-based cohort study. Clin Endocrinol (Oxf). 2013. Sep;79(3):333–41. [DOI] [PubMed] [Google Scholar]
- 21.Sohl E, Heymans MW, de Jongh RT, den Heijer M, Visser M, Merlijn T, Lips P, van Schoor NM. Prediction of vitamin D deficiency by simple patient characteristics. Am J Clin Nutr. 2014. May;99(5):1089–95. [DOI] [PubMed] [Google Scholar]
- 22.Choi R, Kim S, Yoo H, Cho YY, Kim SW, Chung JH, Oh SY, Lee SY. High prevalence of vitamin D deficiency in pregnant Korean women: the first trimester and the winter season as risk factors for vitamin D deficiency. Nutrients. 2015. May 11;7(5):3427–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall I, Mehta R, Ayers C, Dhumal S, Petrova A. Prevalence and risk factors for vitamin D insufficiency and deficiency at birth and associated outcome. BMC Pediatr. 2016. Dec 8;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolcott CG, Giguère Y, Weiler HA, Spencer A, Forest JC, Armson BA, Dodds L. Determinants of vitamin D status in pregnant women and neonates. Can J Public Health. 2016. Dec 27;107(4–5):e410–e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Yu Y, Li H, Chang Z, Li Y, Duan Y, Wang J, Jiang S, Yang Z, Yin SA. Vitamin D status and the prevalence of deficiency in lactating women from eight provinces and municipalities in China. PLoS One. 2017. Mar 23;12(3):e0174378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013. Jul-Aug;29(7–8):953–7. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Chen J, Wang R, Li M, Yun C, Li W, Yang Y, Piao J, Yang X, Yang L. Vitamin D Nutritional Status and its Related Factors for Chinese Children and Adolescents in 2010–2012. Nutrients. 2017. Sep 15;9(9). pii: E1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Yun C, He Y, Piao J, Yang L, Yang X. Vitamin D status among the elderly Chinese population: a cross-sectional analysis of the 2010–2013 China national nutrition and health survey (CNNHS). Nutr J. 2017. Jan 14;16(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popova P, Dronova A, Sadikova E, Parkkinen M, Bolshakova M, Grineva E. Vitamin D deficiency in Russian pregnant women and risk for gestational diabetes. Endocrine Abstracts. 2014. May;35:142. [Google Scholar]
- 30.Irina Z, Tatyana B, Pawel P, Tatyana T, Ekaterina S, Narine S, Natalia A, Nadejda B, Nadejda K, Vera M, Svetlana P, Valentina P, Natalia S, Irina S, Svetlana V, Maria M, Ekaterina K, Alina R, Yulia D, Leonid K, Victoria K. Seasonality of Vitamin D Insufficiency in Children of Moscow. Am J Pediatr. 2017;3(6):83–88. [Google Scholar]
- 31.Kozlov A, Khabarova Y, Vershubsky G, Ateeva Y, Ryzhaenkov V. Vitamin D status of northern indigenous people of Russia leading traditional and “modernized” way of life. Int J Circumpolar Health. 2014. Dec 2;73:26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karonova T, Andreeva A, Nikitina I, Belyaeva O, Mokhova E, Galkina O, Vasilyeva E, Grineva E. Prevalence of Vitamin D deficiency in the North-West region of Russia: A cross-sectional study. J Steroid Biochem Mol Biol. 2016. Nov;164:230–234. [DOI] [PubMed] [Google Scholar]
- 33.International Osteoporosis Foundation. The Eastern European & Central Asian Regional Audit: Epidemiology, costs & burden of osteoporosis in 2010. NaturaPrint, Geneva: 2011, pp. 41–46. [Google Scholar]
- 34.Dai Q, Zhu X, Manson JE, Song Y4, Li X, Franke AA, Costello RB, Rosanoff A, Nian H, Fan L, Murff H, Ness RM, Seidner DL, Yu C, Shrubsole MJ. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr. 2018. Dec 1;108(6):1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginde AA, Sullivan AF, Mansbach JM, Camargo CA Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010. May;202(5):436.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010. Apr;140(4):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]