Abstract
Background:
Pharmacovigilance (PV) is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine-related problem. Since its inception in the 1960s, PV has undergone continuous evolution, progressing from a basic level mainly focused on the collection and analysis of cases in its earliest years to a complex system regulated by rigorous standards and laws with modern PV. In recent years, PV has faced the challenge of adapting to rapid scientific advancements, the complexity of the pharma industry, and the digital revolution. To better understand the current state and future developments of PV within pharma companies, the PV working group “Ernesto Montagna” of the Italian Society of Pharmaceutical Medicine (SIMeF ETS) conducted a national survey in Italy.
Objectives:
The main objective of this survey was to explore the current state and future developments of PV within Pharmaceutical Companies in Italy.
Design:
This study was designed as a national survey targeting members of the Italian Society of Pharmaceutical Medicine (SIMeF ETS).
Methods:
The survey utilized computer-assisted web interview (CAWI) technology to collect data from SIMeF members across affiliate and corporate companies, aiming to explore expectations for PV. A simplified version of the questionnaire was also sent to members of the Clinical Research and Medical Affairs (RICMA) and Real-World Evidence working groups of SIMeF to gather input from RICMA professionals regarding the role of PV in pharma companies.
Results:
The survey revealed that PV in pharma companies is undergoing a transformation, with the potential for greater strategic alignment with business objectives and stakeholder focus. However, there is still room for improvement, particularly in terms of perception within other company departments. It is evident that PV’s evolution has only just begun.
Conclusion:
A critical factor in the evolution of PV is the adoption of a holistic and comprehensive approach to activities and processes. Scientific associations such as SIMeF can play a valuable role in cultivating new skills and capabilities among PV professionals, assisting, and supporting this change.
Keywords: innovation, patient centricity, pharmacovigilance
Plain language summary
The evolution of the Pharmacovigilance department in the pharmaceutical industry: results of an Italian national survey
Background:
Pharmacovigilance departments within the Pharmaceutical Companies have been structured several years ago, with the main objective to respond to the regulatory requirements and guarantee the correct and safe use of marketed products. The digitalization and the new approach to drug development drove to a rapid evolution of the healthcare environment. This change led the shift of Pharmacovigilance activities (initially mainly focused on the collection of the adverse events), to better ways to collect and use safety data, combined with the possibility to share them with decision makers, improving the engagement of patients and healthcare professionals. The Pharmacovigilance Working Group “Ernesto Montagna” of the Italian Society of Pharmaceutical Medicine (SIMeF ETS) carried out an online Italian national survey to have a better understanding of this new role of Pharmacovigilance in the new scenario and the perception of the Pharmacovigilance experts about this evolution. The digital world allows and makes easy sharing of information, including that related to the health status of patients and side effects of drugs. Healthcare/Pharmaceutical Companies are faced with both opportunities and challenges provided by such new ways of interaction among patients and healthcare professionals. It is precisely the emergence of new challenges in the scientific and technological fields and, not less important, the multiple and different operational and management skills required by Pharmacovigilance team members, that led us to reflect on which direction Pharmacovigilance is taking and what is expected for the near future.
Methods:
The Pharmacovigilance Working Group “Ernesto Montagna” of SIMeF ETS distributed a questionnaire to the members of SIMeF addressing questions to the Pharmacovigilance dedicated associates. The questionnaire was composed of open and closed questions to explore the affiliate and corporate scenario. A simplified version of the questionnaire was also sent to the members of Clinical Research and Medical Affairs (RICMA) and Real Word Evidence (RWE) Working Groups of SIMeF ETS to collect the perspective by clinical research and medical affairs on the role of Pharmacovigilance in the Pharmaceutical Companies.
Results:
The results show that the Pharmacovigilance departments in Pharmaceutical Companies are changing their positioning and have a greater attitude to be influential not only by executing the traditional compliance related tasks but also on more strategic and stakeholder-oriented activities. Despite this, there are still big areas of opportunities, and the change has just started.
Conclusion:
In a scenario evolving very quickly, it is critical to manage Pharmacovigilance in a more holistic and comprehensive way, adding to the compliance related activities, a more customer-oriented perspective. Scientific Pharmaceutical Associations like SIMeF ETS itself could give a valuable contribution to the development of new skills and capabilities to Pharmacovigilance personnel, to help and support this change, in an ecosystem rapidly evolving under the pressure of new technology and regulations, a more patient oriented approach and a more complex drug development.
Background
Pharmacovigilance (PV) is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine-related problem. 1 It is a critical component of the drug development and regulatory process, and it plays a vital role in ensuring the safety of patients.
Since its inception in the 1960s, PV has undergone continuous evolution, progressing from a basic level mainly focused on the collection and analysis of cases in its earliest years to a complex system regulated by rigorous standards and laws with modern PV.2,3 In recent years, PV has faced the challenge of adapting to rapid scientific advancements, the complexity of the pharmaceutical industry, and the digital revolution. Patient centricity, artificial intelligence (AI), automation and digitalization, plus advanced therapy medicinal products (ATMPs), are now key drivers of healthcare innovation, conditioning, and transforming PV, too. These changes have been accelerated during and following the recent pandemic period; for example, effective risk communication during public health emergencies/crises (e.g., COVID situation) showed the importance of topics such as patient centricity and more effective communications around safety aspects.
Patient centricity is the healthcare approach putting the patient at the center of all the decisions pertaining to their healthcare environment; they are directly involved and consulted for their needs and preferences when developing and delivering healthcare services. 4 Such an approach is now taking more and more space in the Pharmaceutical Companies, resulting in a significant impact on PV, for example, by driving a change from the original PV main purpose of identifying and reporting adverse events (AEs) to making PV concepts as an integral part of the employees and company culture with the ambition to support initiatives aimed at increasing PV awareness for final stakeholders.
Therefore, patient empowerment is pushing PV and probably will push even more significantly in the future to go beyond its traditional role, by increasingly considering the impact of medicines on patients’ quality of life, where the AEs experienced during disease treatment may play an important role in patient adherence and acceptability of the treatment. 5
Moving to the impact of AI and digitalization transformation in the healthcare sector, PV is not an exception. AI is being already used to develop new methods for detecting and analyzing AEs, and to identify risk factors. For example, AI-powered systems are being developed to scan electronic health records checking for potential AEs. These systems can identify patterns and trends that would be difficult or impossible for humans to detect at a convenient time.
Digital technologies are also being used to improve the collection and reporting of AE data,6,7 for example, by allowing the direct reporting of AEs by patients to their healthcare provider or to a Pharmaceutical Company using a smartphone app or website. This facilitates the intake of new AE data for the Pharmaceutical Companies. 8
Other important drivers for innovation in healthcare and in PV are the advanced therapies, ATMP,9,10 the new class of medicines derived from living cells, genes, or tissues. Advanced therapies are developed to treat a wide range of diseases, including cancer, rare diseases, and infectious diseases 11 , and present unique challenges for PV for the complexity of their AEs, not to mention that the small amount of population treated with advanced therapies can make difficult detecting and analyzing AEs.5,12
Therefore, PV teams are working with clinicians to develop patient reported outcomes measures to assess the impact of advanced therapies on patients’ quality of life and also new statistical methods for detecting and analyzing AEs in small patient populations and from new Real-World Data sources.13,14
Considering the above-discussed trends, PV has the potential to be a critical asset for increasing product value and to play a more strategic role for Pharmaceutical Companies while keeping the traditional role of protecting patient’s safety.4,15,16 This will influence even the perception of the PV personnel roles within the Pharmaceutical Companies, leading to impactful and challenging changes in the skills and capabilities required for the PV roles.
Having said that, we were interested to address two main points:
✓ Should we work on a different set of competencies?
✓ Should we work on a different perception of the role also with our internal and external stakeholders?
Bearing in mind the above questions, the PV working group “Ernesto Montagna” of the Italian Society of Pharmaceutical Medicine (SIMeF ETS) 17 conducted an Italian national survey to measure the dimension and perception of the change in the Pharmaceutical Companies, irrespective of being affiliate or corporate.
The PV working group “Ernesto Montagna” is part of SIMeF ETS (an Italian scientific society that promotes and coordinates scientific initiatives and encourages the dissemination of knowledge in both preclinical, clinical, and scientific and professional training of young researchers).
The “Ernesto Montagna” working group is dedicated to PV with the scope to stimulate continuous evolution and awareness of PV and to sustain capabilities and development for new PV professionals, transparently working with other scientific societies and institutions.
The results of the survey are presented in this paper.
Design and methods
Two open voluntary surveys (one directed to PV professionals, and one directed to medical affairs as a main stakeholder of PV) were designed and internally tested by the SIMeF PV working group; they were distributed through a computer platform using the computer-assisted web interview (CAWI) technology, with the aim to measure evolution of the role of PV inside pharma companies, listening from the function itself and from another function usually strictly linked to the PV department and more focused on the external stakeholder like physicians or patients.
The first survey was meant for PV personnel and was distributed in May 2023 to the members of SIMeF ETS through dedicated email invitations to SIMeF members and social media promotion, to explore the expectations of PV associates working in both affiliate and corporate companies. SIMeF ETS members included associates from Pharmaceutical Companies (both international and national), Medical Device Manufacturers, Consulting Companies, and Contract Research Organizations (CROs) working in the PV area.
This first survey consisted of 15 questions exploring the following thematic areas:
Cluster 1: Role of PV in Pharmaceutical Companies,
Cluster 2: Relationship with internal and external stakeholders,
Cluster 3: Evolution of PV.
Table A1 shows questions and clusters.
The second survey (Table A2), with similar questions, was meant to collect information from internal functions in pharma companies interacting with PV. The survey was addressed in October 2023 to the members of Clinical Research and Medical Affairs (RICMA) and Real-Word Evidence (RWE) working groups of SIMeF ETS to get the point of view of some of the main interacting functions with PV within the Pharmaceutical Companies specifically aiming to collect information on activities not linked to mandatory aspects (we aimed in our survey mostly to RWE groups involved in observational and RWE studies more relevant in a post-marketing setting).
The surveys did not include any patient data and did not require any informed consent or Ethics Committee approval. No incentives were offered, and the surveys were voluntary. Checklist for Reporting Results of Internet E-Surveys (CHERRIES) guidelines were followed for all applicable sections. 18
Completeness of the surveys was ensured as all fields necessary for the analysis were mandatory to be completed in both surveys. Respondents had the possibility to review data and change their answers, if needed, before submitting their answers. For both surveys, we considered the responses from Pharmaceutical Companies only, to have comparable information. No view rate/participation rate collection was foreseen.
Results are shown in the figures to compare from a qualitative point of view.
Results
Overall, 75 SIMeF ETS members provided answers to the first survey. Six (belonging to consultancy agencies and CROs) out of 75 respondents were excluded, as the analysis was focused on the Pharmaceutical Companies. Data shown are therefore coming from 69 valid respondents.
The answers to the survey confirmed the interest in the topic for both Pharmaceutical Companies (subdivided into Italian headquarters—36/69, equal to 52% of respondents—and personnel of multinational companies, 33/69 equal to 48%) and service providers companies (as presented in Figure 1).
Figure 1.
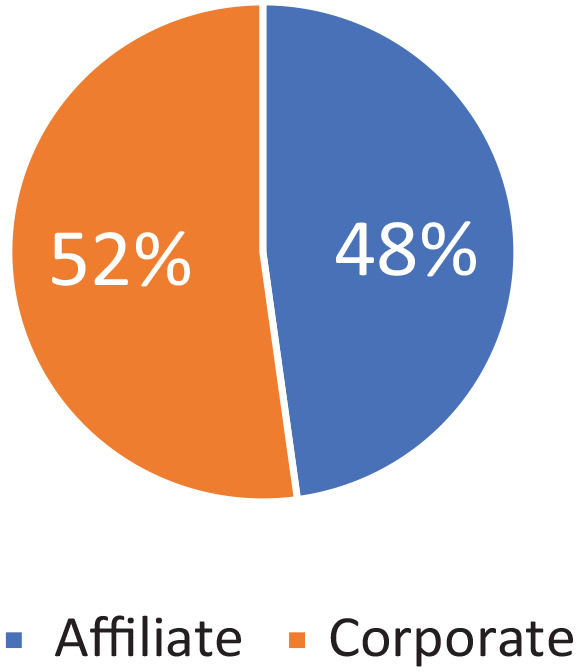
Location of responders (headquarter or an affiliate).
As PV is closely linked with other functions, the reporting line of the department in the company has been investigated (Figure 2). In the majority of cases, the PV personnel in the affiliates reports directly to global functions.
Figure 2.
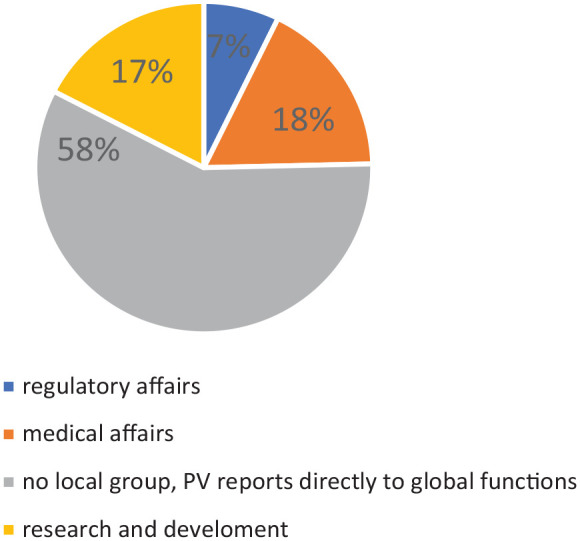
Reporting line of the PV department within the company.
PV, Pharmacovigilance.
Cluster 1: Role of PV in Pharmaceutical Companies
After the evaluation of the function positioning in the organization charts, the internal perception of the department within the company was investigated.
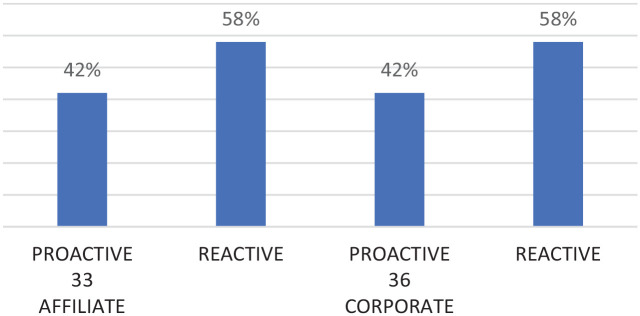
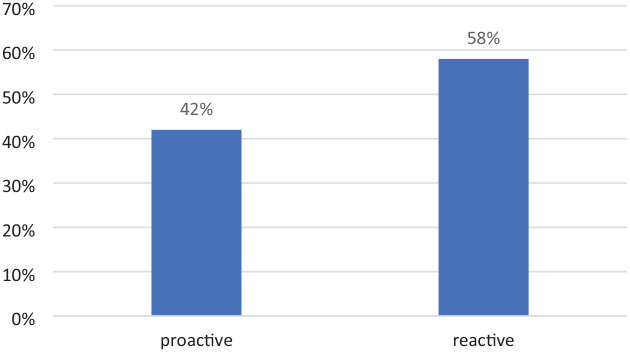
Figure 3 shows that PV is still perceived as a reactive function by 58% of survey responders, and the scenario does not change from affiliates to headquarters. Surprisingly, the PV department itself sees its approach as still mainly reactive (Figure 4). Although changes are already ongoing as it has been recognized by the majority of respondents (59/69), they also underlined that the PV proactive role is a bit far from being fully recognized at the internal level according to their perception (53.6%—37/69 responders felt not or little recognized).
Figure 3.
Perception of the PV role (reactive/proactive) according to the type of company (affiliate/corporate).
PV, Pharmacovigilance.
Figure 4.
How is the PV role perceived by the PV department itself?
PV, Pharmacovigilance.
On the other hand, answer in Figure 5 shows the significant PV evolution over the last 5 years as underlined by the 64% of responders.
Figure 5.
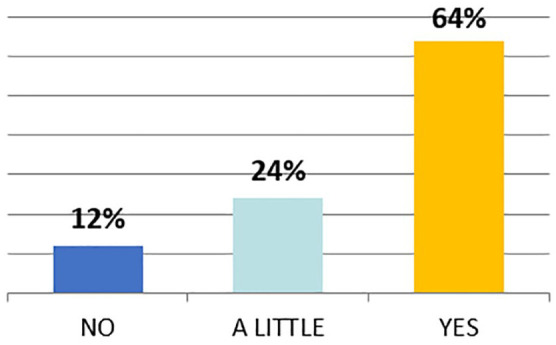
Evolution of the PV department in the last 5 years.
PV, Pharmacovigilance.
Looking at the answers provided to the question related to possible improvements and actions that can be put in place to improve PV perception in the company (Figure 6), the most important ones were focused on increasing training and awareness (32%), being more proactive in participating to cross-functional initiatives (25%) and carefully considering the resources and capabilities allocated to PV (16%).
Figure 6.
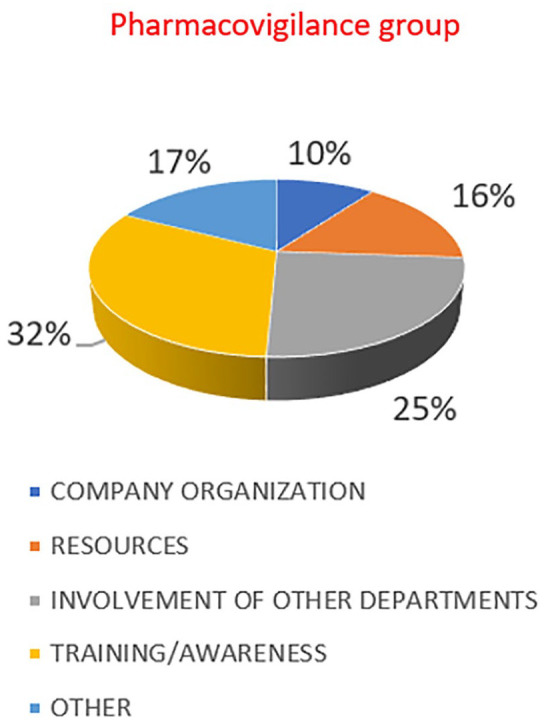
Actions that can be put in place to improve PV perception in the company.
PV, Pharmacovigilance.
Reflecting on the answers, it appears that, even though pharma companies have usually in place good training programs (mainly focused on the AEs collection) according to the EU Good Pharmacovigilance Practices, there is still a need to work more within the pharma companies on the awareness of scientific relevance of risk-benefit concept, especially for departments outside PV. As for external stakeholders, there is a need to promote a culture of PV. This need for more awareness has also been evidenced by patient associations and institutions, in particular after the pandemic period 19 : here pharma companies may have a role in working together with institutions.
The reactive role of the PV department has been also confirmed by the RICMA/RWE survey: 65% of respondents in the affiliate and 56% of respondents in the headquarters mainly considered the PV department still as a compliance-driven “regulatory” role.
Despite this perception, 58% of responders in the RICMA/RWE group highlighted an evolution of the PV roles in the last 5 years and suggested to improve the training and awareness (52%) together with a change in the company organization (22%) to increase the visibility of the department within the company.
Cluster 2: Relationship with internal and external stakeholders
The relationship of PV department with internal and external stakeholders was also investigated to evaluate the network within the company and the strategic role of PV.
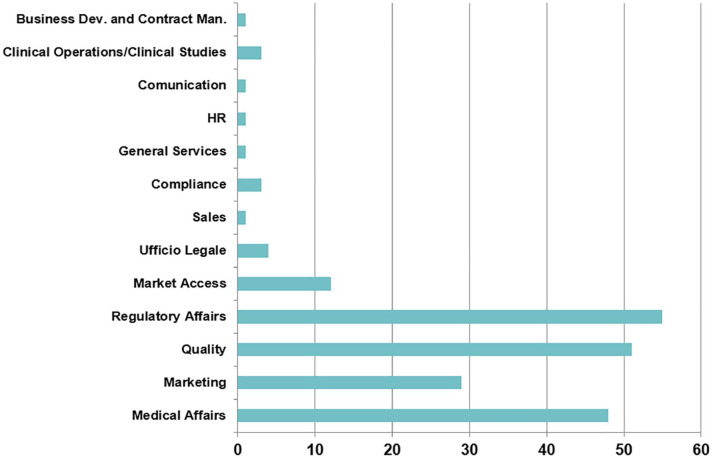
Figure 7 shows that Regulatory Affairs and Quality are the most represented departments that partner closely with PV; as expected the collaboration with Medical Affairs (48%) and with Marketing (nearly 30%) is also remarkable.
Figure 7.
Interaction between PV department and the other departments within the company.
PV, Pharmacovigilance.
Additional considerations should be made for the low percentage of interactions with Human Resources and Clinical Operations teams: such decreased interactions could be indicative of higher level of systems automation associated with the digital transformation within the companies (for example automatization between electronic-Case Report Form (e-CRF) and safety database, technical trainings frequently delivered through digital platforms).
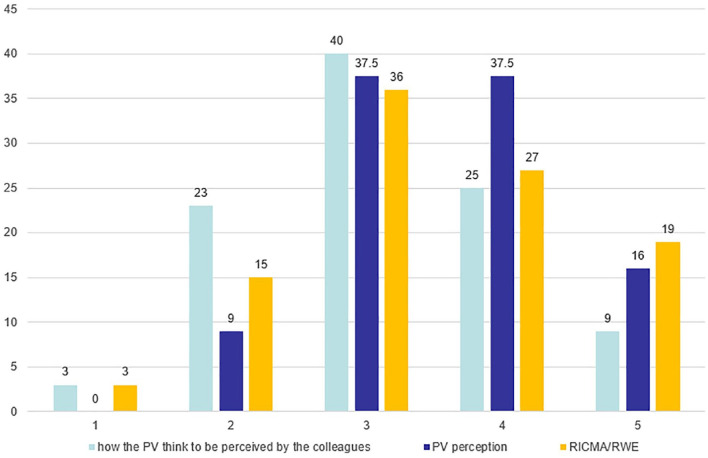
The perception of the strategic support given by PV is evaluated in both surveys, asking the responders to provide a rate from 1 to 5 (1-low—5-very high). Considering the responses provided by the PV employees on the perception of PV from other internal departments and the external perception provided by the responders of RICMA/RWE, it has been highlighted that the PV department could contribute with a medium-high level to the strategic decisions of the company and that the perception by the PV employees is also confirmed by the medical department (RICMA/RWE) responders (Figure 8). Considerable contribution to strategic decision making is indeed linked to a risk-benefit analysis of products as well as customer-centric management of relevant safety information.
Figure 8.
Perception of the PV department.
PV, Pharmacovigilance.
According to the change in the company’s mindset increasingly focused on the stakeholder’s involvement, the participation of the PV department in activities of external stakeholder engagement has been investigated and results showed in Figure 9: 29% of responders from the first survey confirmed their involvement in these activities, suggesting a possible evolution of the PV department to a proactive role, already and increasingly recognized as a strategic role for company decision.
Figure 9.
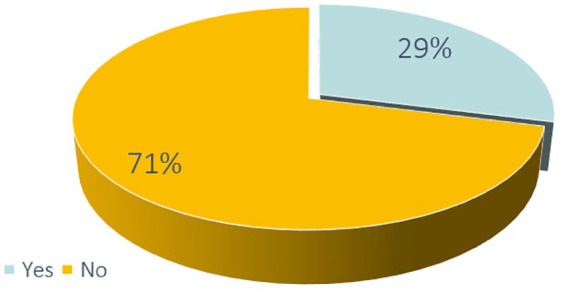
Participation of the PV department in activities of external stakeholder engagement.
PV, Pharmacovigilance.
Cluster 3: Evolution of PV
Considering the positioning of the PV department within the company and its evolving role, the expectations for the future have been investigated in both surveys.
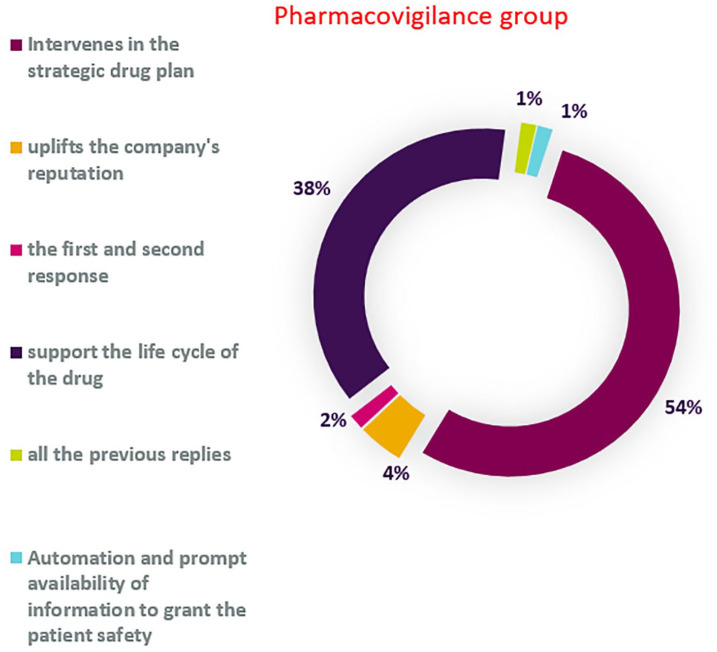
Figure 10 shows that PV employees consider the role of supporting the strategic plan for drug development (54%) and the drug lifecycle (38%). Interestingly, the RICMA/RWE survey shows opposite results (52% PV supporting the lifecycle of the drugs, 45% supporting the strategic plan of the drugs).
Figure 10.
Definition of PV in 2030.
PV, Pharmacovigilance.
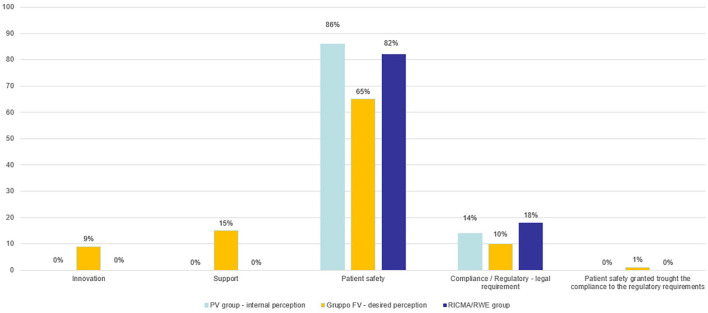
Figure 11 shows some keywords associated with PV. According to the respondents’ opinion, patient safety is the first one, followed by compliance/law obligations/regulatory requirement, Support, and Innovation by PV expectations as the third and fourth word, respectively.
Figure 11.
Keywords associated with PV.
PV, Pharmacovigilance.
The data presented reflect an expectation from PV to evolve and be recognized as a function with the distinctive aspect of protecting patient safety, but able to provide strategic and innovative support as well.
If we compare the results obtained when asking RICMA and RWE groups, the strong focus on patient safety is present, with a slightly higher number of respondents perceiving PV as mainly a regulatory compliance function.
This mismatch may probably be addressed with a better focus on safety strategic activities and on the added value of the safety profile of the product for patient outcomes.
Discussion
To our knowledge, this is the first systematic survey of the evolution of Italian Pharmaceutical Companies in the field of PV, with the purpose of assessing the level of evolution of the PV function within Pharmaceutical Companies, including the change in its relationship with internal and external stakeholders, and the perception of its future perspectives.
Among respondents, personnel from both Italian headquarters and from affiliates of international Pharmaceutical Companies have been included, thus providing a comprehensive picture of Pharmaceutical Companies overall. In addition, a second survey conducted with personnel belonging to the Medical Affairs and RWE departments brings additional qualitative insights to the data collection, allowing for the provision of additional context.
One of the strengths of our research is that SIMeF ETS is among the leading Italian scientific associations for professionals in the field of pharmaceutical medicine: it includes different companies, mainly from the pharmaceutical industry and CROs operating in Italy; therefore, these results can reasonably be considered as highly representative of the Italian situation in terms of numbers, distribution across the territory and relevance of the companies involved.
Another important element of our research was the use of the CAWI methodology, which allowed a straightforward collection of data, minimizing mistakes, and maximizing participation in the survey.
Limitations
One of the limitations of our research could be that it does not include the opinion of the top management of the companies (the survey was specifically addressed to PV personnel) which might provide insight into the next steps the companies are planning.
Another limitation to consider is the national level: only Italian respondents, although many are representatives of International Pharma Companies.
Conclusion
The overall results of our survey return the picture of PV as an evolving function within pharma companies, with the ambition to add to the strong focus on regulations and laws compliance a more strategic position, although with different paces and nuances in different companies: companies are clearly starting to consider PV departments more strategically in their structures.
This tendency may also be accelerated by technological evolution offering possibilities to automate activities related to data entry allowing more time for PV personnel for more strategic activities.
We may also affirm that medical affairs are welcoming the evolution of the PV role and look at this to improve partnerships on relevant activities specifically for strategic support: while automation is modifying interactions between departments at the technical level, 20 the value of strategic focus on patient safety aspects is becoming more and more relevant.
It is quite clear in our survey that PV awareness initiatives are still relevant and needed, both internally in Pharmaceutical Companies and externally since sometimes PV is not well known beyond the traditional role in AE reporting, as PV training to the company’s staff is generally more focused on this topic, rather than to the whole of PV activities.
We believe that, in this context, scientific associations (such as SIMeF ETS) should play a relevant role in providing full education on the role of PV, by promoting awareness and creating opportunities for uplifting skills and capabilities among PV personnel, especially considering the evolving scenario, where digitalization, 21 big data and patient involvement, together with new therapeutic options such as advanced therapies, are accelerating the evolution trends.
Acknowledgments
Mrs Sabrina Lucioni of SIMeF ETS secretariat supported the authors in the organization and conduct of the surveys. Carla Cottone, Carmela Casino, Grazia Sirizzotti, Pierluigi Crisà, Valentina Calderazzo, and Giacomo Pirisino supported the authors in discussing the results.
Appendix A: Evolution of Pharmacovigilance questionnaires
Table A1.
First survey—clusters of questions for PV department.
| Clusters | Questions |
|---|---|
| Demo | Are you based in a headquarter or an affiliate? |
| In the case of affiliate, which function does the PV department belong to in your company? | |
| Pharmacovigilance role | According to your understanding, how is the PV role perceived in your company (proactive or reactive)? |
| According to your understanding, how is the PV role perceived by the PV department itself (proactive or reactive)? | |
| Are there activities already ongoing with a proactive role? Can you provide some examples? | |
| Is this recognized by other colleagues? | |
| Has your PV role evolved in the last 5 years? | |
| Based on your experience, please provide 1–3 actions to improve the PV role in your company (open question) | |
| PV department relationship with internal and external stakeholders | Which are the departments with which you usually collaborate? |
| If you should rate (1–5) how your colleagues perceive your strategic support, which is your evaluation? | |
| If you should rate (1–5) yourself for the strategic support provided in your company, which is your evaluation? | |
| Have you ever developed in your company initiatives for external stakeholder engagement (e.g., PV awareness for physicians or patients, Health Care Professional (HCP) support from safety perspective, . . .)? If yes, can you please specify how many per year? | |
| Evolution of PV role in the Pharmaceutical Companies | If you think about PV function in 2030, how would you define it? |
| If we say Pharmacovigilance, which is the first word you think about? | |
| If we say Pharmacovigilance, which is the first word you would like your colleagues to think about? |
PV, Pharmacovigilance.
Table A2.
Second survey—clusters of questions for RICMA/RWE (medical affairs personnel).
| Clusters | Questions |
|---|---|
| Demo | Are you based in a headquarter or an affiliate? |
| In case of affiliate, which department belongs to your company? | |
| Pharmacovigilance role | According to your understanding, how is the PV role perceived in your company (proactive or reactive)? |
| How do you consider the role of the PV department (proactive or reactive)? | |
| Do you think that the PV is recognized by other colleagues? | |
| Has your PV role evolved in the last 5 years? | |
| Based on your experience, please provide 1–3 actions to improve PV role in your company (open question) | |
| PV department relationship with internal and external stakeholders | If you should rate (1–5) the strategic support provided by the PV department in your company, which is your evaluation? |
| Evolution of PV role in Pharmaceutical Companies | If you think about PV function in 2030, how would you define it? |
| If we say Pharmacovigilance, which is the first word you think about? |
PV, Pharmacovigilance; RICMA, Clinical Research and Medical Affairs; RWE, Real-Word Evidence.
Footnotes
ORCID iDs: Ilenia Bocchi  https://orcid.org/0000-0001-5762-2466
https://orcid.org/0000-0001-5762-2466
Daniela Bernardini  https://orcid.org/0009-0003-7689-5488
https://orcid.org/0009-0003-7689-5488
Contributor Information
Lisa Stagi, Roche S.p.A., Monza, Italy.
Ilenia Bocchi, Bayer S.p.A., Milano, Italy.
Daniela Bernardini, Novartis Farma S.p.A., Viale Luigi Sturzo, n. 43, Milano 20154, Italy.
Marika Ciappa, AstraZeneca S.p.A., Rho, Italy.
Stefania Dellon, Idorsia Pharmaceuticals Italy S.r.l., Milano, Italy.
Gian Nicola Castiglione, Free Lance, Pharmacovigilance Consultant, Italy.
Silvia Romano, Sanofi S.r.l., Milano, Italy.
Eros Fabrizi, Rhythm Pharmaceuticals, Roma, Italy.
Amanda Mattavelli, Johnson & Johnson Innovative Medicine, Milano, Italy.
Ilaria Grisoni, Jazz Pharmaceuticals, Villa Guardia, Italy.
Gabriella Finizia, Free Lance, Pharmacovigilance Consultant, Verona, Italy.
Stefano Bonato, Free Lance, Pharmacovigilance Consultant, Lissone, Italy.
Declarations
Ethics approval and consent to participate: The surveys did not include any patient data and did not require any informed consent or Ethics Committee approval. No incentives were offered, the surveys were voluntary. CHERRIES guidelines were followed for all applicable sections.
Consent for publication: Not applicable.
Author contributions: Lisa Stagi: Writing – original draft; Writing – review & editing.
Ilenia Bocchi: Writing – original draft; Writing – review & editing.
Daniela Bernardini: Writing – original draft; Writing – review & editing.
Marika Ciappa: Writing – review & editing.
Stefania Dellon: Writing – original draft; Writing – review & editing.
Gian Nicola Castiglione: Writing – original draft; Writing – review & editing.
Silvia Romano: Writing – original draft; Writing – review & editing.
Eros Fabrizi: Writing – original draft; Writing – review & editing.
Amanda Mattavelli: Writing – original draft; Writing – review & editing.
Ilaria Grisoni: Writing – review & editing.
Gabriella Finizia: Writing – review & editing.
Stefano Bonato: Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. WHO PV definition, https://www.who.int/teams/regulation-prequalification/regulation-and-safety/Pharmacovigilance.
- 2. For EU legislation please see for example: Directive 2010/84/EU; Directive 2012/26/EU; Regulation 1235/2010; Regulation 520/2012; European Medicines Agency (EMA) Good Pharmacovigilance Practices (GVP), https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/Pharmacovigilance-post-authorisation/good-Pharmacovigilance-practices-gvp.
- 3. For US legislation please see: Code of Federal Regulation (CFR) Title 21; Center for Drug Evaluation and Research (CDER) guidelines. [Google Scholar]
- 4. Report of the CIOMS working group XI, 2022: Patient involvement in the development, regulation, and safe use of medicines. [Google Scholar]
- 5. European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. EMA/CHMP/292464/2014, https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf (accessed 1 April 2016).
- 6. Whalen E, Hauben M, Bate A. Time series disturbance detection for hypothesis-free signal detection in longitudinal observational databases. Drug Saf 2018; 41: 565–577. [DOI] [PubMed] [Google Scholar]
- 7. Klein K, Scholl JHG De, Bruin ML, et al. When more is less: an exploratory study of the precautionary reporting bias and its impact on safety signal detection. Clin Pharmacol Ther 2018; 103: 296–303. [DOI] [PubMed] [Google Scholar]
- 8. Jokinen JD, Walley RJ, Colopy MW, et al. Pooling different safety data sources: impact of combining solicited and spontaneous reports on signal detection in Pharmacovigilance. Drug Saf 2019; 42: 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regulation (EC) No. 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No. 726/2004. [Google Scholar]
- 10. European Medicines Agency. Draft guideline on Pharmacovigilance for advanced therapy medicinal products. EMA/CMP/EWP/276139/2018, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-safety-and-efficacy-follow-risk-management-advanced-therapy-medicinal-products_en.pdf (accessed 20 November 2008).
- 11. Hauben M, Reynolds R, Caubel P. Deconstructing the Pharmacovigilance hype cycle. Clin Ther 2018; 40: 1981–1990. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization. Artificial intelligence in the healthcare sector, https://iris.who.int/bitstream/handle/10665/373421/9789240078871-eng.pdf?sequence=1&isAllowed=y (2023).
- 13. European Medicines Agency. Guideline on the use of electronic health record data for the Pharmacovigilance of medicinal products for human use. EMA/CHMP/EWP/700183/2020. 2020. [Google Scholar]
- 14. Lavertu A, Vora B, Giacomini KM, et al. A new era in pharmacovigilance: toward real-world data and digital monitoring. Clin Pharmacol Ther 2021; 109: 1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arlett P, Straus S, Rasi G. Pharmacovigilance 2030: invited commentary for the January 2020 “Futures” edition of Clinical Pharmacology and Therapeutics. Clin Pharmacol Ther 2020; 107(1): 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeck-Thole S, Enejo B. The future of pharmacovigilance. Arthur D. Little, 2023. [Google Scholar]
- 17. Società Italiana di Medicina Farmaceutica. https://www.simef.it.
- 18. Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004; 6(3): e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandiera P, Gianetta M, Leone S, et al. Patient Associations as key players in Pharmacovigilance: results of an Italian survey from the Patient Safety Council. Pharmadvances 2021; 3(3): 568–576. [Google Scholar]
- 20. Stagi L, Bocchi I, Bianco S, et al. Pharmacovigilance and the digital world in Italy: presentation of the results of a national survey, Ther Adv Drug Saf 2021; 12: 2042098620985991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernardini D, Bocchi I, Bonato S, et al. Guideline proposal for pharma companies to manage pharmacovigilance activities in digital media. AboutOpen 2022; 9: 21–28. [Google Scholar]