Abstract

Herein, we report that α-fluorocarboxylic acids undergo manganese-mediated oxidative 18F-fluorodecarboxylation with [18F]fluoride affording biologically relevant 18F-difluoromethyl(ene)-containing molecules. This no-carrier added process provides a solution to a known challenge in radiochemistry and expands the radiochemical space available for positron emission tomography (PET) ligand discovery. Scalability on a fully automated radiosynthetic platform is exemplified with the production of [18F]4,4-difluoropiperidine that, we demonstrate, is amenable to postlabeling functionalization including N-heteroarylation and amide as well as sulfonamide bond formation.
The geminal difluoro motif is highly prevalent in pharmaceuticals, agrochemicals and functional materials (Figure 1).1 Its incorporation into bioactive compounds has been demonstrated to impart profound pharmacokinetic and physicochemical effects, including modulation of lipophilicity, metabolic stability, and the pKa of adjacent functional groups.2 The unique properties of the gem-difluoro group also render it an ideal bioisostere of various functionalities, such as carbonyl, sulfonyl, and oxygen atoms.3 More specifically, the difluoromethyl (CF2H) group is able to exert conformational effects and engage in hydrogen-bonding interactions, providing opportunities for the enhancement of drug potency and selectivity.4 These unique characteristics have encouraged the development of numerous synthetic routes to gem-difluoroalkanes. Beyond the well-established deoxyfluorination of carbonyl groups, novel and orthogonal methodologies have been successfully pursued.5
Figure 1.
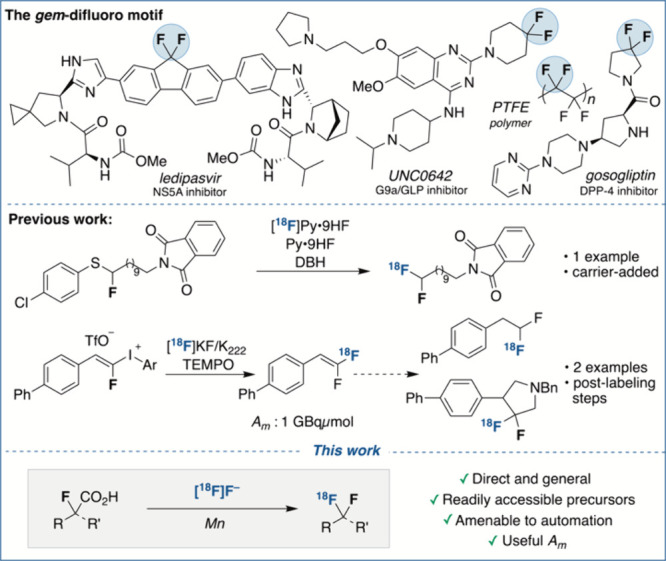
Prevalence of the gem-difluoro motif, previous methods for the synthesis of the 18F-difluoromethyl(ene) motif, and this work: synthesis of 18F-difluoromethyl(ene) motifs via fluorodecarboxylation with [18F]fluoride.
Positron emission tomography (PET) imaging is a highly sensitive, quantitative imaging technology that can greatly accelerate drug development.6 The technology hinges on the detection of γ rays generated by the decay of a positron (β+)-emitting radionuclide incorporated within a radiotracer and administered to patients. Fluorine-18 is ideally suited to this application due to its outstanding properties (e.g., 97% β+ decay, 0.635 MeV β+ energy) and is consequently routinely deployed in the clinic.7 Furthermore, its half-life of 109.8 min permits the multistep radiosynthesis of radiopharmaceuticals and their transportation to satellite imaging centers. While the synthesis of aryl and α-heteroatom 18F-difluoromethyl compounds has been well explored, access to molecules featuring the gem-18F-difluoromethyl(ene) motif at less activated positions remains a challenge in radiochemistry.8 At present, the synthesis of gem-18F-difluoroalkanes is indeed limited to two examples. In 2010, Haufe and co-workers disclosed a protocol for the synthesis of terminal gem-difluoroalkanes via a desulfurization-difluorination reaction of thioethers in the presence of pyridine polyhydrofluoride (Py·9HF) and 1,3-dibromo-5,5-dimethylhydantoin serving as an oxidant.9 Translation of this methodology to the radiofluorination of an α-fluorinated thioether with no-carrier-added [18F]KF proved unsuccessful, but the use of carrier-added [18F]Py·HF led to a single example of a gem-18F-difluoroalkane in 9% RCY (Figure 1). More recently, Tredwell and co-workers reported the synthesis of gem-18F-difluoroalkenes with [18F]KF, using fluoroalkenyl-(aryl)iodonium triflates, a class of substrates accessible from aldehydes in three steps.10 Molar activity (Am) reached 1 GBq/μmol. Further product derivatization reactions, including reduction and 1,3-dipolar cycloaddition, enabled the radiosynthesis of two gem-18F-difluoroalkanes (Figure 1).
Our aim was to develop a protocol, ideally amenable to automation, for the synthesis of geminal 18F-difluoro(cyclo)alkanes from easily accessible precursors and [18F]fluoride. We envisioned subjecting a monofluorinated substrate class to 18F-fluorination in order to avoid postlabeling 19F-fluorination or the requirement to prepare preformed 18F-difluoromethylene transfer reagents.8,11 This direct approach allows for a shorter radiosynthesis time, a key benefit in 18F-radiochemistry due to the loss of radioactivity due to the decay of fluorine-18 (t1/2 = 109.8 min). In terms of reaction design, traditional two-electron pathways featuring the displacement of leaving groups such as (pseudo)halides by [18F]fluoride pose significant challenges, due to the diminished reactivity of fluorinated carbon centers toward nucleophilic substitution reactions.8,12 Instead, we selected α-fluorocarboxylic acids guided by a previous report in our group describing the beneficial effect of α-fluoro substitution for a 18F-fluorodecarboxylative process leading to 18F-difluoromethyl arenes.13 α-Fluorocarboxylic acid precursors present the additional advantage of being commercially available or easily accessible from ubiquitous esters via electrophilic fluorination followed by hydrolysis, among other methods,14 minimizing synthetic bottlenecks.
Preliminary experiments focused on assessing the reactivity of 1-benzoyl-4-fluoropiperidine-4-carboxylic acid 1 toward 18F-fluorodecarboxylation with [18F]TEAF (Table 1). Pleasingly, in the presence of Mn(tmp)Cl (3) and the oxidant PhIO, the desired radiolabeled gem-difluorinated product [18F]2 was obtained in 46% RCY upon heating the reaction mixture at 80 °C over 20 min in 1,2-DCE (Table 1, entry 1). Alternative solvents, such as DMF and CHCl3, were also compatible albeit slightly less suitable for this transformation (Table 1, entries 2, 3). Performing the reaction at lower temperatures, e.g., 50 °C instead of 80 °C, still enabled the formation of the desired radiofluorinated product [18F]2 in lower RCY (Table 1, entry 4). Different loadings of the Mn species 3 and the oxidant PhIO led to [18F]2 in similar or diminished RCY (Table 1, entries 5–8).
Table 1. Optimization of the Reaction Conditionsa.
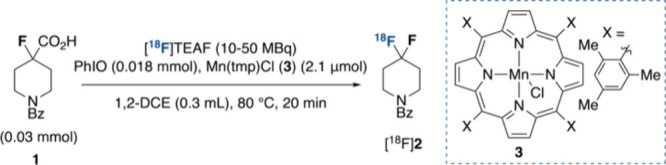
| entry | deviation from standard conditions | RCY (%) |
|---|---|---|
| 1 | none | 46 ± 14n=4 |
| 2 | DMF as solvent | 44n=1 |
| 3 | CHCl3 as solvent | 40 n=1 |
| 4 | 50 °C instead of 80 °C | 27n=1 |
| 5 | 9 μmol PhIO | 16n=1 |
| 6 | 36 μmol PhIO | 49n=1 |
| 7 | 1 μmol 3 | 32n=1 |
| 8 | 4 μmol 3 | 26n=1 |
[18F]TEAF was prepared with a NEt4HCO3 (9 mg) elution protocol. Bz: benzoyl. RCY: radiochemical yield, determined by radioHPLC analysis of the crude reaction mixture.
With the optimized conditions in hand, the scope of our 18F-fluorodecarboxylative protocol was investigated next (Scheme 1). In line with the outcome of a preliminary robustness screen (Figure s1),15,16 the reaction was found to be compatible with numerous functional groups, such as amide ([18F]2), carbamate ([18F]4, [18F]6, [18F]8, [18F]9), sulfonamide ([18F]7), aryl chloride ([18F]10), and phthalimide ([18F]13). The geminal 18F-difluoro motif was successfully installed within the ubiquitous piperidine scaffold,17 both at the 4- ([18F]2, ([18F]4) and 3-positions ([18F]6), as well as exocyclically ([18F]7). Additional N-heterocyclic geminal difluorides, such as Boc-protected pyrrolidine ([18F]8) and azetidine ([18F]9), were radiolabeled in moderate RCY. Furthermore, a cyclohexane derivative underwent 18F-fluorodecarboxylation in good RCY ([18F]10). Notably, these structures feature prominently in compounds of biological interest.2a,2b,18 Beyond cyclic substrates, fluorine-18 was successfully introduced within alkyl chains of varying lengths and substitution patterns ([18F]11–13). The adamantane moiety, often considered as a lead structure in the development of novel pharmaceuticals,19 was also compatible with our conditions, furnishing [18F]14 in 38% RCY. The reaction was suitable for 18F-labeling at the benzylic position, enabling the radiolabeling of 9,9-difluoro-9H-fluorene ([18F]15), a motif contained in the core structure of the antiviral ledipasvir (Figure 1). Last, the 18F-trifluoromethyl group was also within reach of this transformation, as exemplified by the radiosynthesis of [18F]16. Unsuccessful substrates can be found in the Supporting Information (Figure S8).
Scheme 1. Scope of the 18F-Fluorodecarboxylation Reaction.

[18F]TEAF was prepared with a NEt4HCO3 (9 mg) elution protocol. Bz: benzoyl. RCY: radiochemical yield determined by radioHPLC analysis of the crude reaction mixture.
Similarly to a seminal report by Groves and co-workers on the Mn-mediated 18F-fluorodecarboxylation of carboxylic acids,13b we suggest that the reaction proceeds via 18F-fluorine atom transfer between a 18F-fluoromanganese(IV) complex and an α-fluoroalkyl radical formed upon decarboxylation of the α-fluorocarboxylic acid substrate.
In line with previous mechanistic observations,13a the presence of a fluorine atom at the α-carbonyl position was found to be crucial for reactivity, likely providing stabilization of the resulting carbon-centered radical via resonance effects.20 In the absence of α-fluoro substitution, an analogue of model substrate 1 led to no 18F-incorporation ([18F]5) under the standard reaction conditions (Scheme 1).
For (pre)clinical applications, automation of the reaction on a commercial platform is crucial as it permits the safe and reliable synthesis of radiotracers on multi-GBq scale. Hence, we set out to explore the feasibility of performing our 18F-fluorodecarboxylation protocol with a TRASIS AllinOne synthesizer. For the efficient transfer of the reaction mixture within the radiosynthetic module, soluble PhI(OAc)2 was selected in place of PhIO. In addition, manganese species 3 was directly employed for the elution of [18F]fluoride from the ion exchange cartridge to afford [18F]Mn(tmp)F with an elution efficiency of 94%.13
An automated program inclusive of semipreparative HPLC purification thus enabled the radiosynthesis of [18F]4 in an AY of 1.74 GBq from 50 GBq starting activity and high chemical and radiochemical purity (>99%) with a total synthesis time of 78 min (Scheme 2). Am reached 6.35 GBq/μmol (d.c. EOS). An added advantage, in contrast to previous reports of 18F-fluorodecarboxylative strategies, is the direct use of an α-fluorocarboxylic acid substrate, bypassing the time-consuming requirement for the isolation of an activated preformed iodine(III) complex.13 The residual amount of manganese in the purified sample of [18F]4 was measured by ICP-MS and was found to be 4.1 μg/L, thereby meeting the ICH guidelines for pharmaceuticals destined for human use.15
Scheme 2. Automation of the the 18F-Fluorodecarboxylation Reaction.
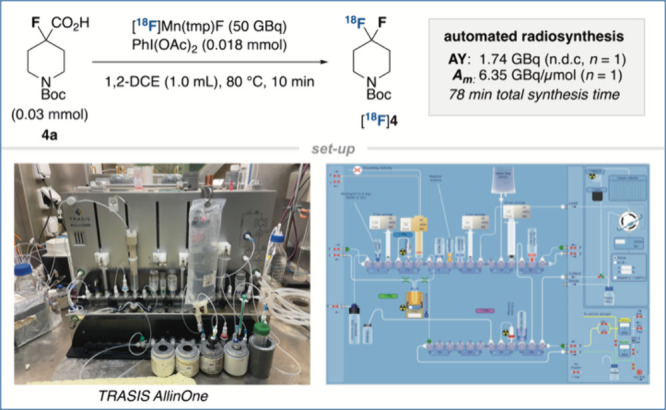
AY (n.d.c.): activity yield (non decay-corrected); Am (d.c. EOS): molar activity (decay-corrected to the end-of-synthesis). Automated radiosynthesis was achieved using a Trasis AllinOne platform.
With an automated radiosynthetic protocol secured for [18F]4, we set out to demonstrate its value as a versatile building block in radiochemistry, encouraged by the privileged role of piperidine in medicinal chemistry campaigns, as highlighted by a recent study identifying it as the most common N-heterocycle in small-molecule pharmaceuticals.17a [18F]4,4-Difluoropiperidine ([18F]18) was expediently accessed upon Boc-deprotection of [18F]4 and engaged in a series of diversification reactions (Scheme 3).15 For example, treatment of [18F]18 with 6-chloro-9-ethyl-9H-purine afforded the SNAr product [18F]19 in excellent RCY. An amide coupling protocol with a N-hydroxysuccinimide ester was also successful, providing a radiofluorinated derivative of the antiepileptic agent ilepcimide ([18F]20) in 65% RCY. Last, sulfonamide [18F]21, displaying PDE5 inhibitory activity, was prepared in 92% RCY via condensation of [18F]18 with the requisite sulfonyl chloride precursor.21
Scheme 3. Derivatization Reactions of [18F]4,4-Difluoropiperidine.
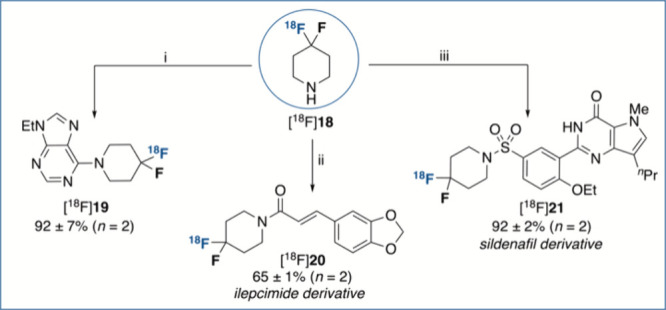
RCY: radiochemical yield determined by radioHPLC analysis of the crude reaction mixture. (i) 6-Chloro-9-ethyl-9H-purine (0.05 mmol), K2CO3 (0.05 mmol), DMSO (0.5 mL), 110 °C, 20 min; (ii) 2,5-dioxopyrrolidin-1-yl (E)-3-(benzo[d][1,3]dioxol-5-yl)acrylate (0.05 mmol), K2CO3 (0.05 mmol), DMA (0.5 mL), 40 °C, 20 min; (iii) 4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene-1-sulfonyl chloride (0.05 mmol), NEt3 (0.1 mmol), and THF (0.5 mL), 40 °C, 20 min.
In conclusion, we have developed a protocol for the synthesis of geminal 18F-difluoroalkanes via manganese-mediated 18F-fluorodecarboxylation of easily accessible α-fluorocarboxylic acids with [18F]fluoride. This first direct radiosynthesis of the geminal 18F-difluoromethyl(ene) motif under no-carrier added conditions was applied to various scaffolds, including medicinally relevant cyclic amines. Scalability and translation to a fully automated radiosynthesis platform were also demonstrated, furnishing radiolabeled products in useful AY and Am. The value of [18F]4,4-difluoropiperidine as a versatile building block is further demonstrated with the assembly of complex and biorelevant radiolabeled scaffolds. Given the significance of the geminal difluoro motif in functional materials as well as medicinal and agrochemistry, we anticipate that the novel radiochemical space accessible with this technology will spark meaningful innovation in the development of radiotracers for applications in PET imaging.
Acknowledgments
V.G. and S.O. acknowledge financial support from Global Discovery Chemistry, Therapeutics Discovery, Johnson & Johnson Innovative Medicine, Janssen-Cilag S.A., Toledo, Spain. J.F. is grateful to the Centre for Doctoral Training in Synthesis for Biology and Medicine for a studentship, generously supported by GlaxoSmithKline, MSD, Syngenta, and Vertex. R.S. is grateful to the Centre for Doctoral Training in Synthesis for Biology and Medicine for a studentship, generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB, and Vertex. B.S. is grateful to the Swiss National Science Foundation for a postdoctoral fellowship. The authors gratefully acknowledge Cecilia Anderson (University of Oxford), Gianluca Destro (University of Oxford), Adeline W. J. Poh (University of Oxford), and Jeroen B. I. Sap (University of Oxford) for useful discussions and assistance with early experiments, as well as Simon Waller (Cardiff University) for assistance with ICP-MS experiments.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.4c03611.
Preparation of starting materials, labeling precursors and reference materials; radiofluorination methods; (radio)HPLC traces; NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Ogawa Y.; Tokunaga E.; Kobayashi O.; Hirai K.; Shibata N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. 10.1016/j.isci.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Inoue M.; Sumii Y.; Shibata N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiménez-Skrzypek G.; González-Sálamo J.; Hernández-Borges J. Analytical methodologies and occurrence of per- and polyfluorinated alkyl substances – a review. J. Chromatogr. Open 2023, 4, 100089. 10.1016/j.jcoa.2023.100089. [DOI] [Google Scholar]
- a Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; b Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]; c Jeffries B.; Wang Z.; Felstead H. R.; Le Questel J.-Y.; Scott J. S.; Chiarparin E.; Graton J.; Linclau B. Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs. J. Med. Chem. 2020, 63, 1002–1031. 10.1021/acs.jmedchem.9b01172. [DOI] [PubMed] [Google Scholar]; d Huchet Q. A.; Kuhn B.; Wagner B.; Kratochwil N. A.; Fischer H.; Kansy M.; Zimmerli D.; Carreira E. M.; Müller K. Fluorination Patterning: A Study of Structural Motifs That Impact Physicochemical Properties of Relevance to Drug Discovery. J. Med. Chem. 2015, 58, 9041–9060. 10.1021/acs.jmedchem.5b01455. [DOI] [PubMed] [Google Scholar]
- a Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]; b Liu Y.; Ahmed V.; Hill B.; Taylor S. D. Synthesis of a non-hydrolyzable estrone sulfate analogue bearing the difluoromethanesulfonamide group and its evaluation as a steroid sulfatase inhibitor. Org. Biomol. Chem. 2005, 3, 3329–3335. 10.1039/b508852f. [DOI] [PubMed] [Google Scholar]; c Zhou Q.; Ruffoni A.; Gianatassio R.; Fujiwara Y.; Sella E.; Shabat D.; Baran P. S. Direct Synthesis of Fluorinated Heteroarylether Bioisosteres. Angew. Chem., Int. Ed. 2013, 52, 3949–3952. 10.1002/anie.201300763. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ivanova M. V.; Bayle A.; Besset T.; Pannecoucke X.; Poisson T. New Prospects toward the Synthesis of Difluoromethylated Phosphate Mimics. Chem.—Eur. J. 2016, 22, 10284–10293. 10.1002/chem.201601310. [DOI] [PubMed] [Google Scholar]
- a Sap J. B. I.; Meyer C. F.; Straathof N. J. W.; Iwumene N.; am Ende C. W.; Trabanco A. A.; Gouverneur V. Late-stage difluoromethylation: concepts, developments and perspective. Chem. Soc. Rev. 2021, 50, 8214–8247. 10.1039/D1CS00360G. [DOI] [PubMed] [Google Scholar]; b Sessler C. D.; Rahm M.; Becker S.; Goldberg J. M.; Wang F.; Lippard S. J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 9325–9332. 10.1021/jacs.7b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Malquin N.; Rahgoshay K.; Lensen N.; Chaume G.; Miclet E.; Brigaud T. CF2H as a hydrogen bond donor group for the fine tuning of peptide bond geometry with difluoromethylated pseudoprolines. Chem. Commun. 2019, 55, 12487–12490. 10.1039/C9CC05771D. [DOI] [PubMed] [Google Scholar]; d Zafrani Y.; Saphier S.; Gershonov E. Utilizing the CF2H Moiety As a H-bond-donating Group in Drug Discovery. Future Med. Chem. 2020, 12, 361–365. 10.4155/fmc-2019-0309. [DOI] [PubMed] [Google Scholar]; e Xing L.; Blakemore D. C.; Narayanan A.; Unwalla R.; Lovering F.; Denny R. A.; Zhou H.; Bunnage M. E. Fluorine in Drug Design: A Case Study with Fluoroanisoles. ChemMedChem. 2015, 10, 715–726. 10.1002/cmdc.201402555. [DOI] [PubMed] [Google Scholar]; f Zafrani Y.; Sod-Moriah G.; Yeffet D.; Berliner A.; Amir D.; Marciano D.; Elias S.; Katalan S.; Ashkenazi N.; Madmon M.; Gershonov E.; Saphier S. CF2H, a Functional Group-Dependent Hydrogen-Bond Donor: Is It a More or Less Lipophilic Bioisostere of OH, SH, and CH3?. J. Med. Chem. 2019, 62, 5628–5637. 10.1021/acs.jmedchem.9b00604. [DOI] [PubMed] [Google Scholar]
- Carvalho D. R.; Christian A. H. Modern approaches towards the synthesis of geminal difluoroalkyl groups. Org. Biomol. Chem. 2021, 19, 947–964. 10.1039/D0OB02374D. [DOI] [PubMed] [Google Scholar]
- a Ametamey S. M.; Honer M.; Schubiger P. A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]; b Katal S.; Eibschutz L. S.; Saboury B.; Gholamrezanezhad A.; Alavi A. Advantages and Applications of Total-Body PET Scanning. Diagnostics 2022, 12, 426. 10.3390/diagnostics12020426. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nerella S. G.; Singh P.; Sanam T.; Digwal C. S. PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front. Med. 2022, 9, 812270. 10.3389/fmed.2022.812270. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ghosh K. K.; Padmanabhan P.; Yang C.-T.; Ng D. C. E.; Palanivel M.; Mishra S.; Halldin C.; Gulyas B. Positron emission tomographic imaging in drug discovery. Drug Discovery Today 2022, 27, 280–291. 10.1016/j.drudis.2021.07.025. [DOI] [PubMed] [Google Scholar]
- a Wang Y.; Lin Q.; Shi H.; Cheng D. Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design. Front. Chem. 2022, 10, 884517. 10.3389/fchem.2022.884517. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jacobson O.; Kiesewetter D. O.; Chen X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18. 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Deng X.; Rong J.; Wang L.; Vasdev N.; Zhang L.; Josephson L.; Liang S. H. Chemistry for Positron Emission Tomography: Recent Advances in 11C-,18F-,13N-, and 15O-Labeling Reactions. Angew. Chem., Int. Ed. 2019, 58, 2580–2605. 10.1002/anie.201805501. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Rong J.; Haider A.; Jeppesen T. E.; Josephson L.; Liang S. H. Radiochemistry for positron emission tomography. Nat. Commun. 2023, 14, 3257. 10.1038/s41467-023-36377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Coenen H. H.; Ermert J. 18F-labelling innovations and their potential for clinical application. Clin. Transl. Imaging 2018, 6, 169–193. 10.1007/s40336-018-0280-0. [DOI] [Google Scholar]; f Ajenjo J.; Destro G.; Cornelissen B.; Gouverneur V. Closing the gap between 19F and 18F chemistry. EJNMMI Radiopharm. Chem. 2021, 6, 33. 10.1186/s41181-021-00143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Campbell M. G.; Mercier J.; Genicot C.; Gouverneur V.; Hooker J. M.; Ritter T. Bridging the gaps in 18F PET tracer development. Nat. Chem. 2017, 9, 1–3. 10.1038/nchem.2693. [DOI] [PubMed] [Google Scholar]; h Halder R.; Ritter T. 18F-Fluorination: Challenge and Opportunity for Organic Chemists. J. Org. Chem. 2021, 86, 13873–13884. 10.1021/acs.joc.1c01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.; Ortalli S.; Gouverneur V. The 18F-Difluoromethyl Group: Challenges, Impact and Outlook. Angew. Chem., Int. Ed. 2024, 63, e202404957 10.1002/anie.202404957. [DOI] [PubMed] [Google Scholar]
- Hugenberg V.; Wagner S.; Kopka K.; Schober O.; Schäfers M.; Haufe G. Synthesis of Geminal Difluorides by Oxidative Desulfurization-Difluorination of Alkyl Aryl Thioethers with Halonium Electrophiles in the Presence of Fluorinating Reagents and Its Application for 18F-Radiolabeling. J. Org. Chem. 2010, 75, 6086–6095. 10.1021/jo100689v. [DOI] [PubMed] [Google Scholar]
- Frost A. B.; Brambilla M.; Exner R. M.; Tredwell M. Synthesis and Derivatization of 1,1-[18F]Difluorinated Alkenes. Angew. Chem., Int. Ed. 2019, 58, 472–476. 10.1002/anie.201810413. [DOI] [PubMed] [Google Scholar]
- a Yuan G.; Wang F.; Stephenson N. A.; Wang L.; Rotstein B. H.; Vasdev N.; Tang P.; Liang S. H. Metal-free 18F-labeling of aryl-CF2H via nucleophilic radiofluorination and oxidative C–H activation. Chem. Commun. 2017, 53, 126–129. 10.1039/C6CC07913J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Trump L.; Lemos A.; Lallemand B.; Pasau P.; Mercier J.; Lemaire C.; Luxen A.; Genicot C. Late-Stage 18F-Difluoromethyl Labeling of N-Heteroaromatics with High Molar Activity for PET Imaging. Angew. Chem., Int. Ed. 2019, 58, 13149–13154. 10.1002/anie.201907488. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sap J. B. I.; Meyer C. F.; Ford J.; Straathof N. J. W.; Dürr A. B.; Lelos M. J.; Paisey S. J.; Mollner T. A.; Hell S. M.; Trabanco A. A.; Genicot C.; am Ende C. W.; Paton R. S.; Tredwell M.; Gouverneur V. [18F]Difluorocarbene for positron emission tomography. Nature 2022, 606, 102–108. 10.1038/s41586-022-04669-2. [DOI] [PubMed] [Google Scholar]
- Martinez H.; Rebeyrol A.; Nelms T. B.; Dolbier W. R. Impact of fluorine substituents on the rates of nucleophilic aliphatic substitution and β-elimination. J. Fluorine Chem. 2012, 135, 167–175. 10.1016/j.jfluchem.2011.10.008. [DOI] [Google Scholar]
- a Sap J. B. I.; Wilson T. C.; Kee C. W.; Straathof N. J. W.; am Ende C. W.; Mukherjee P.; Zhang L.; Genicot C.; Gouverneur V. Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride. Chem. Sci. 2019, 10, 3237–3241. 10.1039/C8SC05096A. [DOI] [PMC free article] [PubMed] [Google Scholar]; For seminal work on Mn-mediated 18F-fluorodecarboxylation, see:; b Huang X.; Liu W.; Hooker J. M.; Groves J. T. Targeted Fluorination with the Fluoride Ion by Manganese-Catalyzed Decarboxylation. Angew. Chem., Int. Ed. 2015, 54, 5241–5245. 10.1002/anie.201500399. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Guo C.-Y.; Yang C.; Chen J.-P. Ag-Catalyzed Chemoselective Decarboxylative Mono- and gem-Difluorination of Malonic Acid Derivatives. J. Am. Chem. Soc. 2019, 141, 5617–5622. 10.1021/jacs.9b00681. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information (SI) for details.
- a Collins K. D.; Glorius F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 2013, 5, 597–601. 10.1038/nchem.1669. [DOI] [PubMed] [Google Scholar]; b Taylor N. J.; Emer E.; Preshlock S.; Schedler M.; Tredwell M.; Verhoog S.; Mercier J.; Genicot C.; Gouverneur V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. 10.1021/jacs.7b03131. [DOI] [PubMed] [Google Scholar]
- a Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]; b Abdelshaheed M. M.; Fawzy I. M.; El-Subbagh H. I.; Youssef K. M. Piperidine nucleus in the field of drug discovery. Futur. J. Pharm. Sci. 2021, 7, 188. 10.1186/s43094-021-00335-y. [DOI] [Google Scholar]
- a Li Petri G.; Raimondi M. V.; Spanò V.; Holl R.; Barraja P.; Montalbano A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379, 34. 10.1007/s41061-021-00347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Parmar D. R.; Soni J. Y.; Guduru R.; Rayani R. H.; Kusurkar R. V.; Vala A. G. Azetidines of pharmacological interest. Arch. Pharm. 2021, 354, 2100062. 10.1002/ardp.202100062. [DOI] [PubMed] [Google Scholar]; c Grygorenko O. O.; Melnykov K. P.; Holovach S.; Demchuk O. Fluorinated Cycloalkyl Building Blocks for Drug Discovery. ChemMedChem. 2022, 17, e202200365 10.1002/cmdc.202200365. [DOI] [PubMed] [Google Scholar]
- a Wanka L.; Iqbal K.; Schreiner P. R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. 10.1021/cr100264t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lamoureux G.; Artavia G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]
- a Ni C.; Hu J. The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441–5454. 10.1039/C6CS00351F. [DOI] [PubMed] [Google Scholar]; b Jiang X.; Li X.; Wang K. Reversal of the nature of substituent effect by changing the number of the α-substituent. Relative ease of formation of the three α-fluoromethyl radicals. J. Org. Chem. 1989, 54, 5648–5650. 10.1021/jo00285a003. [DOI] [Google Scholar]; c Dolbier W. R. Structure, Reactivity, and Chemistry of Fluoroalkyl Radicals. Chem. Rev. 1996, 96, 1557–1584. 10.1021/cr941142c. [DOI] [PubMed] [Google Scholar]
- Reddy G. L.; Dar M. I.; Hudwekar A. D.; Mahajan P.; Nargotra A.; Baba A. M.; Nandi U.; Wazir P.; Singh G.; Vishwakarma R. A.; Syed S. H.; Sawant S. D. Design, synthesis and biological evaluation of pyrazolopyrimidinone based potent and selective PDE5 inhibitors for treatment of erectile dysfunction. Bioorg. Chem. 2019, 89, 103022. 10.1016/j.bioorg.2019.103022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


