Summary
With global climate change, it is essential to find strategies to make crops more resistant to different stresses and guarantee food security worldwide. E3 ubiquitin ligases are critical regulatory elements that are gaining importance due to their role in selecting proteins for degradation in the ubiquitin‐proteasome proteolysis pathway. The role of E3 Ub ligases has been demonstrated in numerous cellular processes in plants responding to biotic and abiotic stresses. E3 Ub ligases are considered a class of proteins that are difficult to control by conventional inhibitors, as they lack a standard active site with pocket, and their biological activity is mainly due to protein–protein interactions with transient conformational changes. Proteolysis‐targeted chimeras (PROTACs) are a new class of heterobifunctional molecules that have emerged in recent years as relevant alternatives for incurable human diseases like cancer because they can target recalcitrant proteins for destruction. PROTACs interact with the ubiquitin‐proteasome system, principally the E3 Ub ligase in the cell, and facilitate proteasome turnover of the proteins of interest. PROTAC strategies harness the essential functions of E3 Ub ligases for proteasomal degradation of proteins involved in dysfunction. This review examines critical advances in E3 Ub ligase research in plant responses to biotic and abiotic stresses. It highlights how PROTACs can be applied to target proteins involved in plant stress response to mitigate pathogenic agents and environmental adversities.
Keywords: ubiquitin‐proteasomal system, E3 Ub ligase, crops, abiotic stress, biotic stress, PROTACs
Introduction
The 21st century is facing unprecedented challenges in the agricultural sector. World population growth and recent climate change are of particular concern. With over 8 billion inhabitants, a critical phase has been reached, with populations increasingly faced with the problem of access to natural resources such as food crops (Vollset et al., 2020). Meeting the food needs of every population is a problem today, as some regions of the world are confronting intense famines, while in others, arable land has shrunk considerably with galloping urbanisation (Dasgupta and Robinson, 2022; Satterthwaite et al., 2010). Worse, climate change makes agriculture unpredictable, and farmers need reliable indicators to program their cultivation campaigns. On top of this, natural disasters are spreading, including fires, droughts and floods, due to climate change. Climate change is associated with multiple stress factors for plants, making global food security a major challenge for the 21st century (Duchenne‐Moutien and Neetoo, 2021). To cope with this demographic and climatic challenge, the agriculture sector needs significant development, and the protection of crops against biotic and abiotic stresses will have to be improved (Dasgupta and Robinson, 2022).
Plants are subject to numerous biotic and abiotic stresses that threaten crop production. Abiotic stress in plants can be caused by various conditions, including meteorological conditions (drought or extreme cold) or physicochemical conditions (high or low salt and water levels) that exceed normal physiological conditions (Francini and Sebastiani, 2019; Oshunsanya et al., 2019). Abiotic stress is the leading cause of reduced crop productivity. Every year, between 51% and 82% of crop yields in global agriculture are lost due to abiotic stress (Oshunsanya et al., 2019). Biotic stress is characterised by the plant's response to invasion by parasitic organisms, including infection by microbial pathogens. The consequences of climate change include new types of stressors that affect the vulnerability of many crops (Duchenne‐Moutien and Neetoo, 2021). Pathogens are also adapting to climate change and evolving their strategies. For example, due to rising temperatures, pathogens move from one region to another, exposing plants to new pathogens and challenging the basic concept of crop resistance (Singh et al., 2023).
Alternatives to plant stress are numerous but remain limited in the face of the current challenge. Rapid breeding technologies can help crops withstand biotic and abiotic stresses. Techniques such as next‐generation technologies, including genotyping, high‐throughput phenotyping, genome editing, marker‐assisted selection and genomic selection, will play a key role. However, how rapid breeding can adapt to a changing environment has yet to be resolved (Hickey et al., 2019). Alternatives to plant stress involve also physical, chemical and biocontrol strategies. Physical control strategies have recently included UV, heat and cold applications. However, these require significant energy resources, limiting them in an energy crisis (Picart‐Palmade et al., 2019). In chemical methods, pesticides and fertilisers control pathogens and enrich soils with essential chemical elements. Nevertheless, this is a growing concern due to increasing residues and the promotion of strain resistance (Ahammed et al., 2021; Kumar et al., 2022). Finally, biological methods are becoming increasingly popular, in particular, the implementation of microbial antagonists in agricultural fields to improve plant health (Ayaz et al., 2023; Collinge et al., 2022) and enhance pathogen control (Godana et al., 2023), but their effectiveness still needs to be improved. Innovative strategies that take advantage of fundamental research into the cellular mechanisms of stress regulation should improve food preservation and safety.
Recent advances in biotechnology have uncovered the fundamental mechanisms of cells, highlighting the role of E3 Ub ligases in plant stress (Stone et al., 2005). E3 Ub ligases represent a regulatory element that can bind to proteins, driving them to the cellular ubiquitin‐proteasome system for their elimination (Vierstra, 2009). In this way, they are essential to reshaping cells' proteome and regulating cell function. Advances in research in this field are mainly due to new biotechnological investigation approaches such as yeast two‐hybrid (Y2H), protein microarrays, global protein stability (GPS) profiling, high‐throughput quantitative microscopy (HCA), differential expression (shotgun) proteomics, cell‐free degradation, immunodetection analyses (Selote et al., 2018), proteomics single and double knockdown, protein localisation, knockout technology, RNA silencing and immunolabelling microscopic methodology (Iconomou and Saunders, 2016). Thanks to this advantage, the study of single or multiple stress induction and cell machinery has highlighted the importance of E3 Ub ligase in stress management. Plant stress and its relevance to biotic and abiotic stress is now a significant research topic (Gingerich et al., 2021). Numerous transcriptomic studies of plants subjected to abiotic and biotic stresses have revealed that E3 Ub ubiquitin ligases are differentially expressed, indicating their prominent role. Moreover, knowledge of their crucial role can improve plant resistance to stress. Research strategies are now focused on harnessing and managing E3 Ub ligases to improve plant resistance to stress.
E3 Ub ligases are critical regulators as they control phytohormone biosynthesis and signalling pathways. Their biological activity is shaped by a specific protein–protein interaction with a short phase of transitions often associated with conformation change. This mechanism, although exceptional, classifies E3 Ub ligases among the so‐called undruggable proteins, as such proteins are challenging to control with conventional inhibitors. E3 Ub ligase activity is pocket‐independent. For this reason, much interest is focused on strategies that can target E3 Ub ligases or proteins that are difficult to destroy and that may be involved in abiotic and biotic stress in plants.
Proteolysis‐targeted chimeras (PROTACs) are a new class of heterobifunctional molecules that have emerged in recent years as relevant alternatives for uncurable human diseases like cancer (Khan et al., 2024) because they can target recalcitrant proteins for destruction. PROTACs can exploit the function of the E3 Ub ligase to select proteins for degradation by the proteasome. PROTACs mainly enhance ubiquitination and proteasomal degradation by facilitating association with an E3 Ub ligase and a protein involved in stress (Li et al., 2022; Raina et al., 2016). The research on PROTACs over the last 10 years available on Web of Science sites shows that the number of publications on PROTACs is increasing rapidly every year, as shown in Figure 1. Developing PROTACs in plants may require consolidating our understanding of E3 Ub ligases, their role, and regulation in plant development and stress control. A major review of E3 Ub ligases' progress and the perspective of applying PROTACs to agriculture is of great interest.
Figure 1.

Evolution of the number of articles related to PROTACs from the year 2003 to the date 2023. Retrieved from PubMed on 2023 12 24. Research term enter ‘Proteolysis‐targeting chimeras (PROTACs)’ https://pubmed.ncbi.nlm.nih.gov/?term=Proteolysis‐targeting%20chimeras%20(PROTACs)%20&timeline=expanded.
The present review will describe recent advances in E3 Ub ligases and include recent study in the proteolytic process of the ubiquitin‐proteasome system, the molecular classification, structure and role of E3 Ub ligases, as well as the importance of E3s in plant response to abiotic and biotic stresses. Finally, this study will prospect the PROTAC methodology in plants to improve crop stress resistance and thus enhance food security worldwide.
Ubiquitin‐proteasome system in plant
Ubiquitin (Ub) is a highly conserved eukaryotic polypeptide of 76 amino acids (Adams and Spoel, 2018; Langin et al., 2023; Stone, 2019). Ubiquitination is the central post‐translational modification of proteins (Sun et al., 2020). It involves modulating a variety of biological functions, including protein homeostasis regulation (Sun et al., 2020), signal transduction (Liu et al., 2020), autophagy and DNA damage response (Huang and Zhang, 2020). During ubiquitination, Ub is attached to a protein target in various forms, resulting in the binding of either single Ub's or complex Ub polymers, causing mono‐, multi‐ and branched poly‐ubiquitylation (Singh et al., 2021). The position of the amino acid on which the Ub is anchored also determines the cellular role of the target (Huang and Zhang, 2020).
The ubiquitination process requires a ‘teamwork’ of three classes of enzymes, namely the E1 Ub activating enzyme, the E2 Ub binding enzyme and the E3 Ub ligase (Brillada and Trujillo, 2022). The main processes are as follows: first, with the participation of ATP, free inactive Ub is activated in the E1 Ub complex, and a high‐energy thioester bond is created between the glycine (Gly) carboxyl terminus of Ub and the E1 cysteine (Cys) residue of the active site; the activated Ub is then moved to the active site Cys residue of E2 Ub‐binding enzyme, forming the complex E2‐Ub thioester (E2‐ Ub); finally, E2 then is recruited and can transfer Ub to the corresponding E3 Ub ligase (Wang et al., 2022). E3 Ub ligase recognises the target protein and catalyses, directly or through a transitional state, the binding of Ub to the target protein (Skieterska et al., 2017), connecting the Ub molecule to the lysine residues of the target protein by forming an isopeptide bond. Figure 2 illustrates the ubiquitination process and proteasome degradation.
Figure 2.

Schematic representation of the ubiquitination process and proteasome degradation. Three classes of enzymes exist – E1 Ub activating enzyme, E2 Ub binding enzyme and E3 Ub ligase. They work as a team to achieve the ubiquitination of a targeted protein. First, with ATP, free inactive Ub is activated in E1 Ub, creating a high‐energy thioester bond between the glycine (Gly) carboxyl terminus of Ub and the active site's E1 cysteine (Cys) residue. The activated Ub is then moved to the active site of the Cys residue of the E2 Ub‐binding enzyme, forming the complex E2‐Ub thioester (E2‐Ub). Finally, E2 is recruited to the corresponding E3 Ub ligase. E3 Ub ligase finds the target protein and either directly or indirectly binds Ub to it by creating an isopeptide bond at the lysine residues of the target protein.
Classification and structure of E3 Ub ligase in plant
E3 Ub ligases classification based on their frame can evolve with advanced research. Hence, E3 Ub ligases are classified according to their functional domain into three structural families: HECT, RING and U‐box (Ryu et al., 2019). Depending on the number of subunits, E3 Ub ligases can be classified into four main types: HECTs, RINGs and U‐boxes, which are single E3 Ub ligase polypeptides, and CRLs, which are made up of several subunits (Chen and Hellmann, 2013; Vierstra, 2009).
HECT family
The HECT family (homologous to the E6‐associated protein C‐terminus) is a highly conserved family of enzymes (Shah and Kumar, 2021). Genomes of various plant and animal species encode different numbers of HECT E3 Ub ligases gene family members, including 7 in Arabidopsis (Downes et al., 2003; Ying, 2018), 10 in Brassica rapa (Alam et al., 2019), 19 in Glycine max (Meng et al., 2015), 28 in Homo sapiens (Sluimer and Distel, 2018), 14 in Solanum lycopersicum (Sharma et al., 2021), 12 in Zea mays (Li et al., 2019) and 19 in Malus domestica (Xu et al., 2016). The C‐terminus of HECT has a HECT structural domain consisting of a N‐lobe and a C‐lobe of approximately 40 kDa. The N‐lobe consists of the E2‐binding domain, and the C‐lobe represents the active site of cysteine (Goto et al., 2021). This structural domain is an α/β structure and has a catalytic role, directly catalysing the binding of Ub to the substrate protein and mediating the covalent transfer of Ub from E2 to the target protein (Sharma et al., 2021b). The HECT‐type E3 Ub ligase first loads the activated Ub of the E2‐Ub intermediate onto its active cysteine residue and then transfers the Ub to the lysine residue of the substrate (Delvecchio et al., 2021). The HECT family is divided into three subfamilies: the NEDD4 (9 members), the HERC (6 members) and other HECT E3 Ub ligases (13 members) (Singh et al., 2021b). The NEDD4 subfamily includes a C2 structural domain and two to four tryptophan–tryptophan (WW) protein interaction domains before the HECT structural domain (Singh et al., 2021b). This WW NEDD4 domain is critical for subtract selection as it can bind to the PPxY motif of the target protein (Weber et al., 2019). The HERC subfamily has two characteristic structural domains: the HECT structural domain and the chromosome condensation regulator (RCC1)‐like structural domain (RLD) (Qian et al., 2020). The third subfamily includes enzymes that contain neither WW nor RLD structural domains with various structural domains at the N‐terminus (Delvecchio et al., 2021).
RING family
The RING family (really interesting new gene) is the most prominent family of E3 Ub ligases (Vander Kooi et al., 2006). The RING structural domain contains a C6HC zinc finger structure, and two RING zinc finger‐like structures exist on both sides of C6HC (Bueso et al., 2014). Depending on whether position 5 of the metal ligand contains a Cys or His residue, RING finger domains have been categorised into two types: RING‐HC (C3HC4) and RING‐H2 (C3H2C3) (FREEMONT, 1993). This structural domain mainly assists the transfer of Ub through the covalent bond formed by the chelation of eight amino acids with zinc ions (Weber et al., 2019). The RING structural domain determines the specific activity of most E3 Ub ligases. Specifically, it was found that while OsSAR1 has E3 Ub ligase activity, MBP‐OsSADR1C168A, whose Cys168 is replaced by Ala168 in the RING structural domain, has no E3 Ub ligase activity (Park et al., 2018). E3 RING ligases may function as allosteric activators of E2, which catalyses the sliding of Ub from its active site to the lysine residues of the target protein. E3 Ub ligases act primarily as scaffolding to bring E2 into proximity to the protein substrate (Weber et al., 2019). Genomes of various plant species encode different numbers of RING protein members, including 477 in Arabidopsis (Stone et al., 2005), 715 in Brassica rapa (Alam et al., 2017), 425 in rice (Oryza sativa) (Lim et al., 2010) and 663 in Malus domestica (Li et al., 2011). RING‐type E3s can form complex multi‐subunit E3s (Al‐Saharin et al., 2022), dividing them into two subtypes: single‐subunit and multi‐subunit families (Wang et al., 2021). Single‐subunit RING E3 Ub ligases specifically possess three RING structural domains in tandem, and RSL1 belongs to the single‐subunit RING E3 Ub ligases with a transmembrane structural domain at the C‐terminus (Bueso et al., 2014). The multiple units, Cullin‐RING Ub ligase (CRL) family consists of a Cullin protein acting as the central scaffold and the N‐terminus binding to the adapter protein‐substrate receptor complex, and the C‐terminus binds to RING proteins (Bueso et al., 2014; Wang et al., 2021).
U‐box family
U‐box protein contains a structural domain of about 70 amino acids and is a single protein widespread in yeast, plants and animals (Wang et al., 2020). Genomes of various plant species encode different numbers of U‐box gene, including 66 in Arabidopsis (Wiborg et al., 2008), 101 in Brassica rapa (Wang et al., 2015), 30 in Chlamydomonas reinhardtii (Luo et al., 2015) (Oryza sativa), 125 in Glycine max (Wang et al., 2016), 21 Homo sapiens (Sharma and Taganna, 2020), 77 in Oryza sativa (Mueller et al., 2005), 2 in Saccharomyces cerevisiae (Koegl et al., 1999), 56 in Vitis vinifera (Jiao et al., 2017) and 62 in Solanum lycopersicum (Sharma and Taganna, 2020). Compared to the RING, the U‐box (PUB) family is less diverse (Smalley and Hellmann, 2022). However, U‐box and RING proteins present both single polypeptides, and both interact non‐covalently with E2‐Ub through the conserved U‐box structural domain and RING structural domain respectively (Mao et al., 2022; Trenner et al., 2022). The primary role of U‐box is to bind to E2‐Ub, mainly involving salt bridges, ion chelation, and hydrogen bonding, and facilitate Ub transfer (Trenner et al., 2022) to the target proteins. The plant U‐box protein family was first identified in Arabidopsis thaliana with 64 members. AtPUB14 protein was the first PUB to be structurally identified and contains a U‐box structural domain and an ARM repeat structure that allows the formation of a U‐box‐mediated featureless structural dimer (Andersen et al., 2004). Both the U‐box structural domain and the RING structural domain have a similar disposition of a three‐stranded chain and a helix as well as a particular long loop, but the U‐box does not have the histidine and cysteine residues as well as two zinc ions (Aravind and Koonin, 2000; Vander Kooi et al., 2006). The U‐box involves electrostatic interactions rather than metal chelation for stabilising the ubiquitin‐E2‐binding pocket (Mudgil et al., 2004; Wiborg et al., 2008). Further classification of plant U‐box protein is in progress (Hu et al., 2018; Li et al., 2019; Wang et al., 2020). Figure 3 illustrates the E3 Ub ligase structure and mechanism.
Figure 3.

E3 ubiquitin ligase structure and mechanism. Each E3‐ligase in plants is composed of multiple subunits. The numbers indicated within each subunit represent the genes in the Arabidopsis thaliana genome that potentially encode the E3 Ub ligase or E3 Ub ligase component. RING (really interesting new gene) and U‐box E3 Ub ligases engage with an E2 and the target, thereby enabling the direct transfer of Ub to the target protein. HECT E3 Ub ligases, which are defined as homologous to the E6AP carboxyl terminus, as well as RBR E3 Ub ligases, known as RING‐in‐Between‐RING E3 Ub ligases, facilitate the transfer of Ub from the E2 to a cysteine residue in the E3. Subsequently, the Ub is transferred from the cysteine to the target protein. The CRLs, called Cullin‐RING Ligases, are complex structures of multiple subunits. These structures consist of a backbone protein called Cullin (CUL), which acts as a binding site for the E2 protein and a target adapter protein or complex. In SCF complexes, which stand for Skp1/CUL1/F‐box complexes, the target adapter proteins known as F‐box proteins bind to a protein called Skp1, called S‐phase Kinase‐associated Protein 1. This Skp1‐like protein then interacts with CUL1. In the CRL3 complexes, a protein containing the BTB domain (broad‐complex, tramtrack and bric‐à‐brac) binds to both the target and CUL3. Within the CRL4 complexes, DWD proteins that contain the WD40 domain (DDB1 binding WD40) bind to the targets and interact with CUL4 through a protein bridge called DDB1 (damaged DNA binding 1). The APC/C (anaphase‐promoting complex/cyclosome) comprises a minimum of 11 subunits. APC2 is a protein similar to Cullin, and APC11 shares similarities with RBX1 (ring box protein 1). Three parts – CDC20 (cell division cycle 20), CDH1 (CDC20 homologue 1) and APC10 – work together to recognise the target (Gingerich et al., 2021).
CRL family
CRLs are indeed the best‐recorded E3 Ub ligases, involved in plants' physiological, developmental and stress responses (Chen and Hellmann, 2013). CRLs are complexes in which the cullin protein acts as an elongated scaffold, linking RING‐FINGER PROTEIN RING BOX PROTEIN 1 (RBX1) and E2 to its carboxyl (C)‐terminal region while linking a substrate adaptor to its amine (N)‐terminal region. In plants, there are four CRL subtypes harbouring distinct cullins: CUL1, CUL3, CUL4 and the cullin‐like protein ANAPHASE‐PROMOTING COMPLEX 2 (APC2) (Ban and Estelle, 2021; Vierstra, 2009). E3 CUL1 ligases, also known as SCF complexes (S‐PHASE KINASE‐ASSOCIATED PROTEIN 1 (SKP1)‐CUL1‐F‐box), are one of the most extensively studied CRL complexes, with the substrate adaptor consisting of SKP1 and an F‐box protein. Genomes of various plant species encode different numbers of F‐box protein members, including 600–700 in Arabidopsis (Gagne et al., 2002; Kuroda et al., 2002), 687/779 in rice (Oryza sativa) (Jain et al., 2007; Xu et al., 2009), 359 in Zea mays (Jia et al., 2013), 517 in Malus domestica (Cui et al., 2015), 337 in poplar (Xu et al., 2009), 226 in Pyrus spp. (Wang et al., 2016), 285 in chickpeas (Gupta et al., 2015) and 972 in Medicago (Song et al., 2015). SCFs are also essential regulators of plant hormone responses (auxin, jasmonic acid (JA) and ethylene) and many other processes (Ban and Estelle, 2021). In CRL3 complexes, the BTB (broad‐complex, tramtrack and bric‐à‐brac) domain binds both the target and CUL3. Genomes of various plants encode different numbers of MATH–BTB gene family members, including 6 in Arabidopsis (Juranić and Dresselhaus, 2014), 10 in Brassica rapa (Zhao et al., 2013), 68 in Oryza sativa (Gingerich et al., 2007), 5 in Solanum lycopersicum (Li et al., 2018), 31 in Zea mays (Juranić et al., 2013) and 19 in Malus domestica (Xu et al., 2016). In CRL4 complexes, DWD proteins incorporate the WD40 (DDB1 binding WD40) domain, which binds targets and interacts with CUL4 via a protein bridge called DDB1 (damaged DNA binding 1). Finally, the APC/C (anaphase‐promoting complex/cyclosome) complex comprises a minimum of 11 subunits. APC2 is a protein similar to Cullin, and APC11 has similarities with RBX1 (ring box protein 1). Classically, three parts – CD20 (cell division cycle 20), CDH1 (CDC20 Homologue 1) and APC10 – work together to recognise the target (Gingerich et al., 2021; Vierstra, 2009).
Role of plant E3 Ub ligase in different stresses
Role of E3 Ub ligase in abiotic stress
Drought stress
Plant species under drought stress express E3 Ub ligases that modify the ABA signalling pathway, disrupt gene expression in specific pathways for bioactive compound synthesis, and contribute to the ubiquitination and degradation of transcription factors responsible for the drought stress response (Cheng et al., 2012; Joo et al., 2017; Lim et al., 2017b; Park et al., 2010; Sun et al., 2022; Wang et al., 2018; Yu et al., 2021). Silencing these genes leads to drought and salt stress resistance (Park et al., 2010).
Ring ligases can positively regulate tolerance to drought stress by interfering with cellular mechanisms to reduce water loss and ion leakage. For example, OsCTR1 can inhibit the trafficking of OsCP12 and OsRP147 to chloroplasts (Lim et al., 2014). Overexpression of some E3 Ub ligases can make crops particularly resistant, such as the pepper E3 Ub ligase CaAIRF1, which regulates ABA and enhances the drought response via degradation of the protein phosphatase CaADIP1 (Brugière et al., 2017; Lim et al., 2017a). E3 Ub ligases regulate pathways in a multi‐level fashion, controlling the regulator‐specific cellular network and potentially producing bioactive compound synthesis. Some genes up‐regulated by drought resistance may also regulate elevated levels during salt stress (Chen et al., 2020; Cho et al., 2022; Joo et al., 2019, 2020; Yang et al., 2016).
PUB proteins play negative and positive roles in abiotic stress resistance (Ryu et al., 2019). Negative regulators, such as PUB11 signalling kinase, alter signal transduction during drought stress (Chen et al., 2021). They also modify the cell's hormone signalling system, particularly in ABA signalling pathways. Positive regulators, like OsPUB67, enhance cell protection against stress by improving reactive oxygen scavenging (Qin et al., 2020).
PUBs can interact with multiple regulators to positively regulate tolerance to drought stress. Water resources are crucial in drought management, and PUBs manage them by facilitating water channels and stomatal closure. Through the molecular mechanism of proteasomal degradation of aquaporins (AQPs), OsRINGzf1, an E3 Ub ligase, acts on the turnover of OsPIP2;1 (Chen et al., 2022). Still, in the strategic action of minimising water waste, PUBs also act on stomatal closure (Xiao et al., 2022). Figure 4 shows the role of E3 Ub in drought stress.
Figure 4.

Regulatory mechanism of E3 Ub in response to drought stress in the plant. E3 Ub ligases during drought stress are involved in (1) activating the response to drought stress and (2) attenuating the response to drought stress. In (1) response to drought stress, the ABA (abscisic acid) pathway is activated by CaAIRE1 (Capsicum annuum ABA induced RING‐type E3 Ub ligase 1), which mediates the elimination of CaAITP1 (protein phosphatase), a stimulator of the ABA pathway. CaASRF1 (E3 Ub ligase containing a C3H2C3‐type RING finger domain) mediates the accumulation of CaATBZ1 (pepper bZIP transcription factor), which can promote ABA pathways. After drought stress is abolished (2), the E3 Ub ligase domain mediates attenuation of the response to drought stress. CaAIRF1 (Capsicum annuum RING‐type E3 Ub ligase) and BRUTUS (iron‐binding E3 Ub ligase containing three hemerythrin (HHE) domains and a Really Interesting New Gene (RING) domain) mediate destabilisation of CaADIP1 (Capsicum annuum type 2C protein phosphatase) and VOZ1/2 (vascular plant one‐zinc finger ½), respectively, which are two positive regulators of the ABA pathway. The E3 Ub ligase CaATIR1 (Capsicum annuum E3 Ub ligase ATBZ1 interacting RING finger protein 1) mediates destabilisation of CaATBZ1 (pepper bZIP transcription factor), RGLG2 (RING domain E3 Ub ligase 2) and AtAIRP1 (cytosolic protein containing a single C3H2C3‐type RING motif with in vitro E3 Ub ligase activity) mediate destabilisation of MAPKKK18 (mitogen‐activated protein kinase kinase kinase 18), OsPUB67 (U‐box E3 Ub ligase) mediates destabilisation of OsRZFP34 (RING zinc‐finger protein (RZFP))/OsDIS1, AtAIRP1 (cytosolic protein containing a single C3H2C3‐type RING motif with in vitro E3 Ub ligase activity) mediates destabilisation of AtKPNB1 (Arabidopsis importin‐β protein), RGLG2 mediates destabilisation of AtERF53 (ETHYLENE RESPONSE FACTOR 53) and TaDIS1 (Triticum aestivum L RING E3 Ub ligase) mediates destabilisation of TaSTP (Triticum aestivum L sugar transporter protein (STP)). CaATBZ, MAPKKK18, OsRZFP34/OsDIS1, AtKPNB1, AtERF53 and TaSTP are all critical for the continuation of the drought stress response.
Salt stress
Salt stress is one of the most damaging stresses for plants. It can cause osmotic, nutritional and metabolic dysfunctions. Salt stress affects plant primary growth and reduces productivity (Ahmad et al., 2023; Fu and Yang, 2023). Ring E3 Ub ligases play a crucial role in plant salt stress tolerance. Some ligases, such as AtPPRT1, are involved in hormone deregulation and activate organelles in ion trafficking (Pei et al., 2019). Others, like IbATL38, positively regulate salt stress tolerance (Du et al., 2021). Specific E3 Ub ligases, like the SpRing gene and SARS1, are inducible and improve tolerance (Qi et al., 2016). Inducible ligases, like CaAIP1 in pepper and OsHKT1;1 in rice, interact with ion transporters and can induce resistance (Xiao et al., 2022). Inhibition of specific E3 Ub ligases can result in opposition, while others, like SKP1‐Cullin/CDC53‐F‐box, are involved in salt and drought stress responses in Arabidopsis (Li et al., 2022). Understanding the complete mechanisms of these E3 Ub ligases is essential for understanding plant growth and stress responses.
PUB ligases play a crucial role in regulating salt stress tolerance in plants. They can affect salt stress by interfering with cell permeability, such as the E3 GmPUB21 ligase, which can enhance stomatal aperture density (Yang et al., 2022). A single PUB gene can regulate various abiotic stresses, such as osmotic and salt stress sensitivity and drought stress tolerance. PUBs also positively regulate phosphate stress tolerance, such as PRU2 enhancing phosphate uptake. Further studies are needed to understand their roles in stress responses by interacting with a CK2α1 kinase and a ribosomal protein RPL10 for degradation (Sun et al., 2022). Figure 5 shows the role of E3 Ub in salt stress.
Figure 5.

Regulatory mechanism of E3 Ub in response to plant salt stress. E3 Ub ligases during salt stress are involved in (1) activation of the salt stress response and (2) attenuation of the salt stress response. In (1), salt stress tolerance is activated by OsSIRP2 (rice gene encoding the RING Ub E3 Ub ligase), which mediates the elimination of OsTKL1 (transketolase), a negative regulator of salt stress tolerance. After removing salt stress (2), the E3 Ub ligase domain mediates the attenuation of the salt stress response. SINAT (RING‐domain containing E3 Ub ligase) mediates the destabilisation of ACS (1‐aminocyclopropane‐1‐carboxylic (ACC) synthase), which positively regulates the ethylene pathway. SRAS1.1 (salt‐responsive alternatively spliced gene one encoding a RING‐Type E3 Ub ligase, generates two splicing variants: SRAS1.1 and SRAS1.2) mediates the destabilisation of CSN5A (COP9 signalosome 5A), a positive regulator of stress response. OsMSRFP (MYBc stress‐related RING finger protein) mediates the elimination of OsMYBc (MYB transcription factor), the latter involved in the activation of HKT (OsHKkT1;1) (member of the high‐affinity K+ transporter (HKT) family in rice (Oryza sativa)). OsSIRP4 (E3 Ub ligase, the Oryza sativa salt‐induced RING finger) mediates the destabilisation of OsPEX11‐1 (Oryza sativa peroxisomal biogenesis factor 11–1), the latter promoting the stress response. XBAT35.2 (RING‐type E3 Ub ligase) mediates the destabilisation of ACD11 (Arabidopsis accelerated cell death 11). PRU2 (a ubiquitin E3 Ub ligase) mediates the destabilisation of RPL10 (ribosomal protein) and KINASE CK2α1 (serine–threonine protein kinase). KINASE CK2α1 is a positive regulator of the MAKP pathway, which is essential for stress tolerance. PQT3 (E3 Ub ligase PARAQUAT TOLERANCE 3) mediates the elimination of PRMT4b (protein arginine methyltransferases), which later activate APX1 (ascorbate peroxidase 1) and GPX1 (glutathione peroxidase‐1) both play essential role intolerance to salt stress. ACD11 is a positive regulator of tolerance to abiotic and biotic stresses.
Cold stress
E3s are crucial in disrupting cold stress tolerance in plants, with inhibitory actions centralised in the nucleus. In Arabidopsis, HOS1, an E3 Ub‐protein ligase, negatively modulates cold stress tolerance by reducing the expression of genes essential to the attenuation of cold (Shkryl et al., 2021). Ring type E3 Ub ligases, such as BBX37 (B‐box protein), also interact with critical hormones, reducing tolerance (An et al., 2021). PUB ligases, such as VpPUB24, positively regulate cold stress tolerance, with overexpression leading to cold stress resistance (Zhang et al., 2017). In A. thaliana, PUB25 and PUB26 operate by poly‐ubiquitination of MYB15, promoting CBF expression under cold conditions (Wang et al., 2019). PUB expression changes according to stress type, with up‐regulation under low‐temperature and up‐regulation under drought stress (Zhou et al., 2021). HECT‐type E3 Ub ligases are thought to affect abiotic stress by modulating the ABA pathway in plants (Sharma and Taganna, 2020). Table 1 shows the E3 Ub ligase genes in response to abiotic stresses in plants. Figure 6 shows the role of E3 Ub in abiotic stresses.
Table 1.
E3 ubiquitin ligase genes involved in plant response to abiotic stresses
| Species | Type of E3 Ub ligase | Year | Gene | Cellular localisation | Type of stress | Cellular target | Role in stress | References |
|---|---|---|---|---|---|---|---|---|
| Arabidopsis thaliana | RING | 2020 | AtAIRP1 | Cytosol | Drought | AtKPNB1 | Inhibit | Oh et al. (2020) |
| Arabidopsis thaliana | RING | 2011 | AtAIRP2 | Cytosol | ABA‐dependent drought stress | Depending on SnRK protein kinase activities | Inhibit | Cho et al. (2011) |
| Arabidopsis thaliana | RING | 2022 | AtAIRP5/GARU | – | Drought | AtSCPL1 | Inhibit | Cho et al. (2022) |
| Arabidopsis thaliana | RING | 2013 | AtATL78 | – | Cold | – | Promote | Kim and Kim (2013) |
| Arabidopsis thaliana | RING | 2016 | AtATL78 | Plasma membrane | Drought | Mediating ABA‐dependent ROS signalling | Promote | Suh et al. (2016) |
| Arabidopsis thaliana | RING | 2015 | AtATL80 | Plasma membrane | Phosphate/Cold | – | Promote | Suh and Kim (2015) |
| Arabidopsis thaliana | RING | 2013 | AtRDUF1 | Cytosol and nuclei | Salt | – | Inhibit | Li et al. (2013) |
| Arabidopsis thaliana | RING | 2021 | AtRZF1 | Nucleus | Drought | Interacts with AtUAP1 | Inhibit | Min et al. (2021) |
| Arabidopsis thaliana | RING | 2011 | AtSAP5 | Primarily in nuclei of root cells | Abiotic stress | – | Inhibit | Kang et al. (2011) |
| Arabidopsis thaliana | RING | 2018 | BRUTUS | Nucleus | Drought/Cold | VOZ1/2 transcription factors | Inhibit | Selote et al. (2018) |
| Arabidopsis thaliana | RING | 2016 | COP1 | Nucleus | Salt/cold/UV‐B exposure | AtSIZ1 | Inhibit | Kim et al. (2016) |
| Arabidopsis thaliana | RING | 2006 | HOS1 | – | Cold | ICE1 | Promote | Dong et al. (2006) |
| Arabidopsis thaliana | RING | 2020 | JUN1 | Nucleus | Drought | – | Inhibit | Yu et al. (2020) |
| Arabidopsis thaliana | RING | 2022 | PRU2 | – | Pi stress | Kinase CK2α1 and a ribosomal protein RPL10 | Inhibit | Sun et al. (2022) |
| Arabidopsis thaliana | RING | 2021 | RGLG1 and RGLG2 | – | Drought | Modulate MAPKKK18 | Promote | Yu et al. (2021) |
| Arabidopsis thaliana | RING | 2012 | RGLG2 | Nucleus | Drought | AtERF53 | Promote | Cheng et al. (2012) |
| Arabidopsis thaliana | RING | 2015 | RZFP34/CHYR1 | Cytoplasm and nucleus | Drought | SNF1‐RELATED PROTEIN KINASE2 (SnRK2) kinases | Inhibit | Ding et al. (2015) |
| Arabidopsis thaliana | RING | 2008 | SDIR1 | – | Drought | – | Inhibit | Zhang et al. (2008) |
| Arabidopsis thaliana | RING | 2015 | SDIR1 | ER membrane | Salt | SDIR1‐INTERACTING PROTEIN1 | Promote | Zhang et al. (2015) |
| Arabidopsis thaliana | RING | 2021 | SRAS1.1 | Cytoplasm and nucleus | Salt | COP9 signalosome subunit 5A | Inhibit | Zhou et al. (2021) |
| Arabidopsis thaliana | RING | 2015 | STRF1 | Plasma membrane and at the intracellular endosomes | Salt | Involved in membrane trafficking | Promote | Tian et al. (2015) |
| Arabidopsis thaliana | RING | 2020 | XBAT35.2 | – | Salt | ACD11 | Promote | Li et al. (2020) |
| Arabidopsis thaliana | F‐box | 2022 | AtSDR | Nucleus | Salt/drought | – | Inhibit | Li et al. (2022) |
| Arabidopsis thaliana | U‐box | 2003 | AtCHIP | – | Temperature | – | Promote | Yan et al. (2003) |
| Arabidopsis thaliana | U‐box | 2021 | AtPUB11 | – | Drought | Degrading the receptor‐like protein kinases LRR1 and KIN7 | Promote | Chen et al. (2021) |
| Arabidopsis thaliana | U‐box | 2012, 2016, 2017 | AtPUB18/19 | Punctate bodies/membrane‐associated protein | ABA‐mediated drought stress | Exocyst subunit Exo70B1 | Promote | Liu et al. (2017), Seo et al. (2012), Seo et al. (2016) |
| Arabidopsis thaliana | U‐box | 2023 | AtPUB2 | Nucleus | Oxidative stress | – | Inhibit | Saini et al. (2023) |
| Arabidopsis thaliana | U‐box | 2008, 2015, 2017 | AtPUB22/23 | Cytosol | Drought | RPN12a; RPN6; targeting ABA receptor PYL9 for degradation | Promote | Ahn et al. (2022), Cho et al. (2008), Zhao et al. (2017) |
| Arabidopsis thaliana | U‐box | 2019, 2023 | AtPUB25/26 | – | Cold | Targeting MYB15 and ICE1 | Inhibit | Wang et al. (2019, 2023) |
| Arabidopsis thaliana | U‐box | 2017 | AtPUB30 | Cytoplasm, nucleus, and plasma membrane | Salt | Mediating BKI1 protein degradation | Promote | Zhang et al. (2017) |
| Arabidopsis thaliana | U‐box | 2017 | AtPUB46 | – | Drought and oxidative stress | – | Inhibit | Adler et al. (2018, 2017) |
| Arabidopsis thaliana | U‐box | 2017 | AtPUB48 | Nucleus | Drought/heat | – | Inhibit | Adler et al. (2017), Peng et al. (2019) |
| Arabidopsis thaliana | U‐box | 2016 | PARAQUAT TOLERANCE3(PQT3) | Nucleus | Oxidative stress | PRMT4b | Promote | Luo et al. (2016) |
| Arabidopsis thaliana | C3HC4 Zinc‐Finger | 2020 | AtPPRT1 | – | Salt | Promote | Liu et al. (2020) | |
| Arabidopsis thaliana | Putative C3HC4 Zinc‐finger | 2019 | AtPPRT1 | Mitochondria | Drought | Negative Regulator in ABA | Promote | Pei et al. (2019) |
| Apple (Malus domestica) | RING | 2024 | MdATL16 | Cell membrane and nucleus | Salt | Promoting ROS scavenging | Inhibit | Yuan et al. (2024) |
| Apple (Malus domestica) | RING | 2017 | MdMIEL1 | – | Salt/oxidative | – | Inhibit | An et al. (2017) |
| Apple (Malus domestica) | RING | 2022 | MdMIEL1 | – | Drought | MdBBX7/MdCOL9 | Inhibit | Chen et al. (2022) |
| Apple (Malus domestica) | RING | 2022 | MdMIEL1 | – | Cold | BBX37 | Inhibit | An et al. (2021) |
| Apple (Malus domestica) | U‐box | 2022 | MdPUB23 | Nucleus | Cold | MdICE1 | Promote | Wang et al. (2022) |
| Apple (Malus domestica) | U‐box | 2022 | MdPUB29 | Salt | – | Inhibit | Han et al. (2019) | |
| Brassica napus | Cullin‐RING | 2023 | CRL3 BPM | – | Salt | – | Promote | Corbridge et al. (2023) |
| Brassica napus | RING | 2014 | BnaJUL1 | – | Drought | BnaTBCC1 | Inhibit | Hu et al. (2024) |
| Brassica napus | RING | 2014 | BnTR1 | Plasma membrane | Heat | Ca2+ dynamics by regulating the activity of calcium channels | Inhibit | Liu et al. (2014) |
| Chinese grapevine (Vitis pseudoreticulata) | U‐box | 2017 | VpPUB24 | All grapevine protoplasts | Cold | VpICE1 | Inhibit | Yao et al. (2017) |
| Coptis chinensis | RING | 2020 | CcSDIR1 | – | Drought | Increasing antioxidant enzyme activities and reducing oxidative damage | Inhibit | Chen et al. (2020) |
| Cowpea (Vigna unguiculata L. Walp) | RING | 2014 | VuDRIP | – | Abiotic stress | TF VuDREB2A | Regulate | Sadhukhan et al. (2014) |
| Cucumber (Cucumis sativus) | RING | 2023 | CsCHYR1 | Nucleus and membrane | Drought | CsATAF1 | Inhibit | Guo et al. (2023) |
| Cucumber (Cucumis sativus) | F‐box | 2021 | CsTLP8 | Plasma membrane and nucleus | Osmotic stresses | ABA signalling pathway | Promote | Li et al. (2021) |
| Grapevine (Vitis vinifera) | RING | 2014 | VvHOS1 | – | Cold/drought/high salt | – | Promote | Li et al. (2014) |
| Grapevine (Vitis vinifera) | U‐box | 2023 | VviPUB19 | – | Drought/salt | VviExo70B | Promote | Wang et al. (2023) |
| Grimmia pilifera | Cullin‐RING | 2016 | GpDSR7 | ER membrane | Drought | – | Inhibit | Wang et al. (2023) |
| Maize (Zea mays) | RING | 2018 | ZmAIRP4 | Cytoplasm and nucleus | Drought | Enhances the expression level of drought responsive genes | Promote | Yang et al. (2018) |
| Maize (Zea mays) | RING | 2012 | ZmRFP1 | Plasma membrane | Drought | ABA‐dependent manner | Inhibit | Xia et al. (2012) |
| Maize (Zea mays) | RING | 2013 | ZmRFP1 | – | Drought | Regulating stomatal aperture and antioxidants | Inhibit | Liu et al. (2013) |
| Maize (Zea mays) | RING | 2017 | ZmXerico1 | Endoplasmic reticulum | Drought | Regulation of ABA homeostasis | Inhibit | Brugière et al. (2017) |
| Nicotiana tabacum | RING | 2013 | NtRHF1 | – | Drought | Regulating several stress‐responsive genes | Inhibit | Xia et al. (2013) |
| Pepper (Capsicum annuum) | RING | 2012 | CaRma1H1 | Endoplasmic reticulum | Drought/salt | – | Inhibit | Seo et al. (2012) |
| Pepper (Capsicum annuum) | RING | 2016 | CaAIP1 | Nucleus and cytoplasm | Drought/osmotic | Drought stress: via changes in the stomatal aperture and leaf temperature; osmotic stresses: via ABA‐mediated signalling | Inhibit | Park et al. (2016) |
| Pepper (Capsicum annuum) | RING | 2015 | CaAIR1 | Nucleus | Drought | Controlling the transpirational rate via stomatal opening/closure | Promote | Park et al. (2016) |
| Pepper (Capsicum annuum) | RING | 2021 | CaAIRE1 | Nucleus | Drought | Degradation of protein phosphatase CaAITP1 | Inhibit | Baek et al. (2021) |
| Pepper (Capsicum annuum) | RING | 2019 | CaASRF1 | Nucleus and cytoplasm | Drought | CaAIBZ1 | Inhibit | Joo et al. (2019) |
| Pepper (Capsicum annuum) | RING | 2020 | CaATIR1 | Nucleus | Drought | CaATBZ1 | Inhibit | Joo et al. (2020) |
| Pepper (Capsicum annuum) | RING | 2017 | CaDIR1 | Nucleus | Drought | Via ABA‐mediated signalling | Promote | Joo et al. (2017) |
| Pepper (Capsicum annuum) | RING | 2018 | CaDSR1 | Nucleus | Drought | CaDILZ1 | Inhibit | Lim et al. (2018) |
| Pepper (Capsicum annuum) | RING | 2016 | CaDTR1 | Nucleus | Drought | Via ABA‐mediated signalling | Inhibit | Joo et al. (2016) |
| Pepper (Capsicum annuum) | RING | 2024 | CaFIRF1 | Cytoplasm and nucleus | High salt | CaFAF1 | Promote | Bae et al. (2024) |
| Pepper (Capsicum annuum) | RING | 2017 | CaREL1 | Cytoplasm and nucleus | Drought | Via the ABA‐signalling pathway | Promote | Lim et al. (2017b) |
| Pepper (Capsicum annuum) | U‐box | 2016 | CaPUB1 | – | Cold/drought stress | – | Inhibit cold; promote drought stress | Min et al. (2016) |
| Pepper (Capsicum annuum) | U‐box | 2024 | CaPUB24 | Nucleus and cytoplasm | Drought | ABA‐dependent manner | Promote |
Bae et al. (2024) |
| Pohlia nutans | U‐box | 2019 | PnSAG1 | Cytoplasm | Salt | – | Promote | Wang et al. (2019) |
| Poplar (Populus trichocarpa) | RING | 2020 | PtXERICO | – | Drought | Through the increase of cellular ABA level | Inhibit | Kim et al. (2020) |
| Populus (Populus alba var. pyramidalis) | U‐box | 2021 | PalPUB79 | Cytoplasm | Drought | PalWRKY77 | Inhibit | Tong et al. (2021) |
| Populus (Populus euphratica) | RING | 2018 | PeCHYR1 | Nucleus and ER | Drought | Increased sensitivity to exogenous ABA, enhanced ROS production and reduced stomatal aperture | Promote | He et al. (2018) |
| Potato (Solanum tuberosum) | RING | 2020 | SbRFP1 | – | Salt | Regulating the expression of stress‐related genes | Promote | Zhao et al. (2020) |
| Potato (Solanum tuberosum) | RING | 2022 | StATL2‐like | Endoplasmic reticulum | Cold | Interacting with StCBF1/4 | Promote | Song et al. (2022) |
| Potato (Solanum tuberosum) | RING | 2020 | StRFP2 | Cell membrane and cytoplasm | Drought | – | Promote | Qi et al. (2020) |
| Potato (Solanum tuberosum) | U‐box | 2010 | StPUB17 | – | NaCl | – | Inhibit | Ni et al. (2010) |
| Potato (Solanum tuberosum) | U‐box | 2024 | StPUB25 | – | Drought | Increased peroxidase (POD) activity | Inhibit | Liu et al. (2024) |
| Potato (Solanum tuberosum) | U‐box | 2020 | StPUB27 | – | Drought | Mediating stomatal conductance | Promote | Tang et al. (2020) |
| Rice (Oryza sativa) | RING | 2018 | OsSAR1 | Cytosol and nucleus | Salt | OsSNAC2 and OsGRAS44 | Promote | Park et al. (2018) |
| Rice (Oryza sativa) | RING | 2019 | H2Bub1 | – | Drought | OsbZIP46 | Inhibit | Ma et al. (2019) |
| Rice (Oryza sativa) | RING | 2017 | OsAIR2 | Golgi apparatus | Arsenic | OsKAT1 | Inhibit | Hwang et al. (2017) |
| Rice (Oryza sativa) | RING | 2022 | OsATL38 | Plasma membrane | Cold | Via mono‐ubiquitination of OsGF14d 14–3‐3 protein | Promote | Cui et al. (2022) |
| Rice (Oryza sativa) | RING | 2019 | OsCLR1 | Cytosol | Salt/drought | ABA‐dependent manner | Inhibit | Park et al. (2019) |
| Rice (Oryza sativa) | RING | 2014 | OsCTR1 | Chloroplasts and cytosol | Drought | Via ABA‐dependent regulation and related systems | Inhibit | Lim et al. (2014) |
| Rice (Oryza sativa) | RING | 2020 | OsDHSRP1 | Microtubule cytoskeleton | Drought/heat/NaCl | O. sativa glyoxalase (OsGLYI‐11.2) and O. sativa abiotic stress‐induced cysteine proteinase 1 (OsACP1) | Promote | Kim et al. (2020) |
| Rice (Oryza sativa) | RING | 2018 | OsDIRP1 | Nucleus | Drought/salt/cold | – | Promote | Cui et al. (2018) |
| Rice (Oryza sativa) | RING | 2011 | OsDIS1 | Nucleus | Drought | OsNek6 | Promote | Ning et al. (2011) |
| Rice (Oryza sativa) | RING | 2010 | OsDSG1 | – | Salt/drought | – | Promote | Park et al. (2010) |
| Rice (Oryza sativa) | RING | 2014 | OsHIR1 | Plasma membrane and nucleus | Arsenic and cadmium uptakes | Degraded the protein level of OsTIP4;1 | Inhibit | Lim et al. (2014) |
| Rice (Oryza sativa) | RING | 2019 | OsHIRP1 | Nucleus | Heat | OsARK4 and OsHRK1 | Inhibit | Kim et al. (2019) |
| Rice (Oryza sativa) | RING | 2016 | OsHTAS | Nucleus and cytoplasm | Heat | Promote H2O2‐induced stomatal closure | Inhibit | Liu et al. (2016) |
| Rice (Oryza sativa) | RING | 2018 | OsMAR1 | Microtubules | Salt | Via the regulation of the OCPI2 | Inhibit | Xiao et al. (2022) |
| Rice (Oryza sativa) | RING | 2022 | OsMSRFP | – | Salt | Through ubiquitination and proteasomal degradation of OsMYBc | Promote | Kim et al. (2022) |
| Rice (Oryza sativa) | RING | 2022 | OsRF1 | Endoplasmic reticulum membrane | Drought/salt | Targets OsPP2C09 for Degradation | Inhibit | Kim et al. (2022) |
| Rice (Oryza sativa) | RING | 2023 | OsRFPHC‐13 | Chloroplasts and cytoplasm | Salt | Via an ABA‐dependent mechanism | Inhibit | Kim et al. (2023b) |
| Rice (Oryza sativa) | RING | 2023 | OsRFPHC‐4 | Plasma membrane | Salt | Via the regulation of changes in Na+/K+ transporters | Inhibit | Kim et al. (2023a), Kim et al. (2023) |
| Rice (Oryza sativa) | RING | 2021 | OsRFPv6 | Plasma membrane and cytosol | Salt | Via Na+ uptake | Inhibit | Kim et al. (2021) |
| Rice (Oryza sativa) | RING | 2014 | OsRHP1 | – | Drought/Salt | Through increased ABA level | Inhibit | Zeng et al. (2014) |
| Rice (Oryza sativa) | RING | 2022 | OsRINGzf1 | Endoplasmic reticulum and plasma membrane | Drought | Degradation of OsPIP2;1 | Inhibit | Chen et al. (2022) |
| Rice (Oryza sativa) | RING | 2015 | OsRMT1 | Nucleus and microtubules | Salt | Interacted with OsSalT, OsCPA1, OsbZIP60, OsFKBP12, OsEDA16, OsDH1, OsPUB53 and OsPB1 | Inhibit | Lim et al. (2015) |
| Rice (Oryza sativa) | RING | 2011 | OsSDIR1 | Plasma membrane and other intracellular membranes | Drought | – | Inhibit | Gao et al. (2011) |
| Rice (Oryza sativa) | RING | 2023 | OsSIRHC‐2 | Cytosol | Salt | Through the low absorption of Na+ | Inhibit | Choi and Jang (2023) |
| Rice (Oryza sativa) | RING | 2016 | OsSIRP1 | Cytoplasm | Salt | – | Promote | Hwang et al. (2016) |
| Rice (Oryza sativa) | RING | 2018 | OsSIRP2 | Nucleus | Salt/Osmotic stress | O. sativa transketolase 1 (OsTKL1) | Inhibit | Chapagain et al. (2018) |
| Rice (Oryza sativa) | RING | 2018 | OsSIRP3 | Cytoplasm | Salt | OsMADS70 and OsABC1P11 | Promote | Park et al. (2018) |
| Rice (Oryza sativa) | RING | 2021 | OsSIRP4 | Cytosol and plasma membrane | Salt | OsPEX11‐1 | Promote | Kim and Jang (2021) |
| Rice (Oryza sativa) | RING | 2015 | OsSRFP1 | Nucleus | Salt/Cold/Oxidative | Regulation of stress‐related genes | Promote | Fang et al. (2015) |
| Rice (Oryza sativa) | U‐box | 2020 | OsPQT3 | – | Oxidative/Salt | Elevated expression of OsGPX1, OsAPX1andOsSOD1 | Promote | Alfatih et al. (2020) |
| Rice (Oryza sativa) | U‐box | 2017 | OsPUB2/3 | OsPUB2: exocyst positive organelle (EXPO)‐like punctate bodies and nuclei, OsPUB3: predominantly EXPO‐like structure | Cold | – | Inhibit | Byun et al. (2017) |
| Rice (Oryza sativa) | U‐box | 2023 | OsPUB7 | – | Drought/salt | – | Inhibit | Kim et al. (2023) |
| Rice (Oryza sativa) | U‐box | 2011 | OsPUB15 | Cytoplasm | Oxidative stress | – | Inhibit | Park et al. (2011) |
| Rice (Oryza sativa) | U‐box | 2011 | OsPUB16 | Nucleus and cytoplasm | Water‐deficit | Attenuates the ABA and JA response | Promote | Lv et al. (2022) |
| Rice (Oryza sativa) | U‐box | 2021 | OsPUB41 | Cytosol and nucleus | Drought | OsCLC6 | Promote | Seo et al. (2021) |
| Rice (Oryza sativa) | U‐box | 2020 | OsPUB67 | Nuclei, cytoplasm, and membrane | Drought | OsRZFP34 and OsDIS1 | Inhibit | Qin et al. (2020) |
| Rice (Oryza sativa) | Cullin4‐based | 2021 | OsCBE1 | – | Abiotic | OsC3H32 a CCCH‐type transcription factor | Inhibit | Choi et al. (2021) |
| Rice (Oryza sativa) | Zinc‐finger domain | 2015 | OsiSAP7 | Nucleus or sub‐nuclear speckles | Water‐deficit stress | Regulates ABA stress signalling | Promote | Sharma et al. (2015) |
| Sickle medick (Medicago falcata) | RING | 2019 | MfSTMIR | Endoplasmic reticulum membrane | Salt | Interacting with MtUBC32 and MtSec61 gamma | Inhibit | Zhang et al. (2019) |
| Sorghum | RING | 2020 | SbHCI1 | Cytosol/golgi apparatus under heat stress | Heat | OsHCI1 | Inhibit | Lim et al. (2020) |
| Soybean (Glycine max) | U‐box | 2020 | GmPUB6 | Peroxisome | Drought | ABA‐dependent | Promote | Wang et al. (2016) |
| Soybean (Glycine max) | U‐box | 2016 | GmPUB8 | Post‐Golgi compartments | Drought/osmotic stress/salt | – | Promote | Wang et al. (2016) |
| Soybean (Glycine max) | U‐box | 2022 | GmPUB21 | Cytoplasm, nucleus, and plasma membrane | Drought/salt | Via the ABA signalling pathway | Promote | Yang et al. (2022) |
| Sweet potato (Ipomoea batatas) | RING | 2021 | IbATL38 | Nucleus and plasm membrane | Salt | Inducible expression of a series of stress‐responsive genes and prominently decrease of H2O2 content | Inhibit | Du et al. (2021) |
| Sweet potato (Ipomoea batatas) | RING | 2021 | IbMREL57 | – | Combined salt and drought stress | WAVE‐DAM‐PENED2‐LIKE7 | Inhibit | Dou et al. (2021) |
| Sweet potato (Ipomoea batatas) | RING | 2024 | IbNIEL | – | Salt/drought | IbNAC087 | Promote | Li et al. (2024) |
| Tomato (Solanum lycopersicum) | RING | 2017 | SlRING1 | Plasma membrane and nucleus | Cadmium | Stimulate both ROS and metal detoxification mechanisms | Inhibit | Ahammed et al. (2021) |
| Tomato (Solanum lycopersicum) | U box | 2021 | SlCHIP | – | Heat | Targeting degradation of misfolded proteins | Inhibit | Zhang et al. (2021) |
| Tomato (Solanum pimpinellifolium) | RING | 2016 | SpRing | Endoplasmic reticulum | Salt | – | Inhibit | Qi et al. (2016) |
| Wheat (Triticum aestivum) | RING | 2022 | TaSADR1 | Nucleus | Drought | Regulating the expression of stress‐related genes | Promote | Zhang et al. (2017) |
| Wheat (Triticum aestivum) | RING | 2017 | TaSAP5 | Cytosol and nucleus | Drought | TaDRIP, as well as AtDRIP1 and AtDRIP2 | Promote | Zhang et al. (2017) |
| Wheat (Triticum aestivum) | U‐box | 2021 | TaPUB1 | – | Cadmium | Degradation of TaIRT1 and TaIAA17 | Inhibit | Zhang et al. (2021) |
| Wheat (Triticum aestivum) | U‐box | 2020 | TaPUB1 | – | Salt | Interacted with TaMP (Triticum aestivssum α‐mannosidase protein) | Inhibit | Wang et al. (2020) |
| Wheat (Triticum aestivum) | U‐box | 2021 | TaPUB2/3 | Cytoplasm and Golgi apparatus | Drought | – | Inhibit | Kim et al. (2021) |
| Wheat (Triticum aestivum) | U‐box | 2022 | TaPUB2/3 | – | Salt | ABA‐dependent | Inhibit | Kim et al. (2022) |
| Wheat (Triticum aestivum) | U‐box | 2023 | TaPUB4 | Nucleus | Drought | – | Inhibit | Kim et al. (2023) |
| Wheat (Triticum aestivum) | U‐box | 2020 | TaPUB26 | Nucleus | Salt | Interacted with TaRPT2a | Promote | Wu et al. (2020) |
Figure 6.

Regulation of E3 Ub in response to plant cold stress. E3 Ub ligases during cold stress are involved in (1) activation of the cold stress response and (2) attenuation of the cold stress response. In (1), cold stress CBF‐dependent cold signalling pathway is activated by the action of PUB25 (U‐box‐type E3 Ub ligase 25) and PUB26 (U‐box‐type E3 Ub ligase 26). PUB25 and PUB26 mediate the destabilisation of MYB15 (transcriptional repressor of the CBF‐dependent cold signalling pathway); the latter regulates the CBF‐dependent cold signalling pathway. BRUTUS (iron‐binding E3 Ub ligase containing three hemerythrin (HHE) domains and a really interesting new gene (RING) domain) mediates the destabilisation of VOZ1/2 (vascular plant one‐zinc finger ½); the latter regulates negative tolerance to cold stress. After removing cold stress (2), the E3 Ub ligase domain mediates the attenuation of the cold stress response. SINAT (RING‐domain containing E3 Ub ligase) mediates the destabilisation of ACS (1‐aminocyclopropane‐1‐carboxylic (ACC) synthase); the latter activates ethylene synthesis, and the last promotes tolerance to cold stress. OsCBE1 (substrate receptor of cullin4‐based E3) mediates destabilisation of OsC3H32 (CCCH‐type transcription factor), the latter contributing to tolerance to cold stress. PQT3 (E3 Ub ligase PARAQUAT TOLERANCE 3) intervenes in the elimination of PRMT4b (protein arginine methyltransferases), which later activate APX1 (ascorbate peroxidase 1) and GPX1 (glutathione peroxidase‐1) both play essential role intolerance to cold stress.
Role of E3 Ub ligase on biotic stress
Biotic stress can have a significant impact on agriculture, reducing, in some cases, average plant productivity by 65%–87% (Francini and Sebastiani, 2019; Sharma et al., 2023). Its factors commonly include harmful microbes, insects, nematodes, weeds and pathogens (Mohapatra et al., 2021). Biotic stress influences plant cellular mechanisms and can increase reactive oxygen species and disrupt physiological and molecular functions (Chaudhary et al., 2022). Different E3 Ub ligases are crucial for molecular regulation and can influence biotic stress tolerance, either positively or negatively.
Plant immunity involves molecular interactions enabling plants to recognise and respond to pathogens, involving systemic acquired and induced resistance. Understanding plant immunity is crucial for disease management and global food security (Bjornson and Zipfel, 2021). HECT‐type E3 Ub ligases are essential for plant immune responses. Most of them, including UPL1, UPL3 and UPL5, interact with the hormone salicylic acid (SA). Microbial pathogens and insects that invade plants can cause plants to activate numerous RING‐type E3 Ub ligases as positive or negative regulators to control biotic stress response (Furniss et al., 2018). For example, Arabidopsis ATL9 is a chitin‐inducible E3 Ub ligase that shapes basal resistance to Golovinomyces cichoracearum (Deng et al., 2017). Recognition of chitin present in the structural membrane of these pathogens will trigger a plant protection response. It can be activated when plants recognise chitin present in the structural membrane of these pathogens. For example, RING‐type E3 Ub ligase MIEL interacts with MYB96, a regulator of ABA signalling, and limits its accumulation. MIEL1 is critical in biotic stress in conjunction with the ABA pathway (Lee and Seo, 2016). Some PUB E3 Ub ligases exhibit adverse regulatory effects on plant disease resistance, contributing to cell death. PTI and PAMPs‐induced signalling are the core defensive mechanisms of plant systems against pathogen infection (Lu et al., 2011). PUB12 and PUB13 were reported to negatively regulate FLS2‐mediated PTI immune response (Lu et al., 2011). Some plant U‐box proteins, such as CMPG1, PUB44 and PUB17, have positive regulatory roles in plant disease resistance. Some pathogens have evolved with plants and manipulated E3 Ub ligase machinery for their benefit (Zhu et al., 2015). Figure 7 shows the role of E3 Ub ligase in biotic stress. Table 2 shows the E3 Ub ligase genes in response to biotic stresses in plants.
Figure 7.
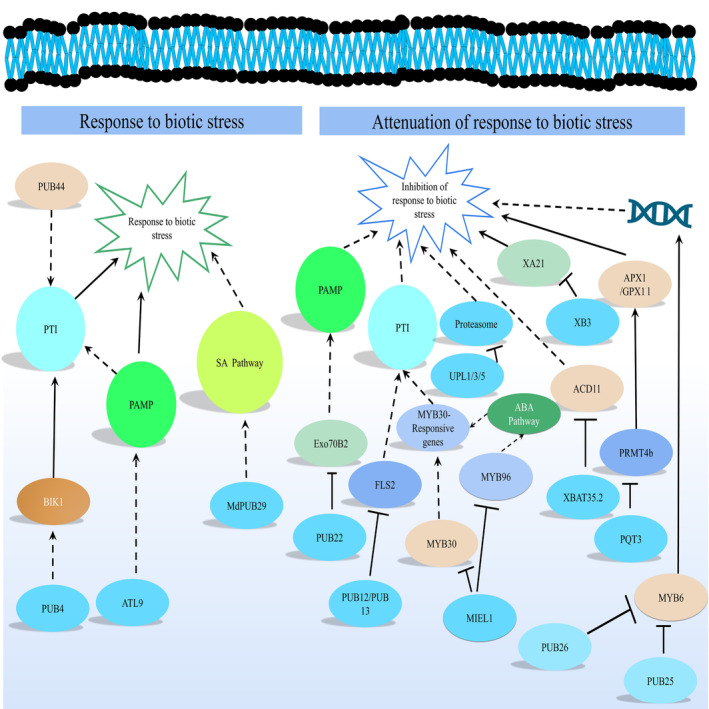
Regulation of E3 Ub in response to plant biotic stress. E3 Ub ligases during biotic stress are involved in (1) activation of the biotic stress response and (2) attenuation of the biotic stress response. In (1), the biotic stress PTI (PAMP‐triggered immunity) is activated by the action of PUB44 (U‐box‐type E3 Ub ligase 44) and PUB4 (U‐box‐type E3 Ub ligase 4). PUB4 mediates the accumulation of BIK1 (receptor‐like cytoplasmic kinase), which is directly involved in the activation of PTI. ATL9 (RING zinc finger protein with E3 Ub ligase) positively regulates PAMPs (pathogen‐ or microbe‐associated molecular patterns) that promote PTI and tolerance to biotic stress. MDPUB29 (apple U‐box E3 Ub ligase) promotes the activation of the SA pathway for biotic stress tolerance. After removing biotic stress (2), the E3 Ub ligase domain mediates the attenuation of the biotic stress response. PUB22 (U‐box‐type E3 Ub ligase 22) mediates the destabilisation of Exo70B2 (exocyst subunit), which activates PAMP. PUB12 (U‐box‐type E3 Ub ligase 12) and PUB13 (U‐box‐type E3 Ub ligase 13) regulate negatively the FLS2 (pattern recognition receptor FLAGELLIN‐SENSING 2), the latter activating PTI. MIEL1 (RING‐type E3 Ub ligase MYB30‐INTERACTING E3 Ub LIGASE 1) directly mediates the elimination of MYB30 (transcription factor), the latter activating the MYB30 responsive genes that activate PTI. MIEL1 also mediates the destabilisation of MYB96 (transcription factor); the latter activates the ABA pathway. The ABA pathway also promotes MYB30‐responsive genes that positively regulate PTI. MIEL1 links abiotic and biotic stress. UPL1/3/5 (HECT domain‐containing family of ubiquitin‐protein ligases 1, 3, 5) mediate the destabilisation of the proteasome, the latter being involved in the continuation of the stress response. PUB26 (U‐box‐type E3 Ub ligase 26) and PUB25 (U‐box‐type E3 Ub ligase 25) mediate the destabilisation of MYB6 (R2R3‐MYB transcription factor), which is involved in the activation of the gene for the response to abiotic stress. PQT3 (E3 Ub ligase PARAQUAT TOLERANCE 3) mediates the elimination of PRMT4b (protein arginine methyltransferases), which later activate APX1 (ascorbate peroxidase 1) and GPX1 (glutathione peroxidase‐1), both essential in tolerance to biotic stress. XBAT35.2 (RING‐type E3 Ub ligase) mediates the destabilisation of ACD11 (arabidopsis accelerated cell death 11), the latter being involved in maintaining tolerance to biotic stress. XB3 mediates the destabilisation of XA21 (receptor‐type protein kinase), which responds to biotic stress.
Table 2.
E3 ubiquitin ligase genes involved in plant response to biotic stresses
| Species | Type of E3 Ub ligase | Year | Gene | Cellular localisation | Type of stress | Cellular target | Role in stress | References |
|---|---|---|---|---|---|---|---|---|
| Arabidopsis thaliana | HECT | 2018 | UPL3 | – | Biotic stress | Interacts with the regulatory particle of the proteasome and with other ubiquitin‐26S proteasome pathway components | Inhibit | Furniss et al. (2018) |
| Arabidopsis thaliana | RING | 2014 | ATL1 | Endomembrane structures | Powdery mildew | Interacts with EDR1 | Promote | Serrano et al. (2014) |
| Arabidopsis thaliana | RING | 2010, 2017 | ATL9 | Endoplasmic Reticulum | Biotic stress | Interacting partner of the PEST domain and the RING domain | Inhibit | Berrocal‐Lobo et al. (2010), Deng et al. (2017) |
| Arabidopsis thaliana | RING | 2022 | AtRDUF1/2 | – | Pseudomonas syringae pv. tomato DC3000 | Involved in SA‐mediated PR1 gene expression | Inhibit | Yi et al. (2022) |
| Arabidopsis thaliana | RING | 2018 | Keep on Going (KEG) | – | Biotic and abiotic stress | Degrade FDH | Inhibit | McNeilly et al. (2018) |
| Arabidopsis thaliana | RING | 2013 | MIEL1 | – | Biotic stress | This leads to MYB30 proteasomal degradation | Promote | Marino et al. (2013) |
| Arabidopsis thaliana | RING | 2012 | RGLG3 and RGLG4 | – | Pseudomonas syringae pv. tomato DC3000 | Controls the accessibility of JA‐Ile (COR) to the coreceptor COI1 | Inhibit | Zhang et al. (2012) |
| Arabidopsis thaliana | RING | 2021 | SDIR1 | ER membrane | Pseudomonas syringae pv. tomato DC3000 | – | Promote | Ramu et al. (2021) |
| Arabidopsis thaliana | RING | 2020 | SINAT4 | – | Pseudomonas syringae pv. tomato DC3000 | Interacts with RD21A | Promote | Liu et al. (2020) |
| Arabidopsis thaliana | RING | 2017 | XBAT35.2 | Golgi | Pseudomonas syringae pv. tomato DC3000 | ACD11 | Inhibit | Liu et al. (2017) |
| Arabidopsis thaliana | U‐box | 2016 | AtCHIP | – | Virulent pathogens | Interacts with HSP Chaperones | Inhibit | Copeland et al. (2016) |
| Arabidopsis thaliana | U‐box | 2022 | AtPUB2/4 | AtPUB2: Plasma membrane; AtPUB4: cytoplasm and plasma membrane | Pseudomonas syringae | Interacts with PTI signalling components, including FLS2, BIK1, PBL27, and RbohD | Inhibit | Wang et al. (2022) |
| Arabidopsis thaliana | U‐box | 2022 | AtPUB4 | – | Biotic stress | BIK1 (targeted by bacterial type‐III effector) | Inhibit | Yu et al. (2022) |
| Arabidopsis thaliana | U‐box | 2011 | AtPUB12/13 | – | Pseudomonas syringae pv. tomato DC3000 and Pseudomonas syringae maculicola ES4326 | FLS2 | Promote | Lu et al. (2011) |
| Arabidopsis thaliana | U‐box | 2024 | AtPUB20/21 | – | Pseudomonas syringae pv. tomato DC3000 | Promote | Yi et al. (2024) | |
| Arabidopsis thaliana | U‐box | 2012 | AtPUB22 | – | Biotic stress | Mediates the ubiquitination and degradation of Exo70B2 | Promote | Stegmann et al. (2012) |
| Arabidopsis thaliana | U‐box | 2008 | PUB22/23/24 | – | Pseudomonas syringe pv tomato DC3000 and Ha isolate Emco5 | – | Promote | Trujillo et al. (2008) |
| Arabidopsis thaliana | U‐box | 2021 | PUB25/PUB26 | – | Verticillium dahliae | MYB6 | Promote | Ma et al. (2021) |
| Arabidopsis thaliana | U‐box | 2018 | PUB25/PUB26 | – | Botrytis cinerea and Pseudomonas syringae pv tomato DC3000 | BIK1 | Promote | Wang et al. (2018) |
| Arabidopsis thaliana | U‐box | 2017 | SAUL1 | Plasma membrane | Pseudomonas syringae pv. tomato DC3000 | Monitored by the TNL SOC3 | Inhibit | Tong et al. (2017) |
| Apple (Malus domestica) | U‐box | 2019 | MdPOB1 | – | Botryosphaeria dothidea | MdPUB29 | Promote | Han et al. (2019) |
| Apple (Malus domestica) | U‐box | 2019 | MdPUB29 | – | Botryosphaeria dothidea | Regulates the SA pathway | Inhibit | Han et al. (2019) |
| Chinese wild grapevine (Vitis yeshanensis) | U‐box | 2024 | VyPUB21 | – | Powdery mildew | Targets VyNIMIN protein hydrolysis through the 26S proteasome system | Inhibit | Wang et al. (2024) |
| Cotton (Gossypium hirsutum) | U‐box | 2021 | GhXB3s (GhXB32A, GhXB35A and GhXB37A) | – | Verticillium dahliae | – | Inhibit | Ge et al. (2021) |
| Grapevine (Vitis vinifera) | U‐box | 2021 | VvPUB17 | – | Powdery mildew | Via the salicylic acid signalling pathway | Inhibit | Li et al. (2021) |
| Kiwifruit (Actinidia chinensis) | U‐box | 2024 | PUB23 | – | Pseudomonas syringae pv. actinidiae | GT1 | Promote | Wang et al. (2024) |
| Medicago truncatula | U‐box | 2010 | PUB1 | Plasma membrane | Infection and nodulation | PUB1 is phosphorylated by LYK3 | Inhibit | Mbengue et al. (2010) |
| Nicotiana benthamiana | U‐box | 2015, 2021 | NbPUB17 | Nucleus | Phytophthora infestans | Promotes specific PTI and PCD pathways | Inhibit | He et al. (2015) |
| Potato (Solanum tuberosum) | U‐box | 2020 | StPUB17 | Nucleus | Phytophthora infestans | Targeting a Negative Regulator, KH17 | Inhibit | McLellan et al. (2020) |
| Rice (Oryza sativa) | U‐box | 2015 | OsPUB15 | Cytosol | Biotic stress | Interacts with the receptor‐like kinase PID2 | Inhibit | Wang et al. (2015) |
| Rice (Oryza sativa) | U‐box | 2022 | OsPUB33/34/39 | – | Magnaporthe grisea | – | Inhibit/Promote | Zhang et al. (2022) |
| Rice (Oryza sativa) | U‐box | 2014, 2022 | OsPUB44 | Entire region | Xanthomonas oryzae pv. oryzae | PBI1 | Inhibit | Ichimaru et al. (2022) |
| Rice (Oryza sativa) | U‐box | 2012, 2015 | OsSPL11 | – | Magnaporthe oryzae and Xanthomonas oryzae pv. oryzae | SPIN6 | Inhibit/Promote | Liu et al. (2012, 2015) |
| Tobacco (Nicotiana tabacum) | U‐box | 2010 | CMPG1 | – | Phytophthora infestans | AVR3a | Inhibit | Bos et al. (2010) |
| Tobacco (Nicotiana tabacum) | U‐box | 2006 | NtCMPG1 | – | Biotic stress | Involved in Pto/AvrPto and Inf1‐mediated cell death | Inhibit | González‐Lamothe et al. (2006) |
| Tomato (Solanum lycopersicum) | U‐box | 2018 | SlPUB13 | – | Biotic stress | Works with group III E2s to ubiquitinate FLS2 | Inhibit | Zhou and Zeng (2018) |
| Tomato (Solanum lycopersicum) | U‐box | 2021 | SlPUB24 | Cytoplasm, plasma membrane and nucleus | Xanthomonas euvesicatoria pv. perforans | SlCWP | Inhibit | Liu et al. (2021) |
| Wheat (Triticum aestivum) | U‐box | 2015 | CMPG1‐V | Nucleus, endoplasmic reticulum, plasma membrane and partially in trans‐Golgi network/early endosome vesicles | Blumeria graminis f. sp. tritici | Reactive oxidative species and the phytohormone pathway | Inhibit | Zhu et al. (2015) |
Research efforts have made considerable progress in the structural characterisation of E3 Ub ligases and their importance in plant resistance to abiotic and biotic stresses. However, it is vital to capitalise on this advance to meet the food security challenge. The following section proposes the PROTAC methodology to improve plant resistance to stress.
PROTACs: Concept, mode of action, origin and development
PROTACs can target the E3 Ub ubiquitin ligase for improved plant stress resistance. This section introduces the concept of PROTACs, their mode of action, origin and development.
The concept of PROTACs
PROTACs are defined as proteolysis‐targeted chemotherapeutics or proteolysis‐targeted protein‐degrading chemotherapeutics. They are small molecules with dual functions. PROTACs are made up of a 01 linker and 02 moieties (Figure 8). One moiety binds the target protein (protein of interest, POI), and the other binds the E3 Ub ligase. By linking a POI to an E3 Ub ligase, PROTACs facilitate the ubiquitination of the POI and, consequently, its degradation by the proteasome. PROTAC class compounds offer a solid complementary alternative for gene silencing or targeted inhibition and represent a viable option for controlling proteins responsible for plant sensitivity to stress. PROTAC may ultimately target the plant E3 Ub ligases that regulate biotic and abiotic stresses in plants.
Figure 8.

The mechanism of PROTAC‐mediated targeted protein degradation. PROTACs act as a bridge between the target protein or protein of interest (POI) and the E3 Ub ligase, creating sufficient proximity to allow the transfer of ubiquitin from the E2 ligase to the target protein. This action will be repeated several times, and the POI will have a ubiquitin chain leading to its recognition and degradation by the proteasome. The PROTAC molecule can conserve its properties after several cycles and eliminate the POI.
The mode of action of PROTACs
The mode of action of PROTACs is innovative and relevant to eliminating E3 Ub ligases important in plant stress, as illustrated in Figure 8. Concisely, the PROTAC mechanism causes the elimination of the target protein from the cell and not just inhibits it, as the conventional chemical pesticides used in agriculture do. PROTACs may not be subject to the traditional stoichiometric ratio like common chemicals applied in crop protection because of their unique chemical architecture. As major heterobifunctional degraders, PROTACs are relevant for targeting the E3 Ub ligases responsible for plant stress. They offer a viable alternative to address pathological conditions of plants where the proteins involved in stress are complex to inhibit by conventional chemical strategies. More interestingly, PROTACs do not require the absolute presence of an active site on the targeted protein to act but instead can be designed to find affinity with different areas of the protein structure. PROTAC technologies are relevant to target molecules that are difficult to inhibit and could be used to treat so‐called incurable diseases involving proteins with smooth surfaces or huge active sites. The unique properties of PROTACs could pave the way for solving the problem of recalcitrant proteins, which account for around 80% of all cellular proteins in eukaryotic cells, including the E3 Ub ligases. According to this perspective, PROTACs have the potential to transform agriculture by controlling the regulation of E3 Ub ligase in plant stress response, which is of great importance in the agricultural sector.
The origin of PROTACs
Plants and viruses originally inspired the concept of exploiting cellular protein degradation systems for therapeutic purposes (Békés et al., 2022). Research into PROTACs dates back some 20 years (since 2001) but has already made a significant contribution, with numerous clinical trials in cancer treatment entering phase 2 (Békés et al., 2022). Today, PROTACs offer vast prospects in human medicine but are still limited in the plant field. PROTAC concept has risen to the challenge of moving from idea to real‐world application. Since humans and plants share a similar eukaryotic system, PROTACs' success story in human health can be projected to plants.
PROTACs development
PROTAC methodology holds great promise for agriculture. In the agricultural sector, PROTACs can be studied on large farms relatively quickly and would not undergo the tedious clinical steps required for human studies. The role of E3 Ub ligases is crucial in the plant kingdom, which possesses over 1400 of them (Mazzucotelli et al., 2006), compared with around 600 in humans (Békés et al., 2022). Recently, the perspective of applying PROTACs in plants has attracted significant biotech and pharmaceutical companies, including Bayer, Novartis, Pfizer, Amgen, GlaxoSmithKline, Merck, PROTAC® and others (Gao et al., 2020). Bayer, a leading pharmaceutical and agricultural company, drives the PROTAC investment for sustainable agriculture. Arvinas is a clinical‐stage biotechnology company innovating in the field of targeted protein degradation therapies. Bayer and Arvinas launched a separate joint venture to develop PROTAC® agricultural applications. Bayer contributes over $55 million to the financing, while Arvinas provides the technology and intellectual property. Nevertheless, the development of PROTACs requires multidisciplinary collaboration. Synthetic methods may evolve toward computational chemistry and can be relevant to designing new linkers in silico (Bemis et al., 2021). Furthermore, molecular prospecting, advances in biotechnology (including chemical biology), and bioinformatics (exploiting existing data from medical research) can also be used to make significant advances in the field of PROTACs in plants (Guedeney et al., 2023). Efforts will be made to overcome the limitations of designing chimeras targeting proteolysis (Leon and Bassham, 2024).
Role of E3 Ub ligases in new methodology for PROTACs development in plant
Exploration of E3 Ub ligases in designing PROTACs for agriculture is in its infancy. This section gives an overview of promising PROTAC methodologies that can be used in plants while considering current challenges in E3 Ub ligase research to improve crop stress resistance.
Characterisation of plant homologues of human E3 Ub ligases to convert medical PROTAC methodology into agricultural applications
Identifying plant homologues of human E3 Ub ligases is essential for developing PROTAC methodology in agriculture. Although PROTAC‐related research in plants may consider all stress‐promoting proteins in cells as potential targets, starting by directing research at plant homologues of the human E3 Ub ligases used by PROTAC methodology in medicine may facilitate PROTAC design for agriculture. For example, CRBN, F‐box and NPR1 are substrate recognition subunits of Cullin RING E3 Ub ligase complexes recruited by PROTACs in human cancer research (Cruz Walma et al., 2022). Homologue substrate recognition subunits of E3 Ub ligases can be found in plants and considered in the PROTAC strategy. In Figure 9, the superposition of the plant CRBN and the human CRBN shows a certain similarity in the folding of the central part representing the Lon protease. The SCF (SCF‐like cullin‐RING complexes), whose substrate receptor subunits are interchangeable, regulates a broad spectrum of cellular functions in plants by ubiquitinating various protein substrates and can be an essential asset for PROTAC strategies. Besides, NPR1 may act as a substrate‐specific adapter of an E3 ubiquitin‐protein ligase complex (CUL3‐RBX1‐BTB). NPR1 was reported to modulate defensive‐related transcription factors and crosstalk between salicylate‐ and jasmonate‐dependent defence pathways (Johnson et al., 2003; Spoel et al., 2003).
Figure 9.

Superposition of two protein cereblon (CRBN) from Arabidopsis thaliana and Human. These two structures are superimposed in the central part, which represents the Lon protease and N‐terminal domain. PROTACs that target this region can be studied in both humans and plants. CRBN_ARATH, in yellow, represents the structure of the protein cereblon from the organism Arabidopsis thaliana (mouse‐ear cress). CRBN_ARATH functions in the protein ubiquitination pathway. The Uniprot code is Q8VXV2. CRBN_HUMAN, in blue, represents the 3D structure of Protein cereblon from organism HUMAN (mouse‐ear cress), which functions as a Substrate recognition component of a DCX (DDB1‐CUL4‐X‐box) E3 protein ligase complex that mediates the ubiquitination and subsequent proteasomal degradation of target proteins, such as MEIS2 or ILF2 (Kim et al., 2019). The Uniprot code is Q96SW2. All the PDB formats were obtained from Alphafold Alignment and were done on PyMOL, PyMOL Molecular Graphics System, Version 2.5.7pre, Schrödinger, LLC.
Consideration in POIs selection and PROTACs design in plant
Proteins targeted by fungicides and herbicides may be relevant as POI in PROTAC strategies for stress resistance. A specific requirement in selecting POIs is that PROTACs that bind to POIs should not obstruct the binding of a partner E3 Ub ligase. Some essential regions to consider for PROTAC binding are unstructured regions since they do not interfere with the proteolytic action of the proteasome. Research into the structure of the linker or E3 Ub ligase moiety of PROTACs can also benefit from data derived from research into PROTAC therapy in cancer. As a partner, E3 Ub ligase for PROTACs can be a similar subunit recovered in plants and animals with conserved regions. Applicability and specific modality in agriculture versus medicine should consider key stress factors, environment, and plant cell structure. For example, E3 Ub ligases and POIs with favourable and cooperative protein–protein interactions (PPIs) between them must be regarded for developing PROTACs. Targeting complexes such as the scaffolding protein will be a difficult task, and an alternative may be to target the nearby or neighbouring protein (Békés et al., 2022).
Specificity of PROTAC methodology in plant
Under stress, the proportions and compositions of E3 Ub ligases depend on the plant organ and plant variety. Omic methods such as transcriptomics and proteomics could be important for characterising E3 Ub ligase expression under specific conditions. While these preliminary studies can help to tailor PROTAC methodology to specific conditions such as different plant varieties or particular plant organs, it is essential that further PROTAC development takes into account the diversity of E3 Ub ligases and may involve better identification of the role and structure of plant E3 Ub ligases (Tables 1 and 2).
In the future, PROTAC strategies and tailor‐made treatments may be developed for any plant susceptible to a pathogen. Targeting specific E3 Ub ligase protein will enable specific plant treatment for particular plant varieties. A worldwide bank of well‐known microbial effectors could be developed and studied for PROTACs. Future considerations involve characterising the complete list of plant and microbial effectors, constructing a ligand library, constructing a target library and studying the involvement of E3 ligands in plant specificities.
The prospect of combining several PROTACs to attenuate the stress response of crops
PROTACs in plants should consider interrupting the stress response after the stress has been abolished. The persistence of the stress response can be detrimental to plants, as it can represent a waste of vital resources, which is why the response must also be regulated after stress inhibition. As shown in Tables 1 and 2, multiple E3 Ub ligases play diverse roles in abiotic stress and are expressed differently during stress induction studies. The prospect of combining multiple PROTACs should be considered seriously, as E3 Ub ligases have numerous targets playing a role in induced stress. Considering the previous discussion on drought, salt, cold and biotic stress, applying PROTACs in plants should focus on developing strategies to activate stress tolerance in plants and deactivate stress tolerance when the pressure is alleviated. A relevant system can be achieved by developing PROTACs that facilitate the elimination of the POI responsible for impairing stress tolerance. Once the stress is alleviated, the inhibition of PROTACs can be lifted to halt the stress response, allowing the plant to revert to its normal physiological state.
Consideration in selecting E3 Ub ligase as a partner of PROTACs and as POIs
The role of E3 Ub ligase as a partner and E3 Ub ligase as a POI will be the two critical perspectives for applying PROTACs in agriculture. Tables 1 and 2 show essential E3 Ub ligases involved in stress response.
Selecting E3 Ub ligase as POI
As POIs, special attention can be given to this E3 Ub ligase, which is differentially regulated during stress conditions. However, research is limited in predicting the overall connection of a particular E3 Ub ligase in plant molecular mechanism, and the possibility of unexpected results or some dysfunction needs to be considered. More importantly, genome‐wide classification of all classes of E3 Ub ligase in various species needs to be completed.
Selecting E3 Ub ligase as a partner of PROTACs
For E3 Ub ligases to be used as partners, their frequency of appearance during stress responses, their quantity, and how easily the ligand can be designed for the PROTACs strategy must be taken into account. For example, SCFs (SCF‐like cullin‐RING complexes), whose substrate‐receiving subunits are interchangeable, regulate a broad spectrum of cellular functions in plants by ubiquitinating various protein substrates and can therefore be an essential asset for PROTAC strategies. Elucidating the interaction mechanism between hormones and E3 Ub ligases can help develop PROTACs adapted to the recruitment of E3 Ub ligases, thus enabling the elimination of proteins that increase sensitivity to stress.
The PROTAC design strategy can learn how hormones interact with the E3 Ub ligase F‐box substrate receptor to design an E3 Ub ligase moiety of the PROTAC essential for recruiting the E3 Ub ligase to degrade the target protein. For example, auxin is an essential hormone for the development of plants. The auxin response requires a proper interaction between the Aux/IAA protein and SCFTIR1. Advances in research have shown that the auxin molecule binds preliminarily and specifically in a restricted space to TIR1 – the F‐box protein acting as a substrate receptor for the SCF complex. Following auxin binding to TIR1, the affinity of the AUX/IAA protein to auxin‐SCFTIR1 is increased. The AUX/IAA protein then binds to the E3 Ub ligase (forming an AUX/IAA‐auxin‐SCFTIR1 complex). Once the AUX/IAA protein complex is bound to TIR1, ubiquitination occurs through the transfer of ubiquitin from the E2 ligase to the AUX/IAA protein; this final step continues until the AUX/IAA protein is attached to a ubiquitin chain. The AUX/IAA protein is then directed to the proteasome for degradation. Degradation of the AUX/IAA protein leads to the accumulation of the ARF/MYB2 transcription factor set, enabling the expression of an appropriate auxin response in the plant (Tan et al., 2007). A similar phenomenon was observed for jasmonic acid, which interacts with SCFCOI1 to eliminate JASMONATE‐ZIM DOMAIN (JAZ) proteins that inhibit MYB2, a transcription factor involved in responses to abiotic stress. MYB2 accumulation plays a regulatory role in salt, cold, and drought tolerance. A structural analogue of auxin or jasmonic acid or their derivatives can be developed and tested based on their superior affinity with F‐BOX TIR1 or F‐BOX COI1 to design a PROTAC capable of recruiting the E3 Ub ligase SCFTIR1 or SCFCOI1 for removal of the POI, probably an E3 Ub ligase known to promote stress in the plant. Eliminating stress‐promoting E3 Ub ligases is more likely to increase plant resistance to stress.
Importance of inducible E3 Ub ligase in strategy of PROTACs in plant
Targeting or recruiting stress‐inducible E3 Ub ligases as part of the PROTAC strategy in plants could help control stress as soon as it occurs (Qi et al., 2016). For example, an inducible E3 Ub ligase partner or POI can be used, including SARS1, CaAIP1 and OsHKT1;1. So, the PROTACs study can consider this reference for more models to control stress and improve tolerance (Qi et al., 2016; Xiao et al., 2022).
Considering multi‐stress E3 Ub ligases in PROTAC strategies
POIs of PROTACs will include various enzymes, membrane receptors such as the ABA receptor or the chloride channel, and transcription factors (Tables 1 and 2) (Zhao et al., 2017). The discovery of specific E3 Ub ligases acting with multiple targets may inspire research into the design of PROTAC chimeras capable of controlling one or more stress response factors simultaneously and allowing plants to be resistant to multiple stresses (Xiao et al., 2022). PROTACs should also consider targeting the elimination of specific E3 Ub ligases effector of multiple stress sensitivity as GmPUB8 (Wang et al., 2016). Some E3 Ub ligases are overexpressed under various stresses, for example, SDIR1 (salt‐ and drought‐induced ring finger1) (Alfatih et al., 2020). Using these E3 Ub ligases as PROTAC partners could be recommended to impact multiple stresses consistently.
PROTACs can target microbial effectors and mimic insects and microbes that hijack the plant ubiquitin system
Plant pathogens cause biotic stress to plants, resulting in significant activation and deactivation of E3 Ub ligases. PROTACs can improve plant sensibility to herbicides and plant resistance to microbial pathogens. PROTACs could mediate ubiquitination and the proteasomal elimination of any infectious effectors introduced in the plant by pathogens. This prospect is attractive as it will contribute to controlling plant pathogens specialised in hijacking the eukaryotic ubiquitin‐proteasome system (Zhao et al., 2019). Beyond triggering immunity during microbial infection, microbes' primary strategy is mimicking essential E3 Ub ligases to induce plant vulnerability. One of PROTAC's exciting prospects will be to target specific microbial E3 Ub ligases that mimic the ubiquitin function of the plant proteasomal pathway.
Pathogens stimulate the plant immune system and release effector molecules to bypass defences. The plant ubiquitin‐proteasome system regulates protein abundance and controls immunity. Pathogen effector molecules disrupt the UPS by hijacking its functions or affecting target degradation. Studying the interactions between microbial effectors and UPS can help develop PROTACs with greater affinity for POI and E3 Ub ligase (Hijacking protein degradation, 2020; Langin et al., 2023). The model of insects and microbes manipulating the E3 Ub ligase system to their advantage can be studied to develop innovative PROTAC ligands. Hijacking protein degradation (2020).
Delivering PROTACs in plant system
The large size (700–1000 Da) and unusual physicochemical properties of PROTACs can significantly limit their plant uptake (Martín‐Acosta and Xiao, 2021). However, PROTACs need to penetrate plant cells and reach their molecular targets to exert their action. The complexity of plant cells and cell walls will likely hinder the uptake of PROTACs (Kastl et al., 2021). Strategies to maximise the uptake and delivery of PROTACs into plant cells may be essential for any breakthrough. Delivery systems or structural modifications of PROTACs could improve solubility, stability and membrane permeability (An and Fu, 2018). Research will be needed to optimise the design and delivery of PROTACs for plant systems.
The challenge of PROTACs in improving plant resistance to stress and the role of E3 Ub ligases beyond PROTACs
PROTACs have a long way to go before being applied to plant stress resistance. Indeed, many challenges must be resolved first (Leon and Bassham, 2024). Among the plausible limitations of PROTACs, implementing PROTACs in agriculture will require the regulation of heterobifunctional molecules and a complete study of their toxicology for plants, animals, humans and the environment. Promoting bioPROTACs or peptide‐PROTACs that integrate biocompatible elements into the structure of PROTACs could reduce toxicity issues (Lim et al., 2020). Low permeability or low stability of PROTACs may be recurrent in the plant system and lead to ineffective PROTACs; development of resistance may also exist with PROTACs as they are based on E3 Ub ligase specificity, and mutation in the recognition site may lead to resistance (Zhang et al., 2019). A similar situation was observed when cancer cells developed resistance to PROTACs. The integrative strategies will remain relevant in the future field of PROTACs and may involve combining pesticides and PROTACs.
A key research challenge is controlling the chemical characteristics of PROTAC chimeras and the ligand identification strategy for screening POIs. Molecular glues are molecules known to stabilise PPIs. They show promise in E3 Ub ligases and their targets. Molecular glues comprise one component and are not heterobifunctional like PROTAC molecules. The molecular glue concept could be applied to PROTACs to achieve more significant target protein degradation. In addition to PROTACs, other heterobifunctional molecules are non‐UPS pathways for the degradation of a POI, such as autophagy‐targeted chimeras. Research in these areas holds great promise for the integral management of plant resistance in the face of increasing disease threats associated with climate change.
Conclusion
Ensuring access to a sufficient and reliable food supply is a global challenge. The world is currently confronted with major threats such as climate change, population growth and the proliferation of stressful factors, including drought, extreme cold, salinity and the unprecedented invasion of pathogens. Developing a sustainable and comprehensive food production strategy requires innovative advancements in the molecular mechanisms of plants. This paper demonstrates how advances in molecular research of E3 Ub ligase can provide opportunities for innovation in biotechnology approaches highly relevant to enhancing plant resistance to stress. The discussion focused on the concept and mechanism of the newly discovered class of heterobifunctional molecules known as PROTACs. The emphasis highlights its significance in enhancing control over plants' abiotic and biotic stress. Targeting E3 Ub Ligase, a crucial factor in stress response, as a therapeutic target and partner will significantly advance plant disease control. This potential will substantially enhance global food security. Hence, there are still crucial prerequisites for implementing PROTACs in crop production. These include overcoming the barriers of fully understanding the properties and stability of PROTAC chimeras, as well as determining the administration method for PROTACs in plants to ensure their entry into cells and contact with the molecular targets, taking into account their significantly larger size compared to pesticide molecules. Exciting breakthroughs in molecular basis E3 Ub ligase in plant research will certainly fasten progress opportunities in food security worldwide.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Author contributions
Y.S. and G.L.N.N. Contributed equally. Designing the framework of the article, Y.S. and G.L.N.N.; writing – original draft, Y.S., G.L.N.N., K.W., Y.L., E.A.G., and M.A.; writing – review & editing, Q.Y., Q.Z., and H.Z.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32102030, 32072276, 32150410368) and the China Postdoctoral Science Foundation (2023M741440).
Data availability statement
The data that support the findings of this study are available in PubMed at https://pubmed.ncbi.nlm.nih.gov/?term=Proteolysis‐targeting%20chimeras%20(PROTACs)%20&timeline=expanded. These data were derived from the following resources available in the public domain: – PubMed, https://pubmed.ncbi.nlm.nih.gov/ – WebofKnowledge, https://www.webofknowledge.com – Google Scholar, https://scholar.google.com – Springer, https://www.springer.com.
References
- Adams, E.H.G. and Spoel, S.H. (2018) The ubiquitin–proteasome system as a transcriptional regulator of plant immunity. J. Exp. Bot. 69, 4529–4537. [DOI] [PubMed] [Google Scholar]
- Adler, G. , Konrad, Z. , Zamir, L. , Mishra, A.K. , Raveh, D. and Bar‐Zvi, D. (2017) The Arabidopsis paralogs, PUB46 and PUB48,encoding U‐box E3 ubiquitin ligases, are essential for plant response to drought stress. BMC Plant Biol. 17, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, G. , Mishra, A.K. , Maymon, T. , Raveh, D. and Bar‐Zvi, D. (2018) Overexpression of Arabidopsis ubiquitin ligase At PUB46 enhances tolerance to drought and oxidative stress. Plant Sci. 276, 220–228. [DOI] [PubMed] [Google Scholar]
- Ahammed, G.J. , Li, C.X. , Li, X. , Liu, A. , Chen, S. and Zhou, J. (2021) Overexpression of tomato RING E3 ubiquitin ligase gene SlRING1 confers cadmium tolerance by attenuating cadmium accumulation and oxidative stress. Physiol. Plant. 173, 449–459. [DOI] [PubMed] [Google Scholar]
- Ahmad, I. , Zhu, G. , Zhou, G. , Younas, M.U. , Suliman, M.S.E. , Liu, J. , Zhu, Y. et al. (2023) Integrated approaches for increasing plant yield under salt stress. Front. Plant Sci. 14, 1215343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, M.Y. , Seo, D.H. and Kim, W.T. (2022) PUB22 and PUB23 U‐box E3 ubiquitin ligases negatively regulate 26S proteasome activity under proteotoxic stress conditions. J. Integr. Plant Biol. 64, 625–631. [DOI] [PubMed] [Google Scholar]
- Alam, I. , Yang, Y.Q. , Wang, Y. , Zhu, M.L. , Wang, H.B. , Chalhoub, B. and Lu, Y.H. (2017) Genome‐wide identification, evolution and expression analysis of RING finger protein genes in Brassica rapa . Sci. Rep. 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam, I. , Cui, D.‐L. , Batool, K. , Yang, Y.‐Q. and Lu, Y.‐H. (2019) Comprehensive genomic survey, characterization and expression analysis of the HECT gene family in Brassica rapa L. and Brassica oleracea L. Genes (Basel) 10, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfatih, A. , Wu, J. , Jan, S.U. , Zhang, Z. , Xia, J. and Xiang, C. (2020) Loss of rice PARAQUAT TOLERANCE 3 confers enhanced resistance to abiotic stresses and increases grain yield in field. Plant Cell Environ. 43, 2743–2754. [DOI] [PubMed] [Google Scholar]
- Al‐Saharin, R. , Hellmann, H. and Mooney, S. (2022) Plant E3 ligases and their role in abiotic stress response. Cells 11, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, S. and Fu, L. (2018) Small‐molecule PROTACs: an emerging and promising approach for the development of targeted therapy drugs. EBioMedicine 36, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, J.P. , Liu, X. , Song, L.Q. , You, C.X. , Wang, X.F. and Hao, Y.J. (2017) Apple RING finger E3 ubiquitin ligase MdMIEL1 negatively regulates salt and oxidative stresses tolerance. Journal of. Plant Biol. 60, 137–145. [Google Scholar]
- An, J.P. , Wang, X.F. , Zhang, X.W. , You, C.X. and Hao, Y.J. (2021) Apple B‐box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ‐BBX37‐ICE1‐CBF pathway and undergoes MIEL1‐mediated ubiquitination and degradation. New Phytol. 229, 2707–2729. [DOI] [PubMed] [Google Scholar]
- Andersen, P. , Kragelund, B.B. , Olsen, A.N. , Larsen, F.H. , Chua, N.‐H. , Poulsen, F.M. and Skriver, K. (2004) Structure and biochemical function of a prototypical Arabidopsis U‐box domain. J. Biol. Chem. 279, 40053–40061. [DOI] [PubMed] [Google Scholar]
- Aravind, L. and Koonin, E.V. (2000) The U box is a modified RING finger—a common domain in ubiquitination. Curr. Biol. 10, R132–R134. [DOI] [PubMed] [Google Scholar]
- Ayaz, M. , Li, C.H. , Ali, Q. , Zhao, W. , Chi, Y.K. , Shafiq, M. et al. (2023) Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 28, 6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, Y. , Baek, W. , Lim, C.W. and Lee, S.C. (2024) A pepper RING‐finger E3 ligase, CaFIRF1, negatively regulates the high‐salt stress response by modulating the stability of CaFAF1. Plant Cell Environ. 47, 1319–1333. [DOI] [PubMed] [Google Scholar]
- Bae, Y. , Cho, J. , Choi, J. , Lim, C.W. and Lee, S.C. (2024) Pepper U‐Box E3 ubiquitin ligase 24, CaPUB24, negatively regulates drought stress response. Physiol. Plant. 176, e14240. [DOI] [PubMed] [Google Scholar]
- Baek, W. , Lim, C.W. and Lee, S.C. (2021) Pepper E3 ligase CaAIRE1 promotes ABA sensitivity and drought tolerance by degradation of protein phosphatase CaAITP1. J. Exp. Bot. 72, 4520–4534. [DOI] [PubMed] [Google Scholar]
- Ban, Z. and Estelle, M. (2021) CUL3 E3 ligases in plant development and environmental response. Nat Plants 7, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Békés, M. , Langley, D.R. and Crews, C.M. (2022) PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis, T.A. , La Clair, J.J. and Burkart, M.D. (2021) Unraveling the role of linker design in proteolysis targeting chimeras. J. Med. Chem. 64, 8042–8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal‐Lobo, M. , Stone, S. , Yang, X. , Antico, J. , Callis, J. , Ramonell, K.M. and Somerville, S. (2010) ATL9, a RING zinc finger protein with E3 ubiquitin ligase activity implicated in chitin‐and NADPH oxidase‐mediated defense responses. PLoS One 5, e14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson, M. and Zipfel, C. (2021) Plant immunity: crosstalk between plant immune receptors. Curr. Biol. 31, R796–R798. [DOI] [PubMed] [Google Scholar]
- Bos, J.I.B. , Armstrong, M.R. , Gilroy, E.M. , Boevink, P.C. , Hein, I. , Taylor, R.M. , Zhendong, T. et al. (2010) Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc. Natl. Acad. Sci. 107, 9909–9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillada, C. and Trujillo, M. (2022) E2 ubiquitin‐conjugating enzymes (UBCs): drivers of ubiquitin signalling in plants. Essays Biochem. 66, 99–110. [DOI] [PubMed] [Google Scholar]
- Brugière, N. , Zhang, W. , Xu, Q. , Scolaro, E.J. , Lu, C. , Kahsay, R.Y. , Kise, R. et al. (2017) Overexpression of RING domain E3 ligase ZmXerico1 confers drought tolerance through regulation of ABA homeostasis. Plant Physiol. 175, 1350–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueso, E. , Rodriguez, L. , Lorenzo‐Orts, L. , Gonzalez‐Guzman, M. , Sayas, E. , Muñoz‐Bertomeu, J. , Ibañez, C. et al. (2014) The single‐subunit RING‐type E3 ubiquitin ligase RSL 1 targets PYL 4 and PYR 1 ABA receptors in plasma membrane to modulate abscisic acid signaling. Plant J. 80, 1057–1071. [DOI] [PubMed] [Google Scholar]
- Byun, M.Y. , Cui, L.H. , Oh, T.K. , Jung, Y.‐J. , Lee, A. , Park, K.Y. , Kang, B.G. et al. (2017) Homologous U‐box E3 ubiquitin ligases OsPUB2 and OsPUB3 are involved in the positive regulation of low temperature stress response in rice (Oryza sativa L.). Front. Plant Sci. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapagain, S. , Park, Y.C. , Kim, J.H. and Jang, C.S. (2018) Oryza sativa salt‐induced RING E3 ligase 2 (OsSIRP2) acts as a positive regulator of transketolase in plant response to salinity and osmotic stress. Planta 247, 925–939. [DOI] [PubMed] [Google Scholar]
- Chaudhary, P. , Agri, U. , Chaudhary, A. , Kumar, A. and Kumar, G. (2022) Endophytes and their potential in biotic stress management and crop production. Front. Microbiol. 13, 933017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. and Hellmann, H. (2013) Plant E3 ligases: Flexible enzymes in a sessile world. Mol. Plant 6, 1388–1404. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Li, J. and He, Y. (2020) Overexpression of a novel E3 ubiquitin ligase gene from Coptis chinensis Franch enhances drought tolerance in transgenic tobacco. Z. Naturforsch. C 75, 417–424. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Wang, T. , Rehman, A.U. , Wang, Y. , Qi, J. , Li, Z. , Song, C. et al. (2021) Arabidopsis U‐box E3 ubiquitin ligase PUB11 negatively regulates drought tolerance by degrading the receptor‐like protein kinases LRR1 and KIN7. J. Integr. Plant Biol. 63, 494–509. [DOI] [PubMed] [Google Scholar]
- Chen, P. , Zhi, F. , Li, X. , Shen, W. , Yan, M. , He, J. , Bao, C. et al. (2022) Zinc‐finger protein MdBBX7/MdCOL9, a target of MdMIEL1 E3 ligase, confers drought tolerance in apple. Plant Physiol. 188, 540–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Xu, K. , Kong, D. , Wu, L. , Chen, Q. , Ma, X. , Ma, S. et al. (2022) Ubiquitin ligase OsRINGzf1 regulates drought resistance by controlling the turnover of OsPIP2; 1. Plant Biotechnol. J. 20, 1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, M.‐C. , Hsieh, E.‐J. , Chen, J.‐H. , Chen, H.‐Y. and Lin, T.‐P. (2012) Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 158, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S.K. , Ryu, M.Y. , Song, C. , Kwak, J.M. and Kim, W.T. (2008) Arabidopsis PUB22 and PUB23 are homologous U‐Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell 20, 1899–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, S.K. , Ryu, M.Y. , Seo, D.H. , Kang, B.G. and Kim, W.T. (2011) The arabidopsis RING E3 ubiquitin ligase AtAIRP2 plays combinatory roles with AtAIRP1 in abscisic acid‐mediated drought stress responses. Plant Physiol. 157, 2240–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, N.H. , Woo, O.G. , Kim, E.Y. , Park, K. , Seo, D.H. , Yu, S.G. , Choi, Y.A. et al. (2022) E3 ligase AtAIRP5/GARU regulates drought stress response by stimulating SERINE CARBOXYPEPTIDASE‐LIKE1 turnover. Plant Physiol. 190, 898–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M.S. and Jang, C.S. (2023) Overexpression of rice C3HC4‐Type RING finger protein gene, OsSIRHC‐2, improves salinity tolerance through low Na+ accumulation. J Plant Biol 66, 147–162. [Google Scholar]
- Choi, J. , Lee, W. , An, G. and Kim, S.‐R. (2021) OsCBE1, a substrate receptor of cullin4‐based E3 ubiquitin ligase, functions as a regulator of abiotic stress response and productivity in rice. Int. J. Mol. Sci. 22, 2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge, D.B. , Jensen, D.F. , Rabiey, M. , Sarrocco, S. , Shaw, M.W. and Shaw, R.H. (2022) Biological control of plant diseases – What has been achieved and what is the direction? Plant Pathol. 71, 1024–1047. [Google Scholar]
- Copeland, C. , Ao, K. , Huang, Y. , Tong, M. and Li, X. (2016) The evolutionarily conserved E3 ubiquitin ligase AtCHIP contributes to plant immunity. Front. Plant Sci. 7, 172905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbridge, E. , MacGregor, A. , Al‐Saharin, R. , Garneau, M.G. , Smalley, S. , Mooney, S. , Roje, S. et al. (2023) Brassica napus plants gain improved salt‐stress tolerance and increased storage oil biosynthesis by interfering with CRL3BPM activities. Plan. Theory 12, 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Walma, D.A. , Chen, Z. , Bullock, A.N. and Yamada, K.M. (2022) Ubiquitin ligases: guardians of mammalian development. Nat. Rev. Mol. Cell Biol. 23, 350–367. [DOI] [PubMed] [Google Scholar]
- Cui, H.R. , Zhang, Z.R. , Lv, W. , Xu, J.‐N. and Wang, X.‐Y. (2015) Genome‐wide characterization and analysis of F‐box protein‐encoding genes in the Malus domestica genome. Mol. Gen. Genomics. 290, 1435–1446. [DOI] [PubMed] [Google Scholar]
- Cui, L.H. , Min, H.J. , Byun, M.Y. , Oh, H.G. and Kim, W.T. (2018) OsDIRP1, a putative RING E3 ligase, plays an opposite role in drought and cold stress responses as a negative and positive factor, respectively, in rice (Oryza sativa L.). Front. Plant Sci. 9, 1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, L.H. , Min, H.J. , Yu, S.G. , Byun, M.Y. , Oh, T.R. , Lee, A. , Yang, H.W. et al. (2022) OsATL38 mediates mono‐ubiquitination of the 14‐3‐3 protein OsGF14d and negatively regulates the cold stress response in rice. J. Exp. Bot. 73, 307–323. [DOI] [PubMed] [Google Scholar]
- Dasgupta, S. and Robinson, E.J.Z. (2022) Attributing changes in food insecurity to a changing climate. Sci. Rep. 12, 4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvecchio, V.S. , Fierro, C. , Giovannini, S. , Melino, G. and Bernassola, F. (2021) Emerging roles of the HECT‐type E3 ubiquitin ligases in hematological malignancies. Discov. Oncol. 12, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, F. , Guo, T. , Lefebvre, M. , Scaglione, S. , Antico, C.J. , Jing, T. , Yang, X. et al. (2017) Expression and regulation of ATL9, an E3 ubiquitin ligase involved in plant defense. PLoS One 12, e0188458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S. , Zhang, B. and Qin, F. (2015) Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2. 6‐mediated phosphorylation. Plant Cell 27, 3228–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, C.H. , Agarwal, M. , Zhang, Y. , Xie, Q. and Zhu, J.K. (2006) The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 103, 8281–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, L. , He, K. , Peng, J. , Wang, X. and Mao, T. (2021) The E3 ligase MREL57 modulates microtubule stability and stomatal closure in response to ABA. Nat. Commun. 12, 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes, B.P. , Stupar, R.M. , Gingerich, D.J. and Vierstra, R.D. (2003) The HECT ubiquitin‐protein ligase (UPL) family in Arabidopsis: UPL3 has a specific role in trichome development. Plant J. 35, 729–742. [DOI] [PubMed] [Google Scholar]
- Du, B. , Nie, N. , Sun, S. , Hu, Y. , Bai, Y. , He, S. , Zhao, N. et al. (2021) A novel sweetpotato RING‐H2 type E3 ubiquitin ligase gene IbATL38 enhances salt tolerance in transgenic Arabidopsis. Plant Sci. 304, 110802. [DOI] [PubMed] [Google Scholar]
- Duchenne‐Moutien, R.A. and Neetoo, H. (2021) Climate change and emerging food safety issues: a review. J. Food Prot. 84, 1884–1897. [DOI] [PubMed] [Google Scholar]
- Fang, H. , Meng, Q. , Xu, J. , Tang, H. , Tang, S. , Zhang, H. and Huang, J. (2015) Knock‐down of stress inducible OsSRFP1 encoding an E3 ubiquitin ligase with transcriptional activation activity confers abiotic stress tolerance through enhancing antioxidant protection in rice. Plant Mol. Biol. 87, 441–458. [DOI] [PubMed] [Google Scholar]
- Francini, A. and Sebastiani, L. (2019) Abiotic stress effects on performance of horticultural crops. Horticulturae 5, 67. [Google Scholar]
- Freemont, P.S. (1993) The RING finger: a novel protein sequence motif related to the zinc finger. Ann. N. Y. Acad. Sci. 684, 174–192. [DOI] [PubMed] [Google Scholar]
- Fu, H. and Yang, Y. (2023) How plants tolerate salt stress. Curr. Issues Mol. Biol. 45, 5914–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss, J.J. , Grey, H. , Wang, Z. , Nomoto, M. , Jackson, L. , Tada, Y. and Spoel, S.H. (2018) Proteasome‐associated HECT‐type ubiquitin ligase activity is required for plant immunity. PLoS Pathog. 14, e1007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne, J.M. , Downes, B.P. , Shiu, S.H. , Durski, A.M. and Vierstra, R.D. (2002) The F‐box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl. Acad. Sci. USA 99, 11519–11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, T. , Wu, Y. , Zhang, Y. , Liu, L. , Ning, Y. , Wang, D. , Tong, H. et al. (2011) OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 76, 145–156. [DOI] [PubMed] [Google Scholar]
- Gao, H. , Sun, X. and Rao, Y. (2020) PROTAC technology: opportunities and challenges. ACS Med. Chem. Lett. 11, 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, D. , Jiang, J. , An, X. , Wang, L. , Pan, T. , Liu, K. , Sun, J. et al. (2021) Genomics, expression, and function analyses of XB3 family genes in cotton. Genomics 113, 245–256. [DOI] [PubMed] [Google Scholar]
- Gingerich, D.J. , Hanada, K. , Shiu, S.H. and Vierstra, R.D. (2007) Large‐scale, lineage‐specific expansion of a bric‐a‐brac/tramtrack/broad complex ubiquitin‐ligase gene family in rice. Plant Cell 19, 2329–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingerich, D.J. , Hellmann, H. , Christians, M.J. and Stone, S.L. (2021) Editorial: Structure, function, and evolution of E3 ligases and targets. Front. Plant Sci. 12, 767281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godana, E.A. , Yang, Q. , Zhang, X. , Zhao, L. , Wang, K. , Dhanasekaran, S. et al. (2023) Biotechnological and biocontrol approaches for mitigating postharvest diseases caused by fungal pathogens and their mycotoxins in fruits: A review. J. Agric. Food Chem. 71, 17584–17596. [DOI] [PubMed] [Google Scholar]
- González‐Lamothe, R. , Tsitsigiannis, D.I. , Ludwig, A.A. , Panicot, M. , Shirasu, K. and Jones, J.D.G. (2006) The U‐box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato. Plant Cell 18, 1067–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, J. , Otaki, Y. , Watanabe, T. and Watanabe, M. (2021) The role of HECT‐Type E3 ligase in the development of cardiac disease. Int. J. Mol. Sci. 22, 6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedeney, N. , Cornu, M. , Schwalen, F. , Kieffer, C. and Voisin‐Chiret, A.S. (2023) PROTAC technology: a new drug design for chemical biology with many challenges in drug discovery. Drug Discov. Today 28, 103395. [DOI] [PubMed] [Google Scholar]
- Guo, L. , Cao, M. , Li, Y. , Wang, J. , He, L. , Li, P. , Lin, X. et al. (2023) RING finger ubiquitin E3 ligase CsCHYR1 targets CsATAF1 for degradation to modulate the drought stress response of cucumber through the ABA‐dependent pathway. Plant Physiol. Biochem. 202, 107928. [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Garg, V. , Kant, C. and Bhatia, S. (2015) Genome‐wide survey and expression analysis of F‐box genes in chickpea. BMC Genomics 16, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, P.L. , Dong, Y.H. , Jiang, H. , Hu, D.G. and Hao, Y.J. (2019) Molecular cloning and functional characterization of apple U‐box E3 ubiquitin ligase gene MdPUB29 reveals its involvement in salt tolerance. J. Integr. Agric. 18, 1604–1612. [Google Scholar]
- Han, P.‐L. , Dong, Y.‐H. , Gu, K.‐D. , Yu, J.‐Q. , Hu, D.‐G. and Hao, Y.‐J. (2019) The apple U‐box E3 ubiquitin ligase MdPUB29 contributes to activate plant immune response to the fungal pathogen Botryosphaeria dothidea . Planta 249, 1177–1188. [DOI] [PubMed] [Google Scholar]
- Han, P.L. , Wang, C.K. , Liu, X.J. , Dong, Y.H. , Jiang, H. , Hu, D.G. and Hao, Y.J. (2019) BTB‐BACK domain E3 ligase MdPOB1 suppresses plant pathogen defense against botryosphaeria dothidea by ubiquitinating and degrading MdPUB29 protein in apple. Plant Cell Physiol. 60, 2129–2140. [DOI] [PubMed] [Google Scholar]
- He, Q. , McLellan, H. , Boevink, P.C. , Sadanandom, A. , Xie, C. , Birch, P.R.J. and Tian, Z. (2015) U‐box E3 ubiquitin ligase PUB17 acts in the nucleus to promote specific immune pathways triggered by Phytophthora infestans . J. Exp. Bot. 66, 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, F. , Wang, H.L. , Li, H.G. , Su, Y. , Li, S. , Yang, Y. , Feng, C.H. et al. (2018) PeCHYR1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA‐induced stomatal closure by ROS production in populus. Plant Biotechnol. J. 16, 1514–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey, L.T. , Hafeez, N.A. , Robinson, H. , Jackson, S.A. , Leal‐Bertioli, S.C.M. , Tester, M. et al. (2019) Breeding crops to feed 10 billion. Nat. Biotechnol. 37, 744–754. [DOI] [PubMed] [Google Scholar]
- Hijacking protein degradation (2020) Hijacking protein degradation. Nat. Chem. Biol. 16, 1151. [DOI] [PubMed] [Google Scholar]
- Hu, H. , Dong, C. , Sun, D. , Hu, Y. and Xie, J. (2018) Genome‐wide identification and analysis of U‐Box E3 ubiquitin‐protein ligase gene family in banana. Int. J. Mol. Sci. 19, 3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. , Luo, M. , Zhou, X. , Wang, Z. , Yan, L. , Hong, D. , Yang, G. et al. (2024) RING‐type E3 ligase BnaJUL1 ubiquitinates and degrades BnaTBCC1 to regulate drought tolerance in Brassica napus L. Plant Cell Environ. 47, 1023–1040. [DOI] [PubMed] [Google Scholar]
- Huang, Q. and Zhang, X. (2020) Emerging roles and research tools of atypical ubiquitination. Proteomics 20, 1900100. [DOI] [PubMed] [Google Scholar]
- Hwang, S.G. , Kim, J.J. , Lim, S.D. , Park, Y.C. , Moon, J.C. and Jang, C.S. (2016) Molecular dissection of Oryza sativa salt‐induced RING Finger Protein 1 (OsSIRP1): possible involvement in the sensitivity response to salinity stress. Physiol. Plant. 158, 168–179. [DOI] [PubMed] [Google Scholar]
- Hwang, S.G. , Chapagain, S. , Han, A.R. , Park, Y.C. , Park, H.M. , Kim, Y.H. and Jang, C.S. (2017) Molecular characterization of rice arsenic‐induced RING finger E3 ligase 2 (OsAIR2) and its heterogeneous overexpression in Arabidopsis thaliana . Physiol. Plant. 161, 372–384. [DOI] [PubMed] [Google Scholar]
- Ichimaru, K. , Yamaguchi, K. , Harada, K. , Nishio, Y. , Hori, M. , Ishikawa, K. , Inoue, H. et al. (2022) Cooperative regulation of PBI1 and MAPKs controls WRKY45 transcription factor in rice immunity. Nat. Commun. 13, 2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iconomou, M. and Saunders, D.N. (2016) Systematic approaches to identify E3 ligase substrates. Biochem. J. 473, 4083–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, M. , Nijhawan, A. , Arora, R. , Agarwal, P. , Ray, S. , Sharma, P. , Kapoor, S. et al. (2007) F‐Box proteins in rice. Genome‐wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 143, 1467–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, F. , Wu, B. , Li, H. , Huang, J. and Zheng, C. (2013) Genome‐wide identification and characterisation of F‐box family in maize. Mol. Gen. Genomics. 288, 559–577. [DOI] [PubMed] [Google Scholar]
- Jiao, L. , Zhang, Y. and Lu, J. (2017) Overexpression of a stress‐responsive U‐box protein gene VaPUB affects the accumulation of resistance related proteins in Vitis vinifera ‘Thompson Seedless.’. Plant Physiol. Biochem. 112, 53–63. [DOI] [PubMed] [Google Scholar]
- Johnson, C. , Boden, E. and Arias, J. (2003) Salicylic acid and NPR1 induce the recruitment of trans‐activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 15, 1846–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, H. , Lim, C.W. and Lee, S.C. (2016) Identification and functional expression of the pepper RING type E3 ligase, CaDTR1, involved in drought stress tolerance via ABA‐mediated signalling. Sci. Rep. 6, 30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, H. , Lim, C.W. , Han, S.‐W. and Lee, S.C. (2017) The pepper RING finger E3 ligase, CaDIR1, regulates the drought stress response via ABA‐mediated signaling. Front. Plant Sci. 8, 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, H. , Lim, C.W. and Lee, S.C. (2019) A pepper RING‐type E3 ligase, CaASRF1, plays a positive role in drought tolerance via modulation of CaAIBZ1 stability. Plant J. 98, 5–18. [DOI] [PubMed] [Google Scholar]
- Joo, H. , Lim, C.W. and Lee, S.C. (2020) The pepper RING‐type E3 ligase, CaATIR1, positively regulates abscisic acid signalling and drought response by modulating the stability of CaATBZ1. Plant Cell Environ. 43, 1911–1924. [DOI] [PubMed] [Google Scholar]
- Juranić, M. and Dresselhaus, T. (2014) Phylogenetic analysis of the expansion of the MATH‐BTB gene family in the grasses. Plant Signal. Behav. 9, e28242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranić, M. , Srilunchang, K.O. , Krohn, N.G. , Leljak‐Levanić, D. , Sprunck, S. and Dresselhaus, T. (2013) Germline‐specific MATH‐BTB substrate adaptor MAB1 regulates spindle length and nuclei identity in maize. Plant Cell 24, 4974–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, M. , Fokar, M. , Abdelmageed, H. and Allen, R.D. (2011) Arabidopsis SAP5 functions as a positive regulator of stress responses and exhibits E3 ubiquitin ligase activity. Plant Mol. Biol. 75, 451–466. [DOI] [PubMed] [Google Scholar]
- Kastl, J.M. , Davies, G. , Godsman, E. and Holdgate, G.A. (2021) Small‐molecule degraders beyond PROTACs—challenges and opportunities. SLAS Discovery 26, 524–533. [DOI] [PubMed] [Google Scholar]
- Khan, S. , Cao, L. , Wiegand, J. , Zhang, P. , Zajac‐Kaye, M. , Kaye, F.J. , Zheng, G. et al. (2024) PROTAC‐mediated dual degradation of BCL‐xL and BCL‐2 Is a highly effective therapeutic strategy in small‐cell lung cancer. Cells 13, 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. and Jang, C.S. (2021) E3 ligase, the Oryza sativa salt‐induced RING finger protein 4 (OsSIRP4), negatively regulates salt stress responses via degradation of the OsPEX11‐1 protein. Plant Mol. Biol. 105, 231–245. [DOI] [PubMed] [Google Scholar]
- Kim, S.J. and Kim, W.T. (2013) Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett. 587, 2584–2590. [DOI] [PubMed] [Google Scholar]
- Kim, J.Y. , Jang, I.‐C. and Seo, H.S. (2016) COP1 controls abiotic stress responses by modulating AtSIZ1 function through its E3 ubiquitin ligase activity. Front. Plant Sci. 7, 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Lim, S.D. and Jang, C.S. (2019) Oryza sativa heat‐induced RING finger protein 1 (OsHIRP1) positively regulates plant response to heat stress. Plant Mol. Biol. 99, 545–559. [DOI] [PubMed] [Google Scholar]
- Kim, J.H. , Lim, S.D. and Jang, C.S. (2020) Oryza sativa drought‐, heat‐, and salt‐induced RING finger protein 1 (OsDHSRP1) negatively regulates abiotic stress‐responsive gene expression. Plant Mol. Biol. 103, 235–252. [DOI] [PubMed] [Google Scholar]
- Kim, M.‐H. , Cho, J.‐S. , Park, E.‐J. , Lee, H. , Choi, Y.‐I. , Bae, E.‐K. , Han, K.H. et al. (2020) Overexpression of a poplar Ring‐H2 zinc finger, Ptxerico, confers enhanced drought tolerance via reduced water loss and ion leakage in Populus. Int. J. Mol. Sci. 21, 9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Kim, M.S. , Kim, D.Y. , Amoah, J.N. and Seo, Y.W. (2021) Molecular characterization of U‐box E3 ubiquitin ligases (TaPUB2 and TaPUB3) involved in the positive regulation of drought stress response in Arabidopsis. Int. J. Mol. Sci. 22, 13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Lim, S.D. and Jang, C.S. (2021) Oryza sativa, C4HC3‐type really interesting new gene (RING), OsRFPv6, is a positive regulator in response to salt stress by regulating Na+ absorption. Physiol. Plant. 173, 883–895. [DOI] [PubMed] [Google Scholar]
- Kim, M.S. , Kim, J.H. , Amoah, J.N. and Seo, Y.W. (2022) Wheat (Triticum aestivum L.) plant U‐box E3 ligases TaPUB2 and TaPUB3 enhance ABA response and salt stress resistance in Arabidopsis. FEBS Lett. 596, 3037–3050. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Park, S.I. , Kwon, H. , Cho, M.H. , Kim, B.G. , Chung, J.H. , Nam, M.H. et al. (2022) The rice abscisic acid‐responsive RING finger E3 ligase OsRF1 targets OsPP2C09 for degradation and confers drought and salinity tolerance in rice. Front. Plant Sci. 12, 797940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Kim, M.S. and Seo, Y.W. (2023) Overexpression of a plant U‐box gene TaPUB4 confers drought stress tolerance in Arabidopsis thaliana . Plant Physiol. Biochem. 196, 596–607. [DOI] [PubMed] [Google Scholar]
- Kim, M.‐S. , Ko, S.‐R. , Jung, Y.J. , Kang, K.‐K. , Lee, Y.‐J. and Cho, Y.‐G. (2023) Knockout mutants of OsPUB7 generated using CRISPR/Cas9 revealed abiotic stress tolerance in rice. Int. J. Mol. Sci. 24, 5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Lim, S.D. , Jung, K.H. and Jang, C.S. (2023a) Overexpression of a C3HC4‐type E3‐ubiquitin ligase contributes to salinity tolerance by modulating Na+ homeostasis in rice. Physiol. Plant. 175, e14075. [DOI] [PubMed] [Google Scholar]
- Kim, J.H. , Lim, S.D. , Jung, K.H. and Jang, C.S. (2023b) Overexpression of a C3HC4‐type RING E3 ligase gene, OsRFPHC‐13, improves salinity resistance in rice, Oryza sativa, by altering the expression of Na+/K+ transporter genes. Environ. Exp. Bot. 207, 105224. [Google Scholar]
- Koegl, M. , Hoppe, T. , Schlenker, S. , Ulrich, H.D. , Mayer, T.U. and Jentsch, S. (1999) A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 96, 635–644. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Diksha, Sindhu, S.S. and Kumar, R. (2022) Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 3, 100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda, H. , Takahashi, N. , Shimada, H. , Seki, M. , Shinozaki, K. and Matsui, M. (2002) Classification and expression analysis of Arabidopsis F‐box‐containing protein genes. Plant Cell Physiol. 43, 1073–1085. [DOI] [PubMed] [Google Scholar]
- Langin, G. , González‐Fuente, M. and Üstün, S. (2023) The plant ubiquitin–proteasome system as a target for microbial manipulation. Annu. Rev. Phytopathol. 61, 351–375. [DOI] [PubMed] [Google Scholar]
- Lee, H.G. and Seo, P.J. (2016) The Arabidopsis MIEL1 E3 ligase negatively regulates ABA signalling by promoting protein turnover of MYB96. Nat. Commun. 7, 12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon, R.G. and Bassham, D.C. (2024) PROTAC for agriculture: learning from human medicine to generate new biotechnological weed control solutions. Pest Manag. Sci. 80, 262–266. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Wu, B. , Yu, Y. , Yang, G. , Wu, C. and Zheng, C. (2011) Genome‐wide analysis of the RING finger gene family in apple. Mol. Gen. Genomics. 286, 81–94. [DOI] [PubMed] [Google Scholar]
- Li, J. , Han, Y. , Zhao, Q. , Li, C. , Xie, Q. , Chong, K. and Xu, Y. (2013) The E3 ligase AtRDUF1 positively regulates salt stress responses in Arabidopsis thaliana . PLoS One 8, e71078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Wang, N. , Wang, L. , Xin, H. and Li, S. (2014) Molecular cloning and characterization of the HOS1 gene from “Muscat Hamburg” grapevine. J. Am. Soc. Hortic. Sci. 139, 54–62. [Google Scholar]
- Li, Y. , Zhai, L. , Fan, J. , Ren, J. , Gong, W. , Wang, X. and Huang, J. (2019) Genome‐wide identification, phylogenetic and expression analysis of the maize HECT E3 ubiquitin ligase genes. Genetica 147, 391–400. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Serio, R.J. , Schofield, A. , Liu, H. , Rasmussen, S.R. , Hofius, D. and Stone, S.L. (2020) Arabidopsis RING‐type E3 ubiquitin ligase XBAT35.2 promotes proteasome‐dependent degradation of ACD11 to attenuate abiotic stress tolerance. Plant J. 104, 1712–1723. [DOI] [PubMed] [Google Scholar]
- Li, M. , Shen, S. , Xing, Y. , Jiao, W. , Zhan, Y. , Sun, Y. , Guo, D.L. et al. (2021) Vitis vinifera VvPUB17 functions as a E3 ubiquitin ligase and enhances powdery mildew resistance via the salicylic acid signaling pathway. J Berry Res 11, 419–430. [Google Scholar]
- Li, J. , Su, X. , Wang, Y. , Yang, W. , Pan, Y. , Su, C. and Zhang, X. (2018) Genome‐wide identification and expression analysis of the BTB domain‐containing protein gene family in tomato. Genes Genomics 40, 1–15. [DOI] [PubMed] [Google Scholar]
- Li, S. , Wang, Z. , Wang, F. , Lv, H. , Cao, M. , Zhang, N. , Li, F. et al. (2021) A tubby‐like protein CsTLP8 acts in the ABA signaling pathway and negatively regulates osmotic stresses tolerance during seed germination. BMC Plant Biol. 21, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B.W. , Gao, S. , Yang, Z.M. and Song, J.B. (2022) The F‐box E3 ubiquitin ligase AtSDR is involved in salt and drought stress responses in Arabidopsis. Gene 809, 146011. [DOI] [PubMed] [Google Scholar]
- Li, X. , Pu, W. , Zheng, Q. , Ai, M. , Chen, S. and Peng, Y. (2022) Proteolysis‐targeting chimeras (PROTACs) in cancer therapy. Mol. Cancer 21, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Wang, Z. , Sun, S. , Dai, Z. , Zhang, J. , Wang, W. , Peng, K. et al. (2024) IbNIEL‐mediated degradation of IbNAC087 regulates jasmonic acid‐dependent salt and drought tolerance in sweet potato. J. Integr. Plant Biol. 66, 176–195. [DOI] [PubMed] [Google Scholar]
- Lim, S.D. , Yim, W.C. , Moon, J.C. , Kim, D.S. , Lee, B.M. and Jang, C.S. (2010) A gene family encoding RING finger proteins in rice: their expansion, expression diversity, and co‐expressed genes. Plant Mol. Biol. 72, 369–380. [DOI] [PubMed] [Google Scholar]
- Lim, S.D. , Hwang, J.G. , Han, A.R. , Park, Y.C. , Lee, C. , Ok, Y.S. and Jang, C.S. (2014) Positive regulation of rice RING E3 ligase OsHIR1 in arsenic and cadmium uptakes. Plant Mol. Biol. 85, 365–379. [DOI] [PubMed] [Google Scholar]
- Lim, S.D. , Lee, C. and Jang, C.S. (2014) The rice RING E3 ligase, OsCTR1, inhibits trafficking to the chloroplasts of OsCP12 and OsRP1, and its overexpression confers drought tolerance in A rabidopsis. Plant Cell Environ. 37, 1097–1113. [DOI] [PubMed] [Google Scholar]
- Lim, S.D. , Jung, C.G. , Park, Y.C. , Lee, S.C. , Lee, C. , Lim, C.W. , Kim, D.S. et al. (2015) Molecular dissection of a rice microtubule‐associated RING finger protein and its potential role in salt tolerance in Arabidopsis. Plant Mol. Biol. 89, 365–384. [DOI] [PubMed] [Google Scholar]
- Lim, C.W. , Baek, W. and Lee, S.C. (2017a) The pepper RING‐type E3 ligase CaAIRF1 regulates ABA and drought signaling via CaADIP1 protein phosphatase degradation. Plant Physiol. 173, 2323–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, C.W. , Park, C. , Kim, J.‐H. , Joo, H. , Hong, E. and Lee, S.C. (2017b) Pepper CaREL1, a ubiquitin E3 ligase, regulates drought tolerance via the ABA‐signalling pathway. Sci. Rep. 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, C.W. , Baek, W. and Lee, S.C. (2018) Roles of pepper bZIP protein CaDILZ1 and its interacting partner RING‐type E3 ligase CaDSR1 in modulation of drought tolerance. Plant J. 96, 452–467. [DOI] [PubMed] [Google Scholar]
- Lim, S. , Khoo, R. , Peh, K.M. , Teo, J. , Chang, S.C. , Ng, S. , Beilhartz, G.L. et al. (2020) BioPROTACs as versatile modulators of intracellular therapeutic targets including proliferating cell nuclear antigen (PCNA). Proc. Natl. Acad. Sci. USA 117, 5791–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S.D. , Oh, D.G. , Park, Y.C. and Jang, C.S. (2020) Molecular characterization of a RING E3 ligase SbHCI1 in sorghum under heat and abscisic acid stress. Planta 252, 1–18. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Li, W. , Ning, Y. , Shirsekar, G. , Cai, Y. , Wang, X. , Dai, L. et al. (2012) The U‐box e3 ligase SPL11/PUB13 is a convergence point of defense and flowering signaling in plants. Plant Physiol. 160, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Xia, Z. , Wang, M. , Zhang, X. , Yang, T. and Wu, J. (2013) Overexpression of a maize E3 ubiquitin ligase gene enhances drought tolerance through regulating stomatal aperture and antioxidant system in transgenic tobacco. Plant Physiol. Biochem. 73, 114–120. [DOI] [PubMed] [Google Scholar]
- Liu, Z.B. , Wang, J.M. , Yang, F.X. , Yang, L. , Yue, Y.F. , Xiang, J.B. , Gao, M. et al. (2014) A novel membrane‐bound E3 ubiquitin ligase enhances the thermal resistance in plants. Plant Biotechnol. J. 12, 93–104. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Park, C.H. , He, F. , Nagano, M. , Wang, M. , Bellizzi, M. , Zhang, K. et al. (2015) The RhoGAP SPIN6 associates with SPL11 and OsRac1 and negatively regulates programmed cell death and innate immunity in rice. PLoS Pathog. 11, e1004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Zhang, C. , Wei, C. , Liu, X. , Wang, M. , Yu, F. , Xie, Q. et al. (2016) The RING finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2‐induced stomatal closure in rice. Plant Physiol. 170, 429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Ravichandran, S. , Teh, O.K. , McVey, S. , Lilley, C. , Teresinski, H.J. , Gonzalez‐Ferrer, C. et al. (2017) The RING‐Type E3 Ligase XBAT35.2 is involved in cell death induction and pathogen response. Plant Physiol. 175, 1469–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.C. , Zhang, H. , Lei, J.J. and Sun, J. (2017) Expression and the subcellular localization of atpub19, a U‐box E3 ligase in Arabidopsis. Pak. J. Bot. 49, 143–146. [Google Scholar]
- Liu, Y. , Pei, L. , Xiao, S. , Peng, L. , Liu, Z. , Li, X. , Yang, Y. et al. (2020) AtPPRT1 negatively regulates salt stress response in Arabidopsis seedlings. Plant Signal. Behav. 15, 1732103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Wang, K. , Cheng, Q. , Kong, D. , Zhang, X. , Wang, Z. , Wang, Q. et al. (2020) Cysteine protease RD21A regulated by E3 ligase SINAT4 is required for drought‐induced resistance to Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 71, 5562–5576. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Peng, L. , Gao, X. , Liu, Y. , Liu, Z. , Li, X. , Yang, Y. et al. (2020) AtPPRT3, a novel E3 ubiquitin ligase, plays a positive role in ABA signaling. Plant Cell Rep. 39, 1467–1478. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Meng, G. , Wang, M. , Qian, Z. , Zhang, Y. and Yang, W. (2021) Tomato SlPUB24 enhances resistance to Xanthomonas euvesicatoria pv. perforans race T3. Hortic Res 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Wang, L. , Li, Y. , Zhu, J. , Li, Z. , Chen, L. , Li, H. et al. (2024) Genome‐wide analysis of the U‐box E3 ligases gene family in potato (Solanum tuberosum L.) and overexpress StPUB25 enhance drought tolerance in transgenic Arabidopsis. BMC Genomics 25, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. , Lin, W. , Gao, X. , Wu, S. , Cheng, C. , Avila, J. , Heese, A. et al. (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science (1979) 332, 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Q. , Li, Y. , Wang, W. , Fei, X. and Deng, X. (2015) Genome‐wide survey and expression analysis of Chlamydomonas reinhardtii U‐box E3 ubiquitin ligases (CrPUBs) reveal a functional lipid metabolism module. PLoS One 10, e0142996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, C. , Cai, X.‐T. , Du, J. , Zhao, T.‐L. , Wang, P.‐F. , Zhao, P.‐X. , Liu, R. et al. (2016) PARAQUAT TOLERANCE3 is an E3 ligase that switches off activated oxidative response by targeting histone‐modifying PROTEIN METHYLTRANSFERASE4b. PLoS Genet. 12, e1006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, Q. , Li, X. , Jin, X. , Sun, Y. , Wu, Y. , Wang, W. and Huang, J. (2022) Rice OsPUB16 modulates the ‘SAPK9‐OsMADS23‐OsAOC’ pathway to reduce plant water‐deficit tolerance by repressing ABA and JA biosynthesis. PLoS Genet. 18, e1010520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, S. , Tang, N. , Li, X. , Xie, Y. , Xiang, D. , Fu, J. , Shen, J. et al. (2019) Reversible histone H2B monoubiquitination fine‐tunes abscisic acid signaling and drought response in rice. Mol. Plant 12, 263–277. [DOI] [PubMed] [Google Scholar]
- Ma, A. , Zhang, D. , Wang, G. , Wang, K. , Li, Z. , Gao, Y. , Li, H. et al. (2021) Verticillium dahliae effector VDAL protects MYB6 from degradation by interacting with PUB25 and PUB26 E3 ligases to enhance Verticillium wilt resistance. Plant Cell 33, 3675–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X. , Yu, C. , Li, L. , Wang, M. , Yang, L. , Zhang, Y. , Zhang, Y. et al. (2022) How many faces does the plant U‐Box E3 ligase have? Int. J. Mol. Sci. 23, 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, D. , Froidure, S. , Canonne, J. , Ben Khaled, S. , Khafif, M. , Pouzet, C. , Jauneau, A. et al. (2013) Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun. 4, 1476. [DOI] [PubMed] [Google Scholar]
- Martín‐Acosta, P. and Xiao, X. (2021) PROTACs to address the challenges facing small molecule inhibitors. Eur. J. Med. Chem. 210, 112993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucotelli, E. , Belloni, S. , Marone, D. , De Leonardis, A. , Guerra, D. , Di Fonzo, N. et al. (2006) The E3 ubiquitin ligase gene family in plants: regulation by degradation. Curr. Genomics 7, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbengue, M. , Camut, S. , de Carvalho‐Niebel, F. , Deslandes, L. , Solène, F. , Klaus‐Heisen, D. , Moreau, S. et al. (2010) The medicago truncatula E3 ubiquitin ligase PUB1 interacts with the LYK3 symbiotic receptor and negatively regulates infection and nodulation. Plant Cell 22, 3474–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan, H. , Chen, K. , He, Q. , Wu, X. , Boevink, P.C. , Tian, Z. and Birch, P.R.J. (2020) The ubiquitin E3 ligase PUB17 positively regulates immunity by targeting a negative regulator, KH17, for degradation. Plant Commun 1, 100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly, D. , Schofield, A. and Stone, S.L. (2018) Degradation of the stress‐responsive enzyme formate dehydrogenase by the RING‐type E3 ligase keep on going and the ubiquitin 26S proteasome system. Plant Mol. Biol. 96, 265–278. [DOI] [PubMed] [Google Scholar]
- Meng, X. , Wang, C. , Ur Rahman, S. , Wang, Y. , Wang, A. and Tao, S. (2015) Genome‐wide identification and evolution of HECT genes in soybean. Int. J. Mol. Sci. 16, 8517–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, H.J. , Jung, Y.J. , Kang, B.G. and Kim, W.T. (2016) CaPUB1, a hot pepper U‐box E3 ubiquitin ligase, confers enhanced cold stress tolerance and decreased drought stress tolerance in transgenic rice (Oryza sativa L.). Mol. Cells 39, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, J.H. , Park, C.R. , Chung, J.S. and Kim, C.S. (2021) Arabidopsis thaliana ubiquitin‐associated protein 1 (AtUAP1) Interacts with redundant RING Zinc Finger 1 (AtRZF1) to negatively regulate dehydration response. Plant Cell Physiol. 62, 1044–1057. [DOI] [PubMed] [Google Scholar]
- Mohapatra, S. , Banerjee, A. , Senapati, R. , Prasanthi, G. , Mohapatra, M. , Nayak, P. , Nayak, A.K. et al. (2021) Current status and future prospects in biotic stress management in rice. Oryza‐An Int J Rice 58, 168–193. [Google Scholar]
- Mudgil, Y. , Shiu, S.H. , Stone, S.L. , Salt, J.N. and Goring, D.R. (2004) A large complement of the predicted arabidopsis ARM repeat proteins are members of the U‐Box E3 ubiquitin ligase family. Plant Physiol. 134, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, L.A. , Solow, T.H. , Taylor, N. , Skwarecki, B. , Buels, R. , Binns, J. , Lin, C. et al. (2005) The SOL Genomics Network. A comparative resource for Solanaceae biology and beyond. Plant Physiol. 138, 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, X. , Tian, Z. , Liu, J. , Song, B. , Li, J. , Shi, X. and Xie, C. (2010) StPUB17, a novel potato UND/PUB/ARM repeat type gene, is associated with late blight resistance and NaCl stress. Plant Sci. 178, 158–169. [Google Scholar]
- Ning, Y. , Jantasuriyarat, C. , Zhao, Q. , Zhang, H. , Chen, S. , Liu, J. , Liu, L. et al. (2011) The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 157, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, T.R. , Yu, S.G. , Yang, H.W. , Kim, J.H. and Kim, W.T. (2020) AtKPNB1, an Arabidopsis importin‐β protein, is downstream of the RING E3 ubiquitin ligase AtAIRP1 in the ABA‐mediated drought stress response. Planta 252, 1–10. [DOI] [PubMed] [Google Scholar]
- Oshunsanya, S.O. , Nwosu, N.J. and Li, Y. (2019) Abiotic stress in agricultural crops under climatic conditions. In Sustainable Agriculture, Forest and Environmental Management( Jhariya, M.K. , Banerjee, A. , Meena, R.S. and Yadav, D.K. , eds), pp. 71-100. Singapore: Springer. [Google Scholar]
- Park, G.‐G. , Park, J.‐J. , Yoon, J. , Yu, S.‐N. and An, G. (2010) A RING finger E3 ligase gene, Oryza sativa Delayed Seed Germination 1 (OsDSG1), controls seed germination and stress responses in rice. Plant Mol. Biol. 74, 467–478. [DOI] [PubMed] [Google Scholar]
- Park, J. , Yi, J. , Yoon, J. , Cho, L. , Ping, J. , Jeong, H.J. et al. (2011) OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 65, 194–205. [DOI] [PubMed] [Google Scholar]
- Park, C. , Lim, C.W. and Lee, S.C. (2016) The pepper RING‐Type E3 ligase, CaAIP1, functions as a positive regulator of drought and high salinity stress responses. Plant Cell Physiol. 57, 2202–2212. [DOI] [PubMed] [Google Scholar]
- Park, Y.C. , Chapagain, S. and Jang, C.S. (2018) A negative regulator in response to salinity in rice: Oryza sativa salt‐, ABA‐and drought‐induced RING finger protein 1 (OsSADR1). Plant Cell Physiol. 59, 575–589. [DOI] [PubMed] [Google Scholar]
- Park, Y.C. , Moon, J.C. , Chapagain, S. , Oh, D.G. , Kim, J.J. and Jang, C.S. (2018) Role of salt‐induced RING finger protein 3 (OsSIRP3), a negative regulator of salinity stress response by modulating the level of its target proteins. Environ. Exp. Bot. 155, 21–30. [Google Scholar]
- Park, Y.C. , Choi, S.Y. , Kim, J.H. and Jang, C.S. (2019) Molecular functions of rice cytosol‐localized RING finger protein 1 in response to salt and drought and comparative analysis of its grass orthologs. Plant Cell Physiol. 60, 2394–2409. [DOI] [PubMed] [Google Scholar]
- Pei, L. , Peng, L. , Wan, X. , Xiong, J. , Liu, Z. , Li, X. , Yang, Y. et al. (2019) Expression pattern and function analysis of AtPPRT1, a novel negative regulator in ABA and drought stress responses in Arabidopsis. Int. J. Mol. Sci. 20, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L. , Wan, X. , Huang, K. , Pei, L. , Xiong, J. , Li, X. and Wang, J. (2019) AtPUB48 E3 ligase plays a crucial role in the thermotolerance of Arabidopsis. Biochem. Biophys. Res. Commun. 509, 281–286. [DOI] [PubMed] [Google Scholar]
- Picart‐Palmade, L. , Cunault, C. , Chevalier‐Lucia, D. , Belleville, M.P. and Marchesseau, S. (2019) Potentialities and limits of some non‐thermal technologies to improve sustainability of food processing. Front. Nutr. 5, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, S. , Lin, Q. , Zhu, H. , Gao, F. , Zhang, W. and Hua, X. (2016) The RING finger E3 ligase SpRing is a positive regulator of salt stress signaling in salt‐tolerant wild tomato species. Plant Cell Physiol. 57, 528–539. [DOI] [PubMed] [Google Scholar]
- Qi, X. , Tang, X. , Liu, W. , Fu, X. , Luo, H. , Ghimire, S. , Zhang, N. et al. (2020) A potato RING‐finger protein gene StRFP2 is involved in drought tolerance. Plant Physiol. Biochem. 146, 438–446. [DOI] [PubMed] [Google Scholar]
- Qian, H. , Zhang, Y. , Wu, B. , Wu, S. , You, S. , Zhang, N. and Sun, Y. (2020) Structure and function of HECT E3 ubiquitin ligases and their role in oxidative stress. J Transl Int Med 8, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Q. , Wang, Y. , Huang, L. , Du, F. , Zhao, X. , Li, Z. , Wang, W. et al. (2020) A U‐box E3 ubiquitin ligase OsPUB67 is positively involved in drought tolerance in rice. Plant Mol. Biol. 102, 89–107. [DOI] [PubMed] [Google Scholar]
- Raina, K. , Lu, J. , Qian, Y. , Altieri, M. , Gordon, D. , Rossi, A.M.K. , Wang, J. et al. (2016) PROTAC‐induced BET protein degradation as a therapy for castration‐resistant prostate cancer. Proc. Natl. Acad. Sci. USA 113, 7124–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu, V.S. , Oh, S. , Lee, H.K. , Nandety, R.S. , Oh, Y. , Lee, S. , Nakashima, J. et al. (2021) A novel role of salt‐ A nd drought‐induced ring 1 protein in modulating plant defense against hemibiotrophic and necrotrophic pathogens. Mol. Plant‐Microbe Interact. 34, 297–308. [DOI] [PubMed] [Google Scholar]
- Ryu, M.Y. , Cho, S.K. , Hong, Y. , Kim, J. , Kim, J.H. , Kim, G.M. , Chen, Y.J. et al. (2019) Classification of barley U‐box E3 ligases and their expression patterns in response to drought and pathogen stresses. BMC Genomics 20, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhukhan, A. , Panda, S.K. and Sahoo, L. (2014) The cowpea RING ubiquitin ligase VuDRIP interacts with transcription factor VuDREB2A for regulating abiotic stress responses. Plant Physiol. Biochem. 83, 51–56. [DOI] [PubMed] [Google Scholar]
- Saini, L.K. , Sharma, M. , Ravi, B. , Ghosh, S. , Pahuja, S. , Singh, N. and Pandey, G.K. (2023) Overexpression of ARM repeat/U‐box containing E3 ligase, PUB2 positively regulates growth and oxidative stress response in Arabidopsis. Biochem. J. 480, 555–571. [DOI] [PubMed] [Google Scholar]
- Satterthwaite, D. , McGranahan, G. and Tacoli, C. (2010) Urbanization and its implications for food and farming. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 365, 2809–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selote, D. , Matthiadis, A. , Gillikin, J.W. , Sato, M.H. and Long, T.A. (2018) The E3 ligase BRUTUS facilitates degradation of VOZ1/2 transcription factors. Plant Cell Environ. 41, 2463–2474. [DOI] [PubMed] [Google Scholar]
- Seo, D.H. , Ryu, M.Y. , Jammes, F. , Hwang, J.H. , Turek, M. , Kang, B.G. , Kwak, J.M. et al. (2012) Roles of four Arabidopsis U‐box E3 ubiquitin ligases in negative regulation of abscisic acid‐mediated drought stress responses. Plant Physiol. 160, 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, Y.S. , Choi, J.Y. , Kim, S.J. , Kim, E.Y. , Shin, J.S. and Kim, W.T. (2012) Constitutive expression of CaRma1H1, a hot pepper ER‐localized RING E3 ubiquitin ligase, increases tolerance to drought and salt stresses in transgenic tomato plants. Plant Cell Rep. 31, 1659–1665. [DOI] [PubMed] [Google Scholar]
- Seo, D.H. , Ahn, M.Y. , Park, K.Y. , Kim, E.Y. and Kim, W.T. (2016) The N‐terminal und motif of the arabidopsis U‐box E3 ligase pub18 is critical for the negative regulation of aba‐mediated stomatal movement and determines its ubiquitination specificity for exocyst subunit Exo70B1. Plant Cell 28, 2952–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, D.H. , Lee, A. , Yu, S.G. , Cui, L.H. , Min, H.J. , Lee, S.E. , Cho, N.H. et al. (2021) OsPUB41, a U‐box E3 ubiquitin ligase, acts as a negative regulator of drought stress response in rice (Oryza sativa L.). Plant Mol. Biol. 106, 463–477. [DOI] [PubMed] [Google Scholar]
- Serrano, I. , Gu, Y. , Qi, D. , Dubiella, U. and Innes, R.W. (2014) The arabidopsis EDR1 protein kinase negatively regulates the ATL1 e3 ubiquitin ligase to suppress cell death. Plant Cell 26, 4532–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S.S. and Kumar, S. (2021) Adaptors as the regulators of HECT ubiquitin ligases. Cell Death Differ. 28, 455–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, B. and Taganna, J. (2020) Genome‐wide analysis of the U‐box E3 ubiquitin ligase enzyme gene family in tomato. Sci. Rep. 10, 9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, G. , Giri, J. and Tyagi, A.K. (2015) Rice OsiSAP7 negatively regulates ABA stress signalling and imparts sensitivity to water‐deficit stress in Arabidopsis. Plant Sci. 237, 80–92. [DOI] [PubMed] [Google Scholar]
- Sharma, B. , Saxena, H. and Negi, H. (2021) Genome‐wide analysis of HECT E3 ubiquitin ligase gene family in Solanum lycopersicum . Sci. Rep. 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, I. , Kashyap, S. and Agarwala, N. (2023) Biotic stress‐induced changes in root exudation confer plant stress tolerance by altering rhizospheric microbial community. Front. Plant Sci. 14, 1132824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkryl, Y. , Yugay, Y. , Avramenko, T. , Grigorchuk, V. , Gorpenchenko, T. , Grischenko, O. and Bulgakov, V. (2021) CRISPR/Cas9‐mediated knockout of HOS1 reveals its role in the regulation of secondary metabolism in Arabidopsis thaliana . Plan. Theory 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. , Kumar, V. , Dhanjal, D.S. , Dhaka, V. , Sonali, B. and Singh, J. (2021a) Mycotoxin metabolites of fungi. In New and Future Developments in Microbial Biotechnology and Bioengineering, pp. 253–265. Amsterdam: Elsevier. [Google Scholar]
- Singh, S. , Ng, J. and Sivaraman, J. (2021b) Exploring the “Other” subfamily of HECT E3‐ligases for therapeutic intervention. Pharmacol. Ther. 224, 107809. [DOI] [PubMed] [Google Scholar]
- Singh, B.K. , Delgado‐Baquerizo, M. , Egidi, E. , Guirado, E. , Leach, J.E. , Liu, H. and Trivedi, P. (2023) Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 21, 640–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skieterska, K. , Rondou, P. and Van Craenenbroeck, K. (2017) Regulation of G protein‐coupled receptors by ubiquitination. Int. J. Mol. Sci. 18, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluimer, J. and Distel, B. (2018) Regulating the human HECT E3 ligases. Cell. Mol. Life Sci. 75, 3121–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley, S. and Hellmann, H. (2022) Exploring possible approaches using ubiquitylation and sumoylation pathways in modifying plant stress tolerance. Plant Sci. 111275, 111275. [DOI] [PubMed] [Google Scholar]
- Song, J.B. , Wang, Y.X. , Li, H.B. , Li, B.W. , Zhou, Z.S. , Gao, S. and Yang, Z.M. (2015) The F‐box family genes as key elements in response to salt, heavy mental, and drought stresses in Medicago truncatula . Funct. Integr. Genomics 15, 495–507. [DOI] [PubMed] [Google Scholar]
- Song, Q. , Wang, X. , Wu, F. , Zhao, J. , Liu, Y. and Yang, X. (2022) StATL2‐like could affect growth and cold tolerance of plant by interacting with StCBFs. Plant Cell Rep. 41, 1827–1841. [DOI] [PubMed] [Google Scholar]
- Spoel, S.H. , Koornneef, A. , Claessens, S.M.C. , Korzelius, J.P. , Van Pelt, J.A. , Mueller, M.J. , Buchala, A.J. et al. (2003) NPR1 modulates cross‐talk between salicylate‐ and jasmonate‐dependent defense pathways through a novel function in the cytosol. Plant Cell 15, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann, M. , Anderson, R.G. , Ichimura, K. , Pecenkova, T. , Reuter, P. , Žárský, V. , McDowell, J.M. et al. (2012) The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP‐triggered responses in Arabidopsis. Plant Cell 24, 4703–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, S.L. (2019) Role of the ubiquitin proteasome system in plant response to abiotic stress. Int. Rev. Cell Mol. Biol. 343, 65–110. [DOI] [PubMed] [Google Scholar]
- Stone, S.L. , Hauksdóttir, H. , Troy, A. , Herschleb, J. , Kraft, E. and Callis, J. (2005) Functional analysis of the RING‐type ubiquitin ligase family of Arabidopsis. Plant Physiol. 137, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, J.Y. and Kim, W.T. (2015) Arabidopsis RING E3 ubiquitin ligase AtATL80 is negatively involved in phosphate mobilization and cold stress response in sufficient phosphate growth conditions. Biochem. Biophys. Res. Commun. 463, 793–799. [DOI] [PubMed] [Google Scholar]
- Suh, J.Y. , Kim, S.J. , Oh, T.R. , Cho, S.K. , Yang, S.W. and Kim, W.T. (2016) Arabidopsis Tóxicos en Levadura 78 (AtATL78) mediates ABA‐dependent ROS signaling in response to drought stress. Biochem. Biophys. Res. Commun. 469, 8–14. [DOI] [PubMed] [Google Scholar]
- Sun, Z. , Lu, H. , Xiao, W. , Li, Y. and Xu, P. (2020) Progress in K27 ubiquitin modification. Sheng Wu Gong Cheng Xue Bao 36, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Sun, H. , Li, J. , Li, X. , Lv, Q. , Chen, L. , Wang, B. and Li, L. (2022) RING E3 ubiquitin ligase TaSADR1 negatively regulates drought resistance in transgenic Arabidopsis. Plant Physiol. Biochem. 170, 255–265. [DOI] [PubMed] [Google Scholar]
- Sun, M.‐M. , Tian, Y. , Chun, M. and Chen, Y.‐F. (2022) The ubiquitin E3 ligase PRU2 modulates phosphate uptake in Arabidopsis. Int. J. Mol. Sci. 23, 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, X. , Calderon‐Villalobos, L.I.A. , Sharon, M. , Zheng, C. , Robinson, C.V. , Estelle, M. and Zheng, N. (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645. [DOI] [PubMed] [Google Scholar]
- Tang, X. , Ghimire, S. , Liu, W. , Fu, X. , Zhang, H. , Zhang, N. and Si, H. (2020) Potato E3 ubiquitin ligase PUB27 negatively regulates drought tolerance by mediating stomatal movement. Plant Physiol. Biochem. 154, 557–563. [DOI] [PubMed] [Google Scholar]
- Tian, M. , Lou, L. , Liu, L. , Yu, F. , Zhao, Q. , Zhang, H. , Wu, Y. et al. (2015) The RING finger E3 ligase STRF1 is involved in membrane trafficking and modulates salt‐stress response in Arabidopsis thaliana . Plant J. 82, 81–92. [DOI] [PubMed] [Google Scholar]
- Tong, M. , Kotur, T. , Liang, W. , Vogelmann, K. , Kleine, T. , Leister, D. , Brieske, C. et al. (2017) E3 ligase SAUL1 serves as a positive regulator of PAMP‐triggered immunity and its homeostasis is monitored by immune receptor SOC3. New Phytol. 215, 1516–1532. [DOI] [PubMed] [Google Scholar]
- Tong, S. , Chen, N. , Wang, D. , Ai, F. , Liu, B. , Ren, L. , Chen, Y. et al. (2021) The U‐box E3 ubiquitin ligase PalPUB79 positively regulates ABA‐dependent drought tolerance via ubiquitination of PalWRKY77 in populus. Plant Biotechnol. J. 19, 2561–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenner, J. , Monaghan, J. , Saeed, B. , Quint, M. , Shabek, N. and Trujillo, M. (2022) Evolution and functions of plant U‐Box proteins: from protein quality control to signaling. Annu. Rev. Plant Biol. 73, 93–121. [DOI] [PubMed] [Google Scholar]
- Trujillo, M. , Ichimura, K. , Casais, C. and Shirasu, K. (2008) Negative regulation of PAMP‐triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr. Biol. 18, 1396–1401. [DOI] [PubMed] [Google Scholar]
- Vander Kooi, C.W. , Ohi, M.D. , Rosenberg, J.A. , Oldham, M.L. , Newcomer, M.E. , Gould, K.L. and Chazin, W.J. (2006) The Prp19 U‐box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry 45, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra, R.D. (2009) The ubiquitin‐26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 10, 385–397. [DOI] [PubMed] [Google Scholar]
- Vollset, S.E. , Goren, E. , Yuan, C.W. , Cao, J. , Smith, A.E. , Hsiao, T. , Bisignano, C. et al. (2020) Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the global burden of disease study. Lancet 396, 1285–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Duan, W. , Riquicho, A.R.M. , Jing, Z. , Liu, T. , Hou, X. and Li, Y. (2015) Genome‐wide survey and expression analysis of the PUB family in Chinese cabbage (Brassica rapa ssp. pekinesis). Mol. Gen. Genomics. 290, 2241–2260. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Qu, B. , Dou, S. , Li, L. , Yin, D. , Pang, Z. , Zhou, Z. et al. (2015) The E3 ligase OsPUB15 interacts with the receptor‐like kinase PID2 and regulates plant cell death and innate immunity. BMC Plant Biol. 15, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G.M. , Yin, H. , Qiao, X. , Tan, X. , Gu, C. , Wang, B.H. , Cheng, R. et al. (2016) F‐box genes: genome‐wide expansion, evolution and their contribution to pollen growth in pear (Pyrus bretschneideri). Plant Sci. 253, 164–175. [DOI] [PubMed] [Google Scholar]
- Wang, N. , Liu, Y. , Cong, Y. , Wang, T. , Zhong, X. , Yang, S. , Li, Y. et al. (2016) Genome‐wide identification of soybean U‐box E3 ubiquitin ligases and roles of GmPUB8 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 57, 1189–1209. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Li, C. , Kong, X. , Li, Y. , Liu, Z. , Wang, J. , Li, X. et al. (2018) AtARRE, an E3 ubiquitin ligase, negatively regulates ABA signaling in Arabidopsis thaliana . Plant Cell Rep. 37, 1269–1278. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Grubb, L.E. , Wang, J. , Liang, X. , Li, L. , Gao, C. , Ma, M. et al. (2018) A regulatory module controlling homeostasis of a plant immune kinase. Mol. Cell 69, 493–504.e6. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Liu, S. , Liu, H. , Chen, K. and Zhang, P. (2019) PnSAG1, an E3 ubiquitin ligase of the Antarctic moss Pohlia nutans, enhanced sensitivity to salt stress and ABA. Plant Physiol. Biochem. 141, 343–352. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Ding, Y. , Li, Z. , Shi, Y. , Wang, J. , Hua, J. , Gong, Z. et al. (2019) PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negative regulator MYB15. Dev. Cell 51, 222–235. [DOI] [PubMed] [Google Scholar]
- Wang, K. , Yang, Q. , Lanhuang, B. , Lin, H. , Shi, Y. , Dhanasekaran, S. , Godana, E.A. et al. (2020) Genome‐wide investigation and analysis of U‐box Ubiquitin–Protein ligase gene family in apple: expression profiles during Penicillium expansum infection process. Physiol. Mol. Plant Pathol. 111, 101487. [Google Scholar]
- Wang, N. , Liu, Y. , Cai, Y. , Tang, J. , Li, Y. and Gai, J. (2020) The soybean U‐box gene GmPUB6 regulates drought tolerance in Arabidopsis thaliana . Plant Physiol. Biochem. 155, 284–296. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Wang, W. , Wu, Y. , Li, Q. , Zhang, G. , Shi, R. , Yang, J. et al. (2020) The involvement of wheat U‐box E3 ubiquitin ligase TaPUB1 in salt stress tolerance. J. Integr. Plant Biol. 62, 631–651. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Song, B. , Dai, Y. , Zhang, S. and Huang, X. (2021) Genome‐wide identification and functional analysis of U‐box E3 ubiquitin ligases gene family related to drought stress response in Chinese white pear (Pyrus bretschneideri). BMC Plant Biol. 21, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Zeng, M. , Wang, Z. , Qin, F. , Wang, Y. , Chen, J. , Christian, M. et al. (2021) Food phenolics stimulate adipocyte browning via regulating gut microecology. Crit. Rev. Food Sci. Nutr. 63, 4026–4052. [DOI] [PubMed] [Google Scholar]
- Wang, D.R. , Zhang, X.W. , Xu, R.R. , Wang, G.L. , You, C.X. and An, J.P. (2022) Apple U‐box‐type E3 ubiquitin ligase MdPUB23 reduces cold‐stress tolerance by degrading the cold‐stress regulatory protein MdICE1. Hortic Res 9, uhac171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Lv, X. , Zhang, J. , Chen, D. , Chen, S. , Fan, G. , Ma, C. et al. (2022) Roles of E3 ubiquitin ligases in plant responses to abiotic stresses. Int. J. Mol. Sci. 23, 2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Wu, Y. , Zhong, H. , Chen, S. , Wong, K.B. and Xia, Y. (2022) Arabidopsis PUB2 and PUB4 connect signaling components of pattern‐triggered immunity. New Phytol. 233, 2249–2265. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Zhang, X. , Tang, Y. , Zhao, T. , Huang, C. , Li, Y. and Zhang, C. (2023) Exocyst subunit VviExo70B is degraded by ubiquitin ligase VviPUB19 and they regulate drought and salt tolerance in grapevine. Environ. Exp. Bot. 206, 105175. [Google Scholar]
- Wang, X. , Zhang, X. , Song, C.P. , Gong, Z. , Yang, S. and Ding, Y. (2023) PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. Plant Cell 35, 3585–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Bian, L. , Shi, Q. , Li, X. , Sun, Y. , Li, M. , Zhao, A. et al. (2024) The Vitis yeshanensis U‐box E3 ubiquitin ligase VyPUB21 enhances resistance to powdery mildew by targeting degradation of NIM1‐interacting (NIMIN) protein. Plant Cell Rep. 43, 93. [DOI] [PubMed] [Google Scholar]
- Wang, T. , Wang, G. , Zhang, J. and Xuan, J. (2024) E3 ubiquitin ligase PUB23 in kiwifruit interacts with trihelix transcription factor GT1 and negatively regulates immune responses against Pseudomonas syringae pv. actinidiae. Int. J. Mol. Sci. 25, 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, J. , Polo, S. and Maspero, E. (2019) HECT E3 ligases: a tale with multiple facets. Front. Physiol. 10, 443919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg, J. , O'Shea, C. and Skriver, K. (2008) Biochemical function of typical and variant Arabidopsis thaliana U‐box E3 ubiquitin‐protein ligases. Biochem. J. 413, 447–457. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Wang, W. , Li, Q. , Zhang, G. , Zhao, X. , Li, G. , Li, Y. et al. (2020) The wheat E3 ligase TaPUB26 is a negative regulator in response to salt stress in transgenic Brachypodium distachyon . Plant Sci. 294, 110441. [DOI] [PubMed] [Google Scholar]
- Xia, Z. , Liu, Q. , Wu, J. and Ding, J. (2012) ZmRFP1, the putative ortholog of SDIR1, encodes a RING‐H2 E3 ubiquitin ligase and responds to drought stress in an ABA‐dependent manner in maize. Gene 495, 146–153. [DOI] [PubMed] [Google Scholar]
- Xia, Z. , Su, X. , Liu, J. and Wang, M. (2013) The RING‐H2 finger gene 1 (RHF1) encodes an E3 ubiquitin ligase and participates in drought stress response in Nicotiana tabacum . Genetica 141, 11–21. [DOI] [PubMed] [Google Scholar]
- Xiao, L. , Shi, Y. , Wang, R. , Feng, Y. , Wang, L. , Zhang, H. , Shi, X. et al. (2022) The transcription factor OsMYBc and an E3 ligase regulate expression of a K+ transporter during salt stress. Plant Physiol. 190, 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G. , Ma, H. , Nei, M. and Kong, H. (2009) Evolution of F‐box genes in plants: Different modes of sequence divergence and their relationships with functional diversification. Proc. Natl. Acad. Sci. USA 106, 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Xing, S. , Cui, H. , Chen, X. and Wang, X. (2016) Genome‐wide identification and characterization of the apple (Malus domestica) HECT ubiquitin‐protein ligase family and expression analysis of their responsiveness to abiotic stresses. Mol. Gen. Genomics. 291, 635–646. [DOI] [PubMed] [Google Scholar]
- Yan, J. , Wang, J. , Li, Q. , Hwang, J.R. , Patterson, C. and Zhang, H. (2003) AtCHIP, a U‐Box‐containing E3 ubiquitin ligase, plays a critical role in temperature stress tolerance in Arabidopsis. Plant Physiol. 132, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Liu, Q. , Liu, Z. , Yang, H. , Wang, J. , Li, X. and Yang, Y. (2016) Arabidopsis C3HC4‐RING finger E3 ubiquitin ligase AtAIRP4 positively regulates stress‐responsive abscisic acid signaling. J. Integr. Plant Biol. 58, 67–80. [DOI] [PubMed] [Google Scholar]
- Yang, L. , Wu, L. , Chang, W. , Li, Z. , Miao, M. , Li, Y. , Yang, J. et al. (2018) Overexpression of the maize E3 ubiquitin ligase gene ZmAIRP4 enhances drought stress tolerance in Arabidopsis. Plant Physiol. Biochem. 123, 34–42. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Karthikeyan, A. , Yin, J. , Jin, T. , Ren, R. , Fang, F. , Cai, H. et al. (2022) The E3 ligase GmPUB21 negatively regulates drought and salinity stress response in soybean. Int. J. Mol. Sci. 23, 6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, W. , Wang, L. , Wang, J. , Ma, F. , Yang, Y. , Wang, C. , Tong, W. et al. (2017) VpPUB24, a novel gene from Chinese grapevine, Vitis pseudoreticulata, targets VpICE1 to enhance cold tolerance. J. Exp. Bot. 68, 2933–2949. [DOI] [PubMed] [Google Scholar]
- Yi, S.Y. , Lee, M. , Kwon, S.Y. , Kim, W.T. , Lim, Y.P. and Kang, S.Y. (2022) RING‐Type E3 ubiquitin ligases AtRDUF1 and AtRDUF2 positively regulate the expression of PR1 gene and pattern‐triggered immunity. Int. J. Mol. Sci. 23, 14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, S.Y. , Nekrasov, V. , Ichimura, K. , Kang, S.Y. and Shirasu, K. (2024) Plant U‐box E3 ligases PUB20 and PUB21 negatively regulate pattern‐triggered immunity in Arabidopsis. Plant Mol. Biol. 114, 7. [DOI] [PubMed] [Google Scholar]
- Ying, M. (2018) Role of HECT ubiquitin protein ligases in Arabidopsis thaliana . J. Plant Sci. Phytopathol., 2, 20–30. [Google Scholar]
- Yu, S.G. , Kim, J.H. , Cho, N.H. , Oh, T.R. and Kim, W.T. (2020) Arabidopsis RING E3 ubiquitin ligase JUL1 participates in ABA‐mediated microtubule depolymerization, stomatal closure, and tolerance response to drought stress. Plant J. 103, 824–842. [DOI] [PubMed] [Google Scholar]
- Yu, J. , Kang, L. , Li, Y. , Wu, C. , Zheng, C. , Liu, P. and Huang, J. (2021) RING finger protein RGLG1 and RGLG2 negatively modulate MAPKKK18 mediated drought stress tolerance in Arabidopsis. J. Integr. Plant Biol. 63, 484–493. [DOI] [PubMed] [Google Scholar]
- Yu, G. , Derkacheva, M. , Rufian, J.S. , Brillada, C. , Kowarschik, K. , Jiang, S. , Derbyshire, P. et al. (2022) The Arabidopsis E3 ubiquitin ligase PUB4 regulates BIK1 and is targeted by a bacterial type‐III effector. EMBO J. 41, e107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, P. , Wei, Y. , Gao, X. , Song, C. , Jiao, J. , Wang, M. , Zhang, K. et al. (2024) The RING‐H2 type E3 ubiquitin ligase gene MdATL16 positively regulates salt tolerance in transgenic tomato and apple. Environ. Exp. Bot. 220, 105689. [Google Scholar]
- Zeng, D.E. , Hou, P. , Xiao, F. and Liu, Y. (2014) Overexpressing a novel RING‐H2 finger protein gene, OsRHP1, enhances drought and salt tolerance in rice (Oryza sativa L.). J. Plant Biology 57, 357–365. [Google Scholar]
- Zhang, Y.Y. , Li, Y. , Gao, T. , Zhu, H. , Wang, D.J. , Zhang, H.W. , Ning, Y.S. et al. (2008) Arabidopsis SDIR1 enhances drought tolerance in crop plants. Biosci. Biotechnol. Biochem. 72, 2251–2254. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Wu, Q. , Ren, J. , Qian, W. , He, S. , Huang, K. , Yu, X.C. et al. (2012) Two novel RING‐type ubiquitin ligases, RGLG3 and RGLG4, are essential for jasmonate‐mediated responses in Arabidopsis. Plant Physiol. 160, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Cui, F. , Wu, Y. , Lou, L. , Liu, L. , Tian, M. , Ning, Y. et al. (2015) The RING finger ubiquitin E3 ligase SDIR1 targets SDIR1‐INTERACTING PROTEIN1 for degradation to modulate the salt stress response and ABA signaling in Arabidopsis. Plant Cell 27, 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Xie, J. , Zhou, Y. , Deng, L. , Yao, S. and Zeng, K. (2017) Inhibitory effect of Pichia membranaefaciens and Kloeckera apiculata against Monilinia fructicola and their biocontrol ability of brown rot in postharvest plum. Biol. Control 114, 51–58. [Google Scholar]
- Zhang, M. , Zhao, J. , Li, L. , Gao, Y. , Zhao, L. , Patil, S.B. , Fang, J. et al. (2017) The arabidopsis U‐box E3 ubiquitin ligase PUB30 negatively regulates salt tolerance by facilitating BRI1 kinase inhibitor 1 (BKI1) degradation. Plant Cell Environ. 40, 2831–2843. [DOI] [PubMed] [Google Scholar]
- Zhang, N. , Yin, Y. , Liu, X. , Tong, S. , Xing, J. , Zhang, Y. , Pudake, R.N. et al. (2017) The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant Physiol. 175, 1878–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Chen, H. , Duan, M. , Zhu, F. , Wen, J. , Dong, J. and Wang, T. (2019) Medicago falcata MfSTMIR, an E3 ligase of endoplasmic reticulum‐associated degradation, is involved in salt stress response. Plant J. 98, 680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G. , Yang, J. , Zhang, M. , Li, Q. , Wu, Y. , Zhao, X. , Zhang, H. et al. (2021) Wheat TaPUB1 regulates Cd uptake and tolerance by promoting the degradation of TaIRT1 and TaIAA17. J. Agric. Food Chem. 69, 5818–5829. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Lai, X. , Yang, S. , Ren, H. , Yuan, J. , Jin, H. , Shi, C. et al. (2021) Functional analysis of tomato CHIP ubiquitin E3 ligase in heat tolerance. Sci. Rep. 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Zheng, D. , Song, F. and Jiang, M. (2022) Expression patterns and functional analysis of 11 E3 ubiquitin ligase genes in rice. Front. Plant Sci. 13, 840360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Huang, Y. , Hu, Y. , He, X. , Shen, W. , Liu, C. and Ruan, Y. (2013) Phylogenetic analysis of Brassica rapa MATH‐domain proteins. Curr. Genomics 14, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Zhao, L. , Zhang, M. , Zafar, S.A. , Fang, J. , Li, M. , Zhang, W. et al. (2017) Arabidopsis E3 ubiquitin ligases PUB22 and PUB23 negatively regulate drought tolerance by targeting ABA receptor PYL9 for degradation. Int. J. Mol. Sci. 18, 1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Rispe, C. and Nabity, P.D. (2019) Secretory RING finger proteins function as effectors in a grapevine galling insect. BMC Genomics 20, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Zhang, H. , Hou, J. , Zhang, J. , Chen, S. and Song, B. (2020) Overexpression of SbRFP1 gene improves salt tolerance of in vitro potato plantlets. Acta Hortic Sin 47, 381. [Google Scholar]
- Zhou, B. and Zeng, L. (2018) The tomato U‐box type E3 ligase PUB13 acts with group III ubiquitin E2 enzymes to modulate FLS2‐mediated immune signaling. Front Plant Sci. 9, 335206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Li, X.‐H. , Guo, Q.‐H. , Liu, P. , Li, Y. , Wu, C.‐A. , Yang, G.D. et al. (2021) Salt responsive alternative splicing of a RING finger E3 ligase modulates the salt stress tolerance by fine‐tuning the balance of COP9 signalosome subunit 5A. PLoS Genet. 17, e1009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Li, Y. , Fei, F. , Wang, Z. , Wang, W. , Cao, A. , Liu, Y. et al. (2015) E3 ubiquitin ligase gene CMPG1‐V from Haynaldia villosa L. contributes to powdery mildew resistance in common wheat (Triticum aestivum L.). Plant J. 84, 154–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in PubMed at https://pubmed.ncbi.nlm.nih.gov/?term=Proteolysis‐targeting%20chimeras%20(PROTACs)%20&timeline=expanded. These data were derived from the following resources available in the public domain: – PubMed, https://pubmed.ncbi.nlm.nih.gov/ – WebofKnowledge, https://www.webofknowledge.com – Google Scholar, https://scholar.google.com – Springer, https://www.springer.com.


